1. Introduction
Mining sites represent a unique combination of industrial, historical, and natural heritage. These sites have been fundamental to the formation of social groups and communities, creating the conditions for economic and social development [
1]. In recent years, the extractive industry has faced significant challenges and difficulties in Italy. The depletion of mining resources, together with higher production costs compared with other countries, has caused the decline of the mining industry, especially for base metal production, which has had an economic and social impact on the country [
2]. The European Union has defined 34 critical raw materials, 16 of which are also considered strategic due to their relevance in ecological and digital transition [
3,
4,
5], intended for aerospace and defense, for the production of electric batteries, solar panels, and wind turbines [
6,
7,
8]. Italy has 15 of these 34 critical raw materials, specifically lithium, cobalt, barite, beryllium, nickel, tungsten, copper, and zinc. In Italy, metallic critical raw materials (CRMs), which represent the majority of critical materials, are not currently extracted, and for their supply, the country is dependent on foreign markets [
9,
10].
The CRM Act, approved by the European Council on March 2024, requires that by 2030, the annual consumption of the EU must be composed of at least 10% locally extracted minerals; at least 40% of the EU’s annual consumption should be from EU-processed minerals; and at least 25% of the supply should be obtained from recycled minerals [
11,
12]. The implementation of policies to secure a stable supply of CRMs and SRMs to minimize the volume of waste sent to disposal sites and landfills encourages the recovery and recycling of resources from waste within the extractive industry, such as in abandoned mining sites, referred to as mining waste or tailings [
13].
Mine waste and tailings represent a significant environmental concern for present and past mining activities and a potential opportunity for reprocessing and obtaining valuable minerals and CRMs [
14,
15,
16,
17,
18]. Various technologies and methodologies are being applied and developed for the reprocessing of mine waste and tailings, including mineral processing and hydrometallurgical and bio-hydrometallurgical techniques [
19,
20,
21]. However, many challenges remain in terms of technical difficulties, economic viability, and regulatory frameworks due to the complex conditions of these liabilities [
22]. The success of mine waste reprocessing projects depends on factors like detailed preliminary mineralogical and chemical characterization and the development of tailored recovery solutions [
19,
23].
In the literature, numerous applied examples of the laboratory-scale reprocessing of mine waste can be found. For instance, Abaka-Wood et al. [
24,
25] described a methodology for characterizing and recovering valuable iron oxides and rare earth element (REE) minerals from iron ore tailings in South Australia. Their approach utilized preliminary detailed mineralogical analyses, while wet high-intensity magnetic separation and flotation were tested for separation and concentration. Iron-bearing minerals were successfully recovered, but REE minerals faced problems with their concentration due to their varying magnetic properties. Similarly, Mulenshi et al. [
26] investigated beneficiation options for tungsten recovery from mine waste in Sweden. This study involved a preliminary sampling campaign, chemical and mineralogical characterization, and magnetic separation tests employing both low- and high-intensity magnetic separation for iron-bearing minerals and gravity separation for the recovery of tungsten minerals. However, there are still few published works on mine waste recovery from former base metal mines in Italy. Among these, Mehta et al. [
27] described sampling methodologies and laboratory-scale reprocessing trials using froth flotation and a shaking table to recover cadmium, gallium, and zinc compounds from mine waste collected in a mining district in Northern Italy. Their work also explored the potential reuse of processed tailings as soil additives to reduce the environmental impact of polluting minerals.
