Dissolution Kinetics of Carbonates in Low-Grade Microgranular Phosphate Ore Using Organic Acids as Leaching Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
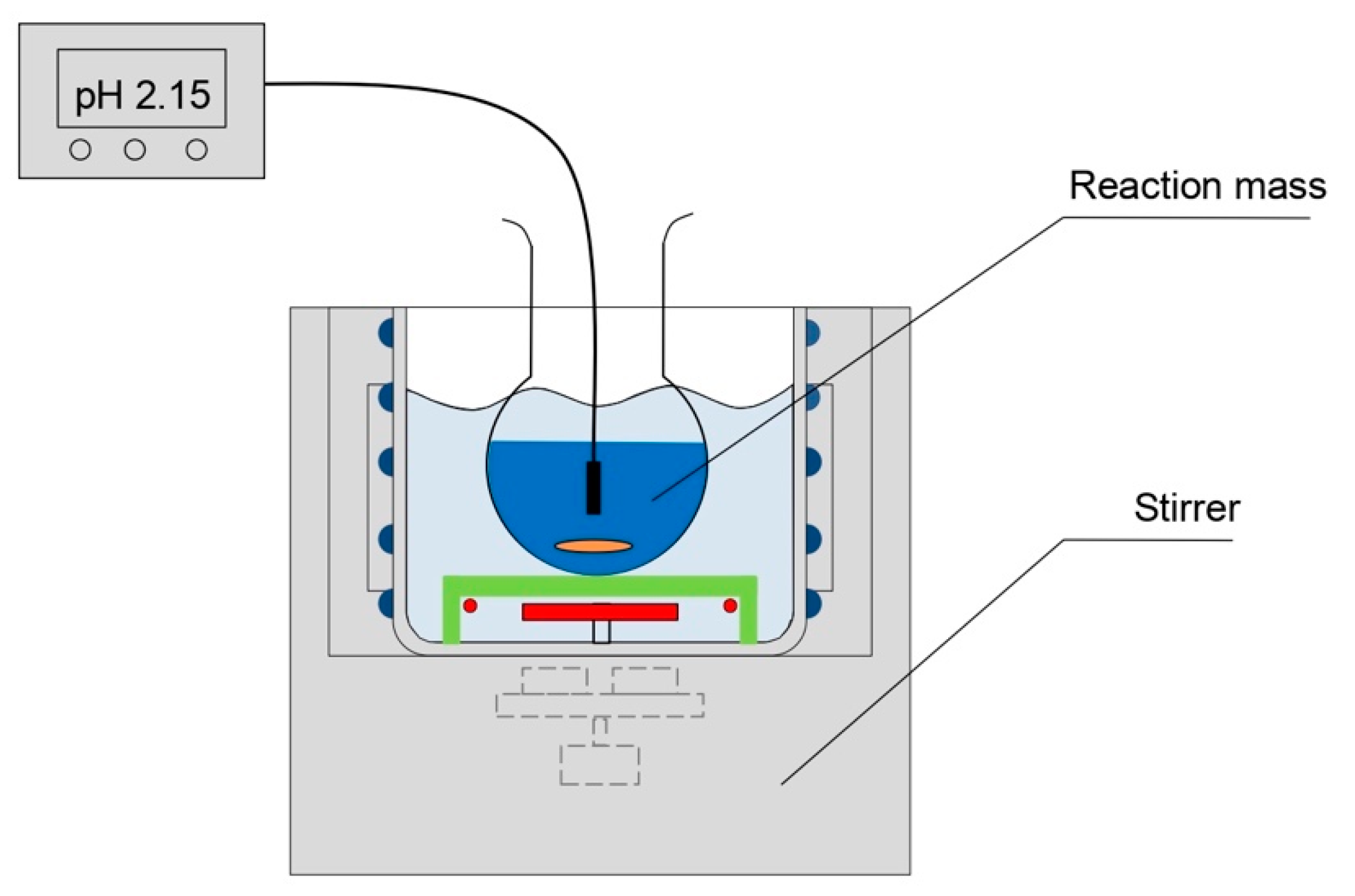
2.2. Experimental Methods
2.3. Analytical Techniques
3. Results and Discussion
3.1. Characterization of the Raw Material
3.2. Acid Leaching Experiments
3.3. Kinetic Patterns
4. Conclusions
- Temperature Impact: formic acid’s reaction rate increased with temperature, while citric acid showed higher rate constants at 55 °C but higher rate constants at 70 °C, indicating a complex mechanism.
