A Review of In Situ Leaching (ISL) for Uranium Mining
Abstract
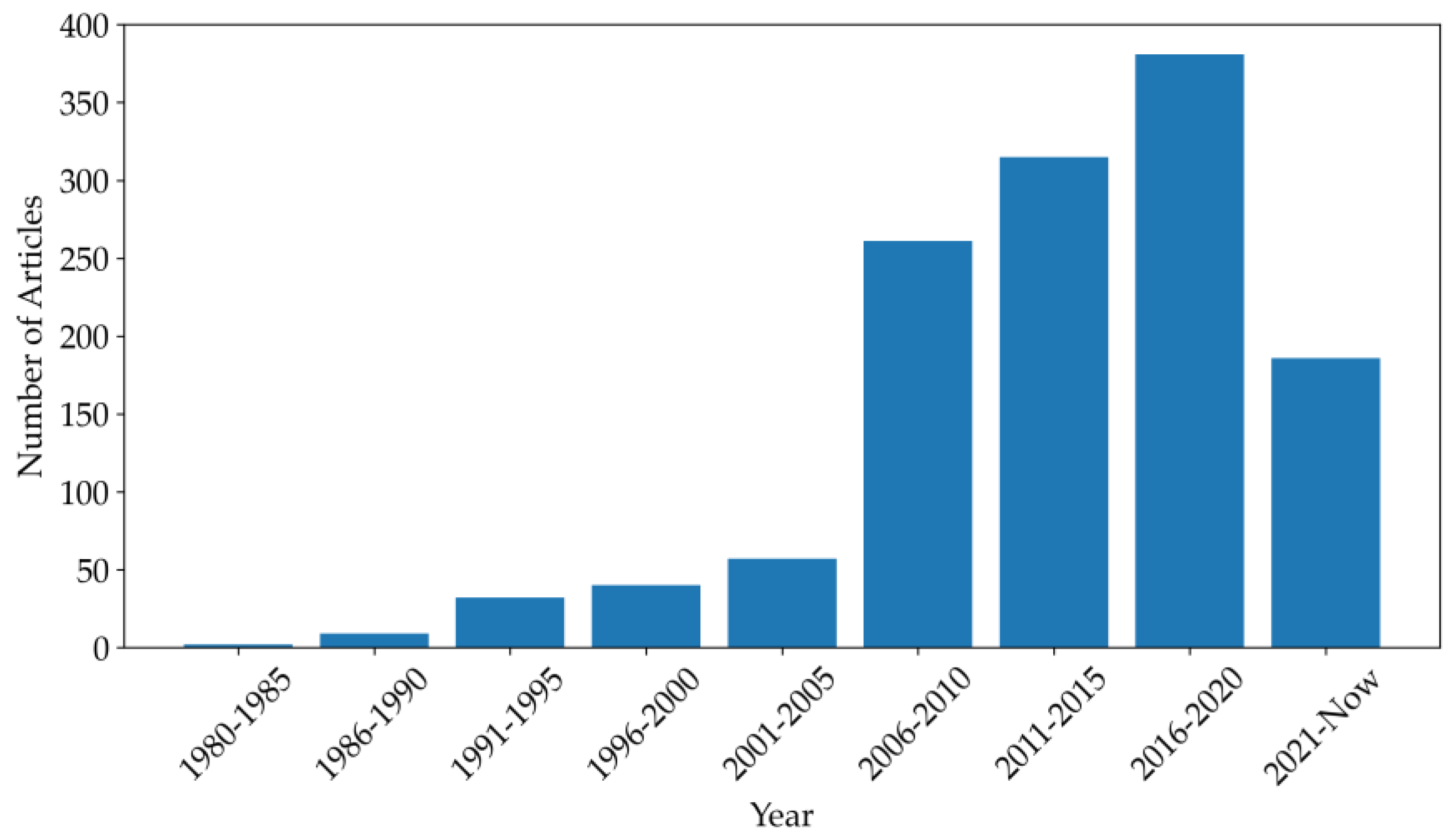
1. Introduction