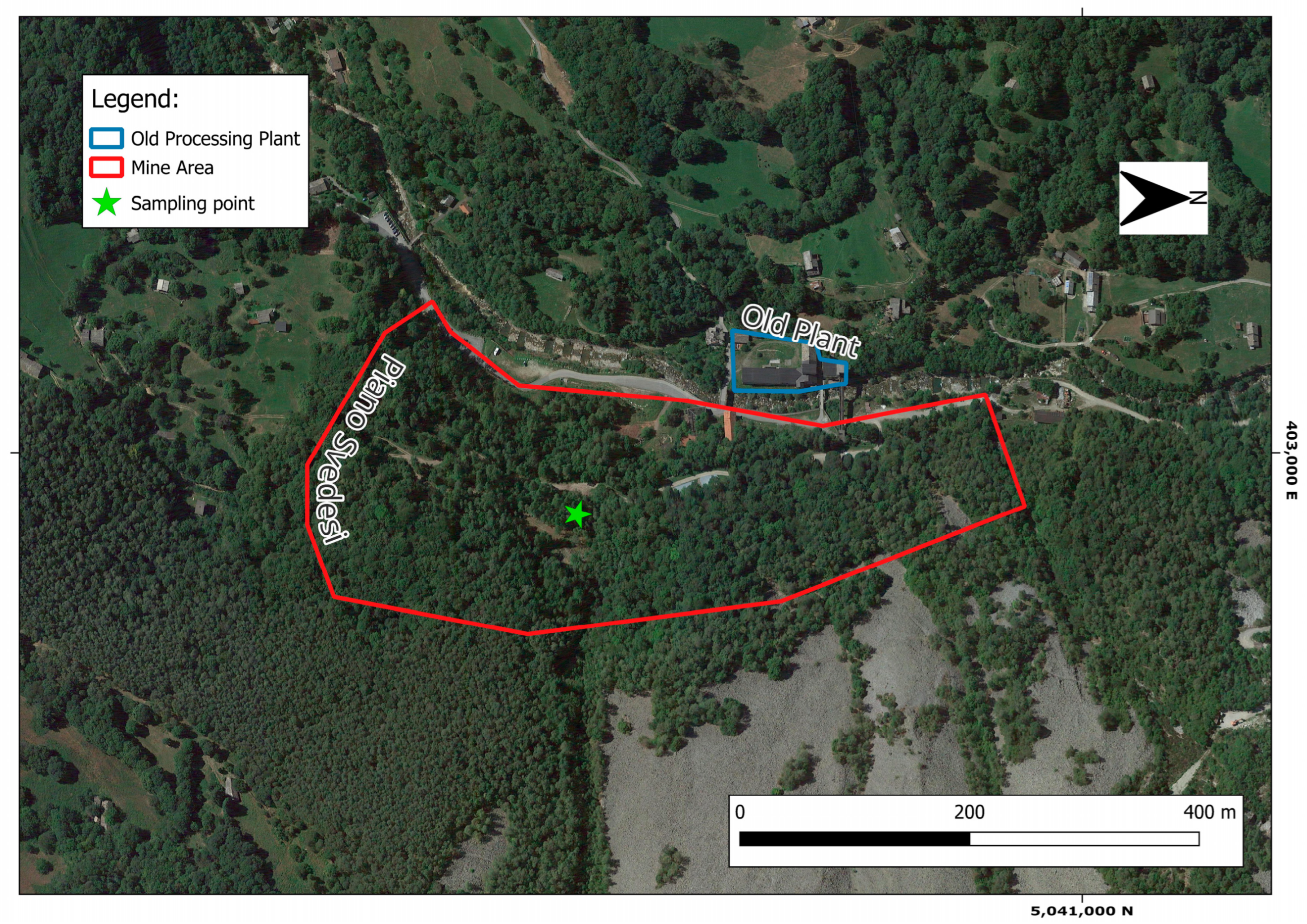
This study aims to explore, for the first time, the potential recovery of CRM and SRM-bearing minerals from a selected mine waste dump area in the historical Traversella mine district (Piedmont, Italy). This site, listed in the National Registry of Extractive Waste Facilities, was identified as containing significant mining waste [
28,
29]. Additionally, this work supports the preliminary study of alternative raw material supplies from relevant Italian mine waste areas, which have not been investigated in recent times for waste valorization.
Mine waste samples were collected from a selected waste rock dump in the Traversella mine area and subjected to mineralogical and chemical characterization using optical microscopy (OM), X-Ray powder diffraction (XRPD), scanning electron microscopy (SEM-EDS) and portable X-Ray fluorescence (p-XRF). Laboratory-scale reprocessing tests were performed in order to assess the possibility of recovering CRMs and SRMs by magnetic and gravimetric separation methods. Finally, the separation performance of the obtained concentrates containing iron (Fe) and minor critical and strategic elements such as copper (Cu) and tungsten (W) was assessed and compared.