- Activation Energy: the activation energy for formic acid was 14.69 kJ/mol, suggesting a diffusion-controlled process, while citric acid had a higher activation energy of 35.78 kJ/mol, indicating a mixed mechanism.
- Efficiency of Acids: citric acid was more effective in dissolving carbonates, particularly at elevated temperatures, compared to formic acid.
- Phosphate Enrichment: the P2O5 content increased to 22.15% with citric acid and to 19.14% with formic acid, demonstrating the effectiveness of both acids in phosphate enrichment.
- Optimal Conditions: formic acid was more efficient at lower temperatures, while citric acid required careful temperature control to maintain reaction efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Mining Review. February 2024. Available online: https://www.globalminingreview.com/magazine/world-fertilizer/february-2024/ (accessed on 25 August 2024).
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Ahmadi, N.; Felhi, M.; El Bahri, D. Beneficiation of low-grade carbonated phosphate ore by reverse flotation technics using anionic and cationic collectors, Sra Ouertane region, Northwest Tunisia. Euro-Mediterr. J. Environ. Integr. 2024. [Google Scholar] [CrossRef]
- Arab, I.; Derqaoui, M.; Abidi, A.; Yaacoubi, A.; El Amari, K.; Etahiri, A.; Bacaoui, A. Direct flotation of low-grade Moroccan phosphate ores: A preliminary micro-flotation study to develop new beneficiation routes. Arab. J. Geosci. 2020, 13, 1252. [Google Scholar] [CrossRef]
- Clifford, P.; Lloyd, M.; Zhang, P. Technology research improves phosphate economics. Min. Eng. 1998, 98, 46–51. [Google Scholar]
- Raiymbekov, Y.; Besterekov, U.; Abdurazova, P.; Nazarbek, U. Review of methods and technologies for the enrichment of low-grade phosphorites. Rev. Inorg. Chem. 2022, 42, 385–395. [Google Scholar] [CrossRef]
- Wilemon, G.M.; Scheiner, B.J. Leaching of the Phosphate Values from Two Central Florida Ores, Using H2SO4-Methanol Mixtures; Buredu of Mines: Washington, DC, USA, 1979; Volume 9094, 9p. [Google Scholar]
- Dahanayake, K.; Senaratne, A.; Subasinghe, S. Potential Use of Naturally Occurring Sulphuric Acid to Beneficiate Poorly Soluble Phosphate from Eppawala, Sri Lanka. Fert. Res. 1991, 29, 197–201. [Google Scholar] [CrossRef]
- Good, P.C.; Goff, T.N.; White, J.C. Acidulation of Florida Phosphate Matrix in a Single-Tank Reactor; Buredu of Mines: Washington, DC, USA, 1979; Volume 8339, 16p. [Google Scholar]
- Zafar, I.Z.; Anwar, M.M.; Pritchard, D.W. A new route for the beneficiation of low-grade calcareous phosphate rocks. Fertil. Res. 1996, 44, 133–142. [Google Scholar] [CrossRef]
- Sadeddin, W.; Abu-Eishah, S.I. Minimization of free calcium carbonate in hard and medium-hard phosphate rocks using dilute acetic acid solution. Int. J. Miner. Process. 1990, 30, 113–125. [Google Scholar] [CrossRef]
- Sengul, H.; Ozer, A.K.; Gulaboglu, M.S. Beneficiation of Mardin-Mazidagi (Turkey) calcareous phosphate rock using dilute acetic acid solution. Chem. Eng. J. 2006, 122, 135–140. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, J.; Chen, J.; Wang, J.; Wu, L. Research on Enrichment of P2O5 from Low-Grade Carbonaceous Phosphate Ore via Organic Acid Solution. J. Anal. Methods Chem. 2019, 2019, 9859580. [Google Scholar] [CrossRef] [PubMed]
- Haweel, C.K.; Abdul-Majeed, B.A.; Eisa, M.Y. Beneficiation of Iraqi Akash at Phosphate Ore Using Organic Acids for the Production of Wet Process Phosphoric Acid. Al-Khwarizmi Eng. J. 2013, 9, 24–38. [Google Scholar]
- Arroug, L.; Elaatmani, M.; Zegzouti, A.; Aitbabram, M. Low-Grade Phosphate Tailings Beneficiation via Organic Acid Leaching: Process Optimization and Kinetic Studies. Minerals 2021, 11, 492. [Google Scholar] [CrossRef]
- GOST 14180-80; Ores and Concentrates. Methods of Sampling and Preparing Samples for Chemical Analysis and Determination of Moisture. Gosstandart of the USSR: Bergamo, Italy, 1980.