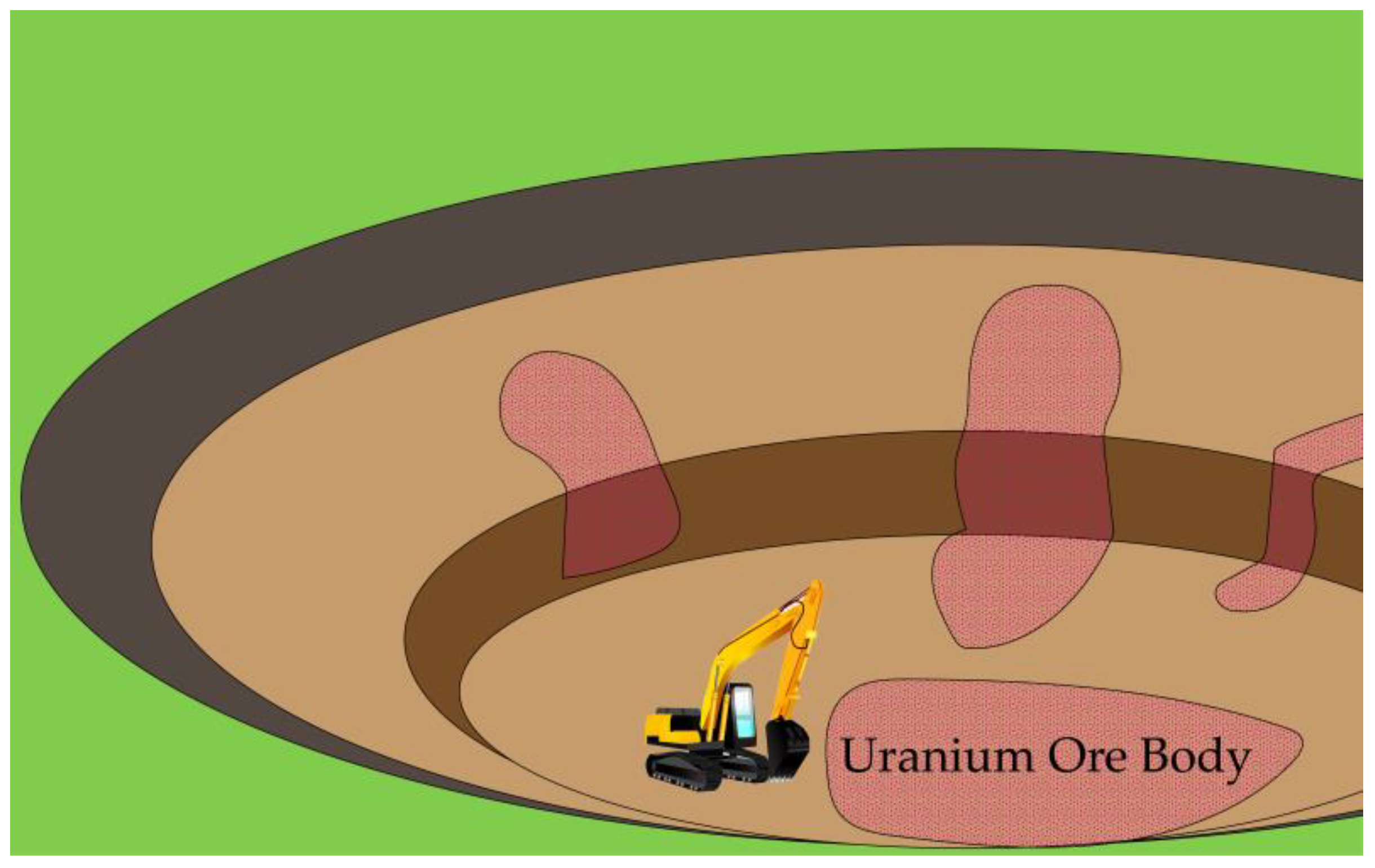
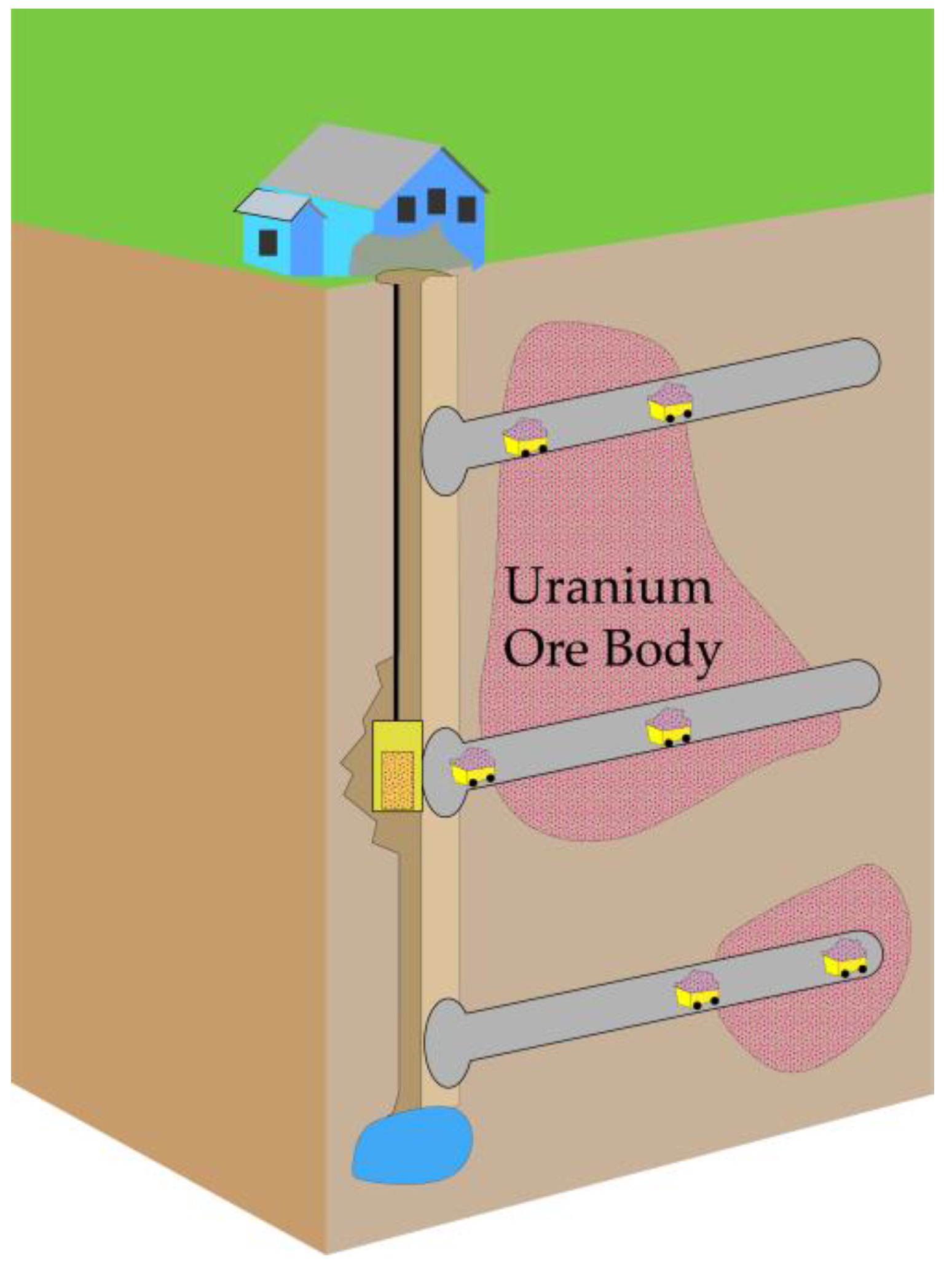