3. Results
3.1. Original Sample Characterization
3.1.1. Optical Microscopy
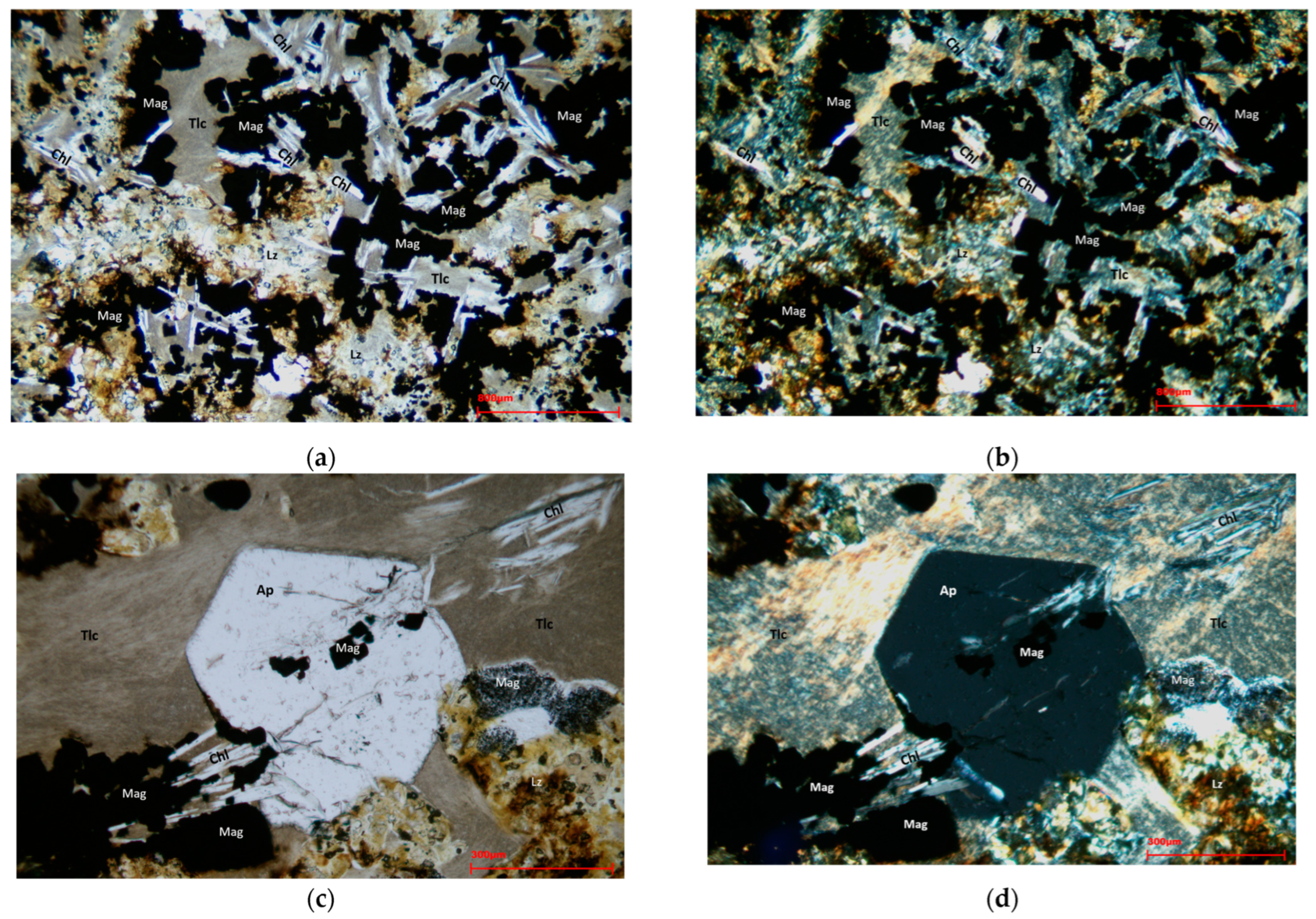
Normal and polarized transmitted light was used to identify the mineral structures and species associated with ferrous mineralization on the 30 µm thin sections realized.
Figure 5a–d show the images obtained from the petrographic observation of the thin sections in transmitted light. The sample appeared highly heterogeneous, characterized by a matrix of silicate minerals such as talc and lizardite, interspersed with chlorite crystals. The mineralization was characterized by opaque magnetite crystals with a regular cubic shape. In some cases, magnetite grains were included in apatite grains.