- ST RK 2211-2012; Phosphate Raw Materials of Fine Grinding Karatau. Technical Conditions. Committee of Technical Regulation and Metrology of the Ministry of Industry and New Technologies of the Republic of Kazakhstan: Astana, Kazakhstan, 2012.
- ST RK 2213-2012; Raw Materials are Crushed Phosphate Karatau. Technical Conditions. Committee of Technical Regulation and Metrology of the Ministry of Industry and New Technologies of the Republic of Kazakhstan: Astana, Kazakhstan, 2012.
- Tarasevich, B.N. IR Spectra of Main Classes of Organic Compounds: Reference Materials; Moscow State University: Moscow, Russia, 2012; 55p. [Google Scholar]
- Korovkin, M.V.; Ananyeva, L.G. Infrared Spectroscopy of Carbonate Rocks and Minerals; TPU: Tomsk, Russia, 2016; 75p. [Google Scholar]
- Seitnazarov, A.; Namazov, S.; Beglov, B. Beneficiation of High-Calcareous Phosphorites of Central Kyzylkum with Organic Acid Solutions. J. Chem. Technol. Metall. 2014, 49, 383–390. [Google Scholar]
- Zafar, I.Z.; Anwar, M.M.; Pritchard, D.W. Selective leaching of calcareous phosphate rock in formic acid: Optimisation of operating conditions. Miner. Eng. 2006, 19, 1459–1461. [Google Scholar] [CrossRef]
- Heidarpour, T. Processing of Dalir Phosphate Samples Using Leaching Method. Master’s Thesis, Department of Mining & Metallurgical Engineering, Amirkabir University of Technology, Tehran, Iran, 2009. [Google Scholar]
- Jander, W. Reaktionen im festen Zustande bei höheren Temperaturen. Reaktionsgeschwindigkeiten endotherm verlaufender Umsetzungen. Z. Anorg. Allg. Chem. 1927, 163, 1–30. [Google Scholar] [CrossRef]
- Makanyire, T.; Jha, A.; Sutcliffe, S. Kinetics of hydrochloric acid leaching of niobium from TiO2 residues. Int. J. Miner. Process. 2016, 157, 1–6. [Google Scholar] [CrossRef]
- Liddel, K.C. Shrinking core models in hydrometallurgy: What students are not being told about the pseudo-steady approximation. Hydrometallurgy 2005, 79, 62–68. [Google Scholar] [CrossRef]
- Taghavi, M.; Gharabaghi, M.; Shafaie, S.Z. Selective leaching of low-grade phosphate ore using a mixture of organic acids. Int. J. Min. Geo-Eng. 2020, 54, 65–70. [Google Scholar]






| P2O5 | CaO | MgO | K2O | Al2O3 | Fe2O3 | SiO2 |
|---|---|---|---|---|---|---|
| 13.36 | 24.12 | 2.87 | 1.04 | 6.01 | 2.39 | 36.14 |
| Temperature, °C | Reaction Rate Constants, min−1 | |
|---|---|---|
| For the Use of HCOOH | For the Use of C6H8O7 | |
| 25 | 0.0049 | 0.0020 |
| 40 | 0.0060 | 0.0124 |
| 55 | 0.0076 | 0.0160 |
| 70 | 0.0108 | 0.0142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiymbekov, Y.; Abdurazova, P.; Nazarbek, U. Dissolution Kinetics of Carbonates in Low-Grade Microgranular Phosphate Ore Using Organic Acids as Leaching Agents. Mining 2024, 4, 766-776. https://doi.org/10.3390/mining4040043
Raiymbekov Y, Abdurazova P, Nazarbek U. Dissolution Kinetics of Carbonates in Low-Grade Microgranular Phosphate Ore Using Organic Acids as Leaching Agents. Mining. 2024; 4(4):766-776. https://doi.org/10.3390/mining4040043
Chicago/Turabian StyleRaiymbekov, Yerkebulan, Perizat Abdurazova, and Ulzhalgas Nazarbek. 2024. "Dissolution Kinetics of Carbonates in Low-Grade Microgranular Phosphate Ore Using Organic Acids as Leaching Agents" Mining 4, no. 4: 766-776. https://doi.org/10.3390/mining4040043
APA StyleRaiymbekov, Y., Abdurazova, P., & Nazarbek, U. (2024). Dissolution Kinetics of Carbonates in Low-Grade Microgranular Phosphate Ore Using Organic Acids as Leaching Agents. Mining, 4(4), 766-776. https://doi.org/10.3390/mining4040043