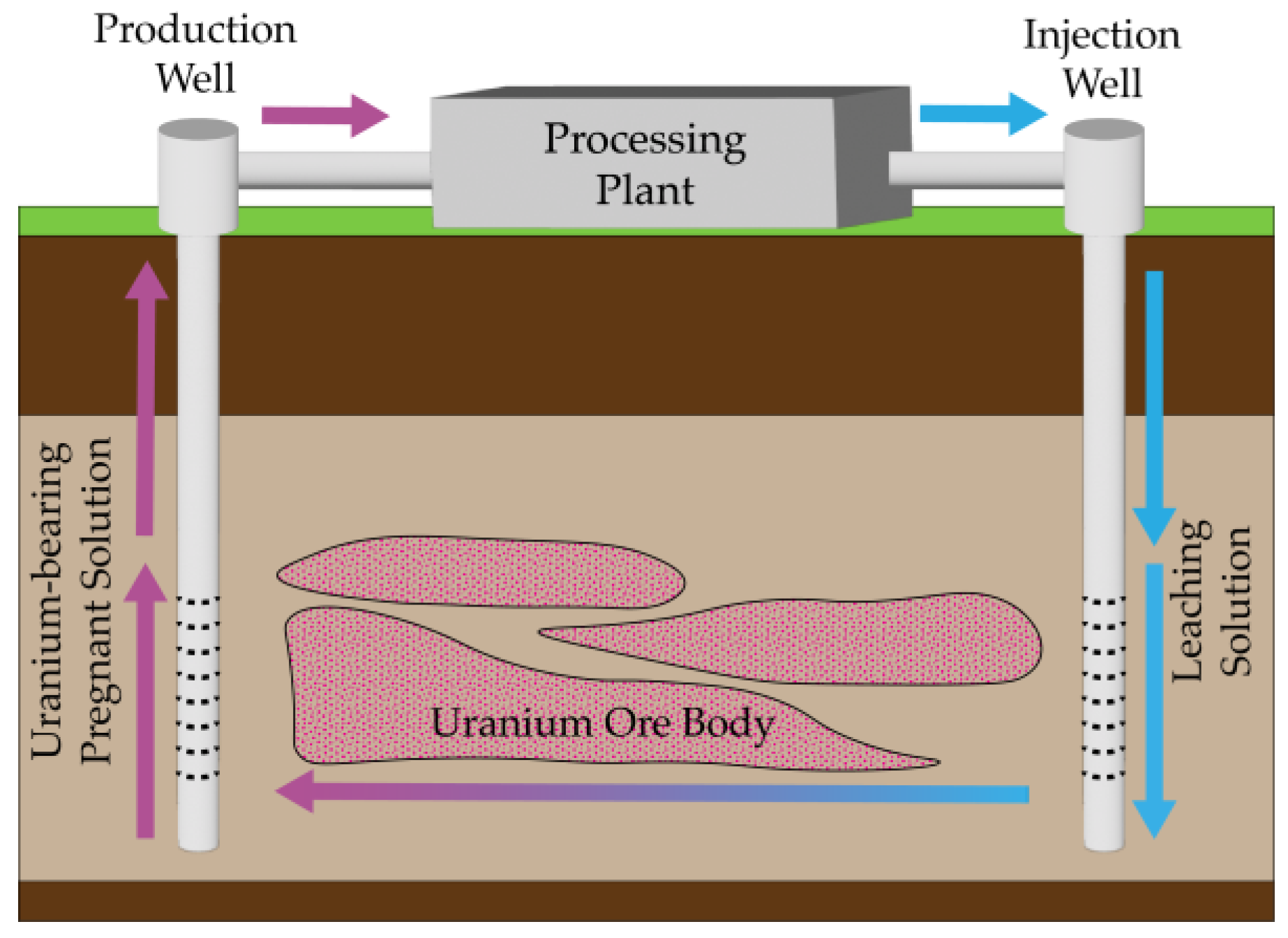