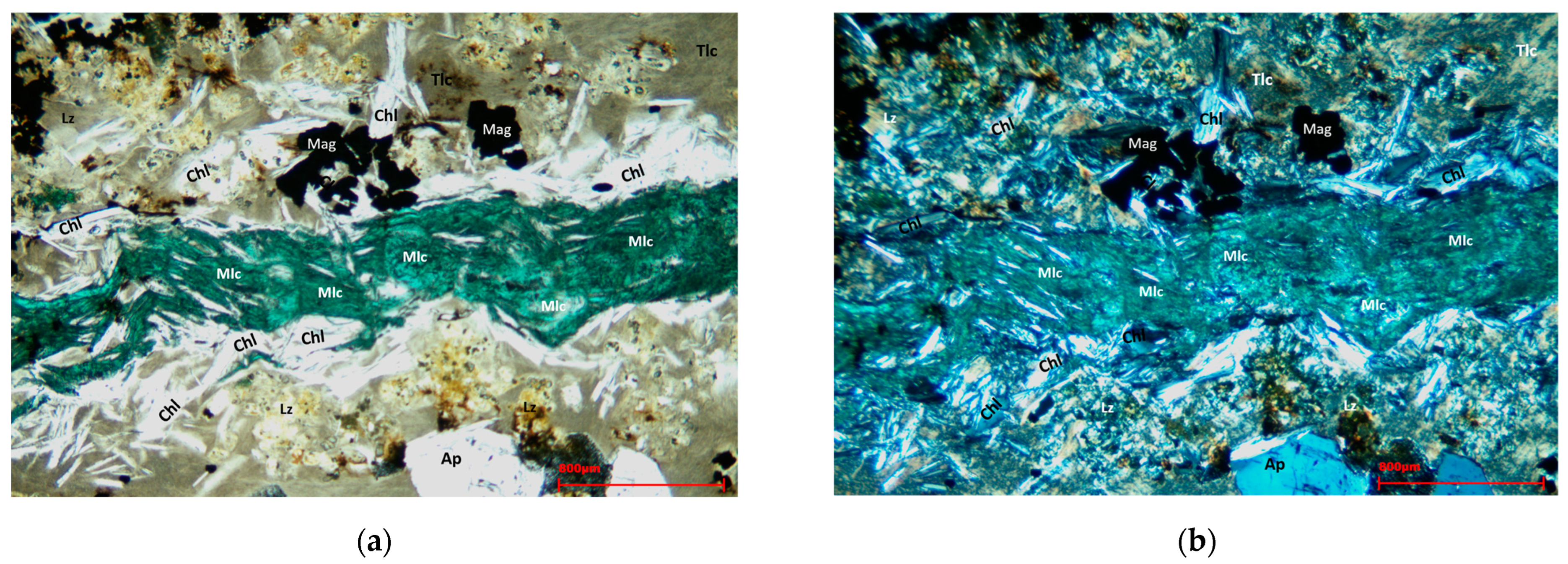
Figure 6a,b show a feature visible to the naked eye on the sample (see
Figure 3), namely a banded inclusion of malachite. This inclusion was surrounded by chlorite minerals and embedded in a matrix composed of talc and lizardite.
3.1.2. X-Ray Powder Diffraction (XRPD)
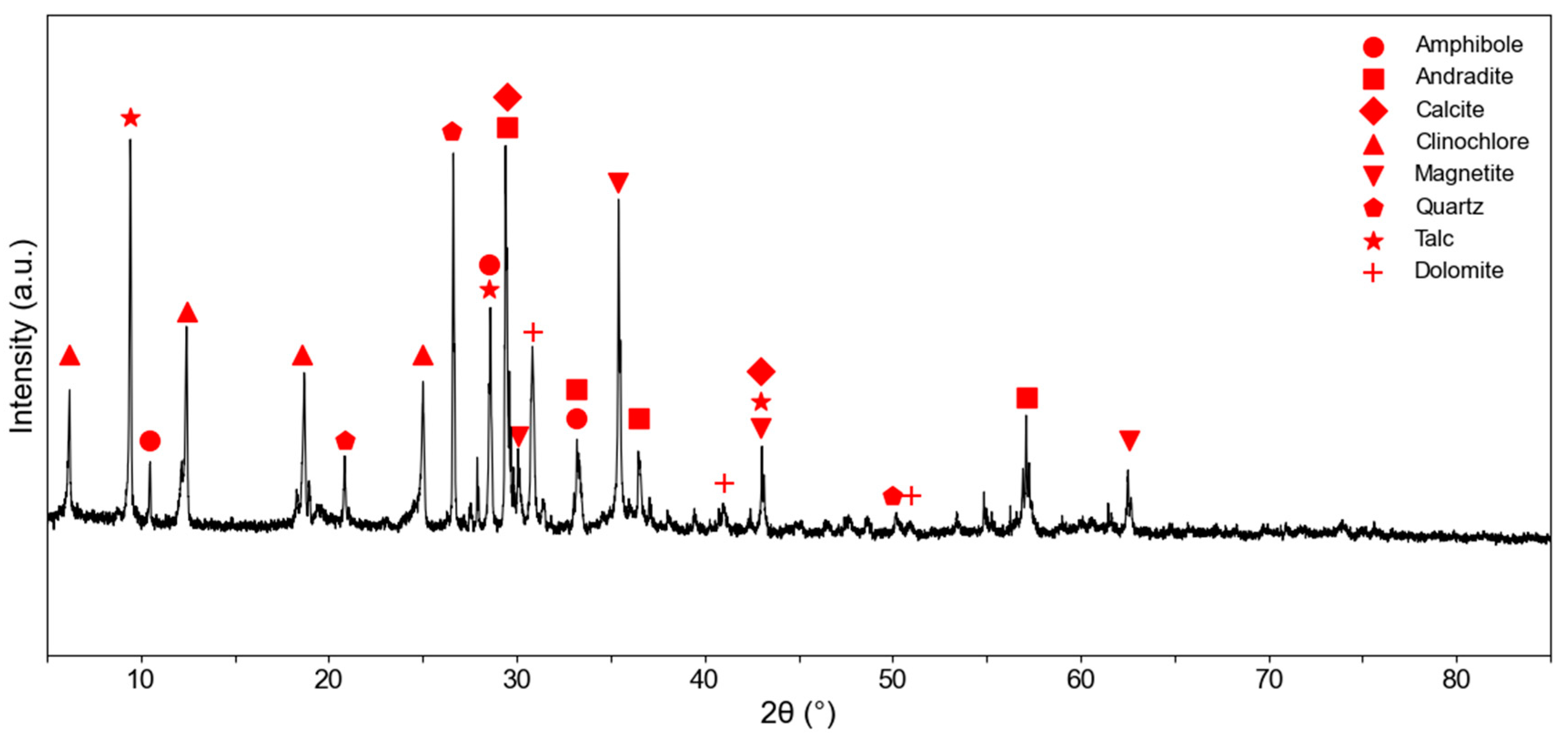
XRPD analyses performed on an untreated and representative portion of the initial sampled material detected the presence of seven mineral phases: amphibole, andradite, calcite, clinochlore, magnetite, quartz, and talc. The XRPD spectrum is reported in
Figure 7.
Reference spectra selected from the consulted database [
38] were compared to experimental ones. The detected peaks of the main mineral phases are reported in
Table 1.
Detected minerals in the initial non-treated waste sample confirmed the presence of mineral phases identified by OM.
3.1.3. Portable X-Ray Fluorescence (p-XRF)
P-XRF analyses investigated the chemical grades of 21 elements in the sampled material, as reported in
Table 2.
The elemental composition of the non-treated material showed that Si and Fe were the most abundant elements, respectively, with grades of 17.2% and 16.9%. Among other major elements, Mg was present in significant amounts (11.4%), while Ca, Al, and P showed lower concentrations of 4.0%, 1.8%, and 1.5%, respectively. S was assayed at 0.3%.
Regarding critical and strategic metals, Cu was the most abundant, with a grade of 1.3%. Mn, Ti, As, and W were present in traces, with concentrations of 0.1%, 544 ppm, 186 ppm, and 96 ppm, respectively. Additionally, Zn and Pb were quantified at 56 ppm and 54 ppm.
3.2. Crushing, Milling, and Sieving
After crushing and milling, sieving was performed to obtain three different granulometric classes.
Table 3 shows the weight fraction of the obtained materials.
The size class 0.125–0.250 mm was selected as the feeding material for the separation tests as it was the most suitable size class for the selected equipment. This material was further investigated in order to better define the mineralogical and chemical features. In addition, the liberation of target minerals was also investigated to confirm the selection of the most suitable feeding material.
3.3. Feeding Material Characterization—0.250–0.125 mm
Following grinding, two size classes were produced and characterized as feeding material for separation tests. Mineralogical characterization was realized by performing XRPD qualitative analysis; the acquired spectra are reported in
Figure 8.
Qualitative mineralogical analyses were performed on the acquired spectra, comparing the identified peaks with the reference database [
38]. The mineral phases identified are reported in
Table 4.
In the size class 0.250–0.125 mm, eight mineral phases were detected. These phases were comparable to the non-treated bulk material sampled in the field. P-XRF analyses were performed to obtain chemical data useful for assessing the grade of the feeding material before separation. Analytical results are reported in
Table 5.
Based on the XRPD and p-XRF results, SEM Energy-Dispersive Spectroscopy (EDS) analyses were performed to better identify the valuable and accessory minerals that may contain valuable elements but which are challenging to detect using XRPD due to their low concentrations.
Figure 9a–d show a BSE image, EDS spectra, and chemical composition of epoxy-embedded size class 0.250–0.125 mm material.
BSE images and EDS spectra showed the widespread presence of grains containing heavy elements, as indicated by the increased brightness intensity, which correlates with a greater average of the atomic number contained in the sample [
52]. Among these minerals, the majority were magnetite (
Figure 9a,c), while pyrite (
Figure 9a,b) grains were sparsely present in the material. Magnetite grains were predominantly observed as single grains, occasionally associated with gangue minerals. Gangue minerals were generally identified as silicates, possibly amphiboles, based on the EDS spectra reported in
Figure 9d.
Similarly,
Figure 10a–d show the BSE images recorded, identifying the main and accessory minerals contained in the samples.
CRM-bearing minerals were detected in the samples, including scheelite, observed as sparse single grains. These grains were characterized by a high brightness in the BSE images and as having relevant W content, as indicated in the EDS spectrum (
Figure 10a,b). Malachite was also detected as the most abundant Cu-bearing mineral in the samples (
Figure 10c,d).
Free-Grain Size Identification and Liberation Assessment
According to the BSE image quality and the extended presence of magnetite grains, BSE images acquired from size class 0.250–0.125 mm were selected as the input images for the semi-automated procedure for the estimation of liberation grade and liberated grain size distribution.
The manual selection of the areas associated with valuable grains on the images was performed by selecting magnetite-bearing grains on the original BSE image (
Figure 11a), producing two different binary masks: the first represented the total magnetite-bearing minerals (
Figure 11b), while the second represented the gangue inclusion of the first image (
Figure 11c).
The output binary masks were processed using the script provided in the
Supplementary Materials, which was designed to automatically calculate the grain-by-grain liberation of magnetite grains.
The script calculated the total area of each grain, determined the magnetite area by subtracting the area of gangue minerals from the magnetite-bearing grains mask, and quantified the proportion of magnetite in each grain to assess the overlap percentage of these areas. Single grains were categorized into three classes based on the following thresholds: liberated grains (≥80% magnetite area), middling grains (<80% and ≥30% magnetite area), and locked grains (<30% magnetite area). Additionally, the script calculated the granulometric distribution using the equivalent diameter derived from the grain-by-grain analysis. Overall, three different BSE images were considered for this evaluation, for a total number of 84 magnetite-bearing grains considered.
Figure 12 shows the results of the grain-by-grain evaluation, indicating the portions of the areas assigned to magnetite and gangue minerals.
In
Figure 13, the liberation analysis outcomes for the 0.250–0.125 mm size class are reported. Magnetite grains were, on average, 85.8% liberated (range 80.5–92.3%), with middling grains accounting for 13.5% (range 7.4–18.7%). Locked magnetite was negligible, averaging 0.6% (range 0.3–0.8%).
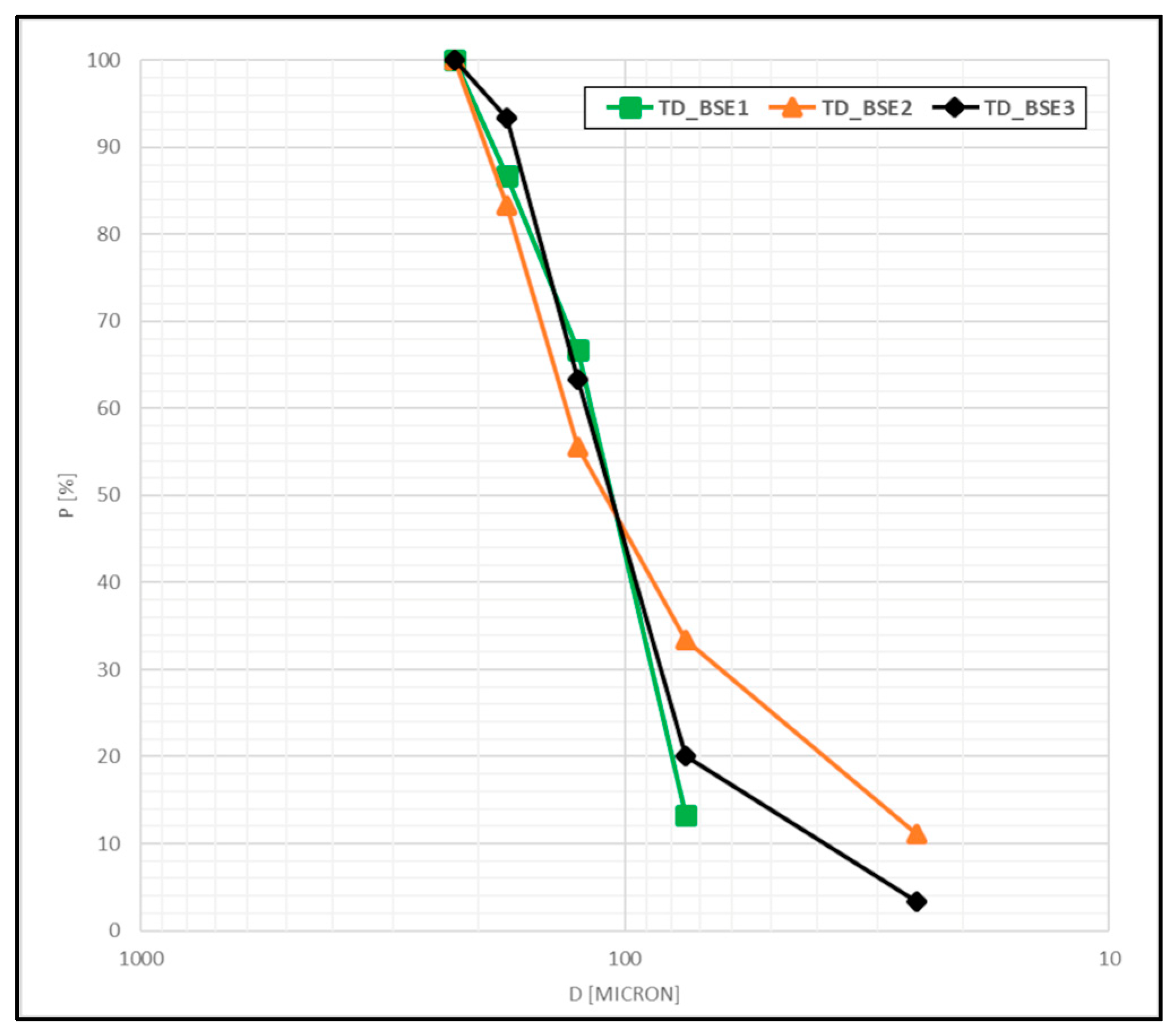
The liberated grain size was evaluated according to the equivalent diameter extrapolated from the identified areas of liberated magnetite grains, which are shown in
Figure 14. According to this granulometric distribution, P80 and P50 were calculated for each sample, as reported in
Table 6.
For P80, values ranged from 153 µm to 169 µm, with a mean value of 160 µm. For P50, values ranged from 109 µm to 113 µm, with a mean of 111 µm.
3.4. Separation Tests and Concentrate Characterization
The two size classes were subjected to separation tests based on their physical and chemical properties after the preliminary characterization.
The yield of each separation product was calculated according to the mass balance, considering a net calculation of losses. The Fe, Cu, and W grades were obtained using p-XRF analyses. XRPD analyses on concentrate were performed to qualitatively assess the mineralogy of the product.
Table 7 summarizes the data collected for the separation tests performed.
The LIMS test performed on the 0.250–0.125 mm size class produced a magnetic concentrate (LIMS-M) with a yield of 26.8%, characterized by a high Fe grade of 66.2%, and a low W concentration of 47 ppm. XRPD analysis identified magnetite with the presence of gangue minerals such as talc and clinochlore. The non-magnetic products (LIMS-NM1 and LIMS-NM2) accounted for 73.2% of the LIMS output, with LIMS-NM1 being the most abundant, with a 67.5% yield. LIMS-NM1 and LIMS-NM2 products contained significantly lower grades of Fe (7.6 wt% and 12.3 wt%) but were characterized by a higher presence of W, respectively, at 218 ppm and 280 ppm. The mineralogy of these materials is characterized by andradite, amphibole, calcite, and dolomite. The Cu grade remained relatively constant across all LIMS outputs, ranging between 0.6% and 0.9%.
The gravity separation test performed by ST produced a heavy concentrate (ST-H) with a yield of 12.1%, characterized by high Fe and W grades of 67.6% and 1289 ppm, respectively. The mineralogical analysis of this concentrate highlighted the presence of magnetite and andradite, which is associated with the presence of gangue minerals such as clinochlore, talc, and quartz. Notably, scheelite was not identified by XRPD. The middling material (ST-M), which accounted for 55.3% of the output, had a moderate Fe grade of 22.2% and a W grade of 256 ppm, with a mineralogy that included silicates, carbonate, and magnetite. The light material (ST-L), accounting for 32.6% of the yield, was characterized by low Fe (12.3%) and W (119 ppm) contents but showed the highest Cu grade among the ST outputs at 1.1%. The mineralogy of the light concentrate included amphibole, talc, carbonates, and andradite.
3.5. Separation Outcomes Evaluation
Following the characterization of the output separation concentrates, ER was calculated for target metals (Fe, W, and Cu), as reported in
Table 8.
The LIMS test produced a magnetic concentrate (LIMS-M) with an enrichment in the Fe grade of 2.8 times while showing very low ER values for W and Cu, 0.3 and 0.8, respectively. The non-magnetic products (LIMS-NM1 and LIMS-NM2) showed comparable results, with ER values for W ranging between 1.2 and 1.6, and for Cu, the values ranged between 1.1 and 1.3. Fe was poorly concentrated in LIMS-NM1 and LIMS-NM2 outputs with an ER of 0.3 and 0.5, respectively.
Gravimetric separation using ST produced a significant enrichment of Fe (2.8) and W (3.8) in the heavy concentrate (ST-H). The middling product (ST-M) showed no significant enrichment in any of the target elements analyzed. The light product (ST-L) showed a minimal concentration of Cu (1.3), while it was not enriched in Fe and W, with an ER of 0.5 and 0.4, respectively.
Table 9 shows the recovery rate (R) values calculated according to the mass balance and grades obtained (
Table 7).
The LIMS test achieved a recovery rate of 75.4% for Fe in the magnetic LIMS-M product, with partial recovery of Cu (20.5%) and low recovery of W (7.2%). Most of the W and Cu recovered in the LIMS-NM1 concentrate, with recovery rates of 83.7% and 72.3%, respectively. The LIMS-M2 concentrate exhibited low recovery rates for all three target elements.
ST gravity separation recovered 46.3% of W and 33.4% of Fe in the ST-H product. The middling concentrate (ST-M) exhibited the highest recovery rates for Fe and Cu at 50.2% and 52.8%, respectively, while W recovery in this fraction was 42.1%. The light product (ST-L) accounted for 42.9% of Cu recovery, but Fe and W recovery rates were the lowest, at 16.4% and 11.6%, respectively.
4. Discussion and Conclusions
Mine waste and tailings could represent a potential opportunity to create alternative sources of CRMs and SRMs [
14,
15,
16,
17,
18]. Due to the high mineralogical and chemical variability of these materials, detailed preliminary characterization and a tailored reprocessing solution could enhance the possibility of the successful recovery of raw materials [
23,
24,
25].
This study investigated the possibility of recovering valuable CRM- and SRM-bearing minerals from waste dumps in the historical Traversella mine (Piedmont, Italy) district, considering the lack of studies in the literature on mine waste recovery potential in this region for officially reported dump areas [
28,
29].
Mineralogical and chemical characterizations of the collected samples from the Piano degli Svedesi area were performed and were in accordance with previous studies about characteristics of the Traversella’s ore bodies described by Costa et al. [
32] and Nimis et al. [
31].
The semi-automated liberation analysis using Matlab showed that in the 0.250–0.125 mm size class, about 86% of magnetite minerals were liberated. This method was proven to be a cost-effective alternative to commercial liberation analysis methods and appeared to be effective in accounting for the liberation grade of target minerals considered [
53,
54].
According to characterization, the primary target mineral was identified as magnetite, while scheelite, as a source of CRMs, was considered an accessory target mineral. Malachite was also considered a minor source of copper. The 0.250–0.125 mm size class was tested using LIMS to concentrate magnetic minerals and evaluate the potential enrichment of scheelite and malachite in the non-magnetic fraction. Additionally, gravimetric separation using ST was performed on this size class to explore concentration strategies for recovering magnetite and scheelite due to their high specific gravity.
In
Figure 15, the grades of target elements and the recovery rates of different output products of LIMS and ST were compared. In addition, products showing an ER higher than one are highlighted.
Assessing the separation performances of the proposed methodologies, LIMS resulted in the production of a magnetic concentrate characterized by a high grade in Fe, recovering about 75% of the total available element in the feed and achieving a concentration 2.8 times that from the beginning. This was proved by mineralogy, in which magnetite was the predominant mineral in the output. Similarly, the non-magnetic product, especially Non-Magnetic 1, reached a recovery of about 85% of W, even if not enriched in this element, resulting in an overall grade (218 ppm) comparable to the original feed (204 ppm). Moreover, in the non-magnetic product, the Fe content decreased from 18.2% to 7.6%. For Cu, more than 75% of this metal was recovered in the LIMS_NM1 non-magnetic product, but no relevant enrichment was achieved (ER = 1.1).
These outcomes from LIMS separation tests highlight the good liberation achieved of magnetite from W-bearing and Cu-bearing minerals, remarking differences in the magnetic susceptibility between magnetite, scheelite and malachite, among other gangue minerals. These results seem to be consistent with the work of Nzeh et al. [
55], which indicates that the effective separation between target minerals could be attributed to this physical characteristic.
ST produced a highly enriched heavy concentrate product, containing 67.6% of Fe and 1289 ppm of W, and showing an ER of 2.5 for Fe and 3.8 for W. Recovery rates remained limited only to 33.4% for Fe and 46.3% for W. The middling product did not succeed in enriching any of the target elements but recovered 50.2% of Fe, 42.1% of W, and 52.8% of Cu. In addition, a slight enrichment in W (1.3) and Cu (1.3) was obtained in the light fraction.
Gravity separation using ST showed typical behavior in enhancing the concentration of heavy minerals at the expense of yield and overall recovery, making it efficient in separating coarse light particles from small dense particles [
42,
56]. In addition, a small difference in density between magnetite (5.15 g/cm
3) and scheelite (6.01 g/cm
3) could result in the inefficient separation of these minerals, which is in agreement with the attempt to recover scheelite from iron-ore mine waste described by Mulenshi et al. [
26].
The present study raises the possibility of recovering CRMs and SRMs from mine waste, promising domestic alternatives for their supply in the EU. However, this work comprised sample collection in a limited accessible sector of the Piano degli Svedesi dump site in the Traversella mine district. Indeed, scientific efforts should be addressed to a wider characterization of mine waste in this area.
Future work should be focused on the assessment of blended separation tests, combining LIMS to efficiently separate magnetite from scheelite and malachite. ST should be tested for the recovery of W-bearing minerals on the non-magnetic product of LIMS in order to enhance the recovery of heavier CRM-bearing minerals. In addition, alternative gravity separation methods, such as mineral jigs [
57] or falcon concentrators [
58] and froth flotation [
59,
60,
61,
62], should be considered for enhancing tungsten mineral recovery.