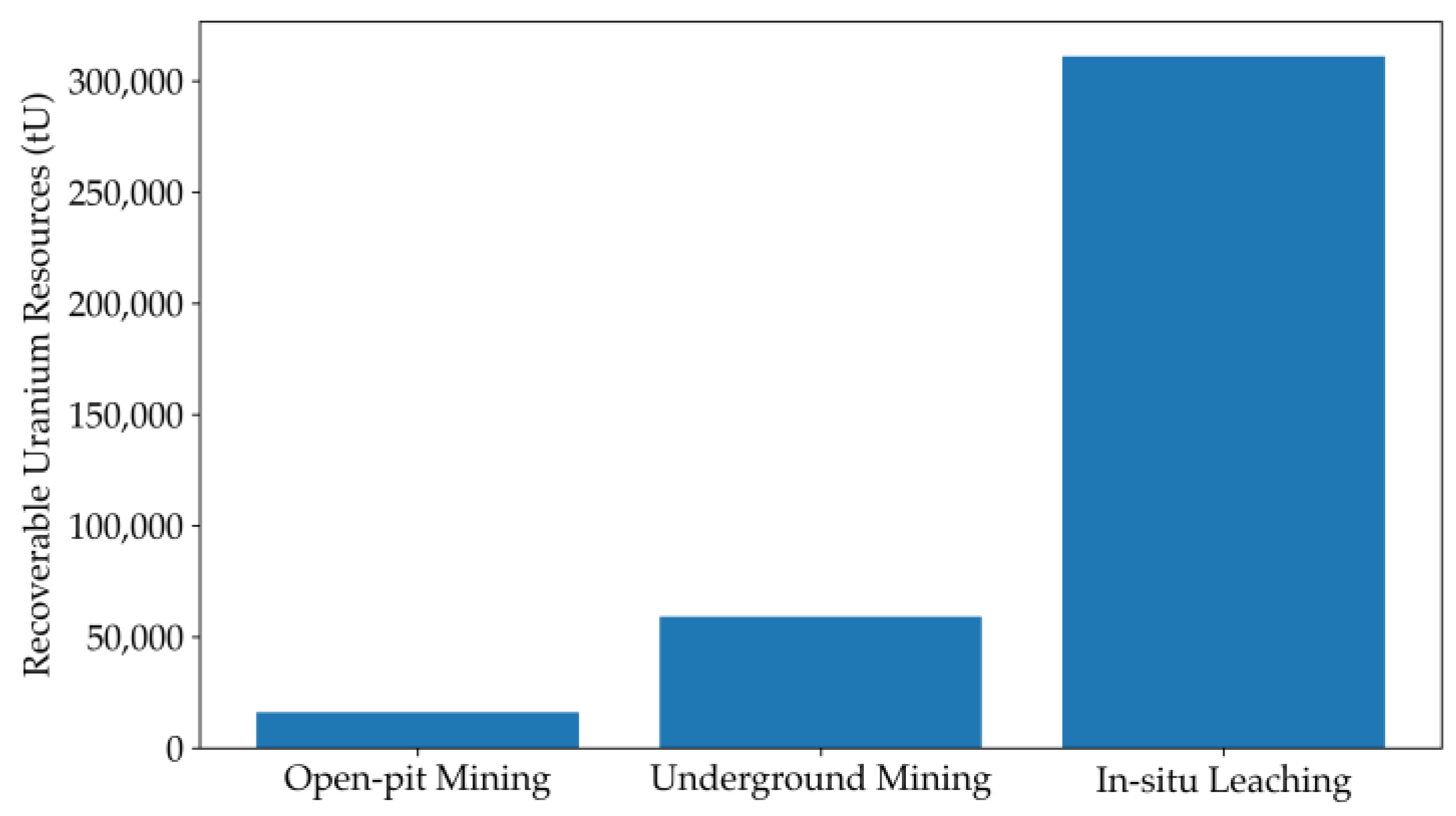
2. Overview of Uranium Mining Methods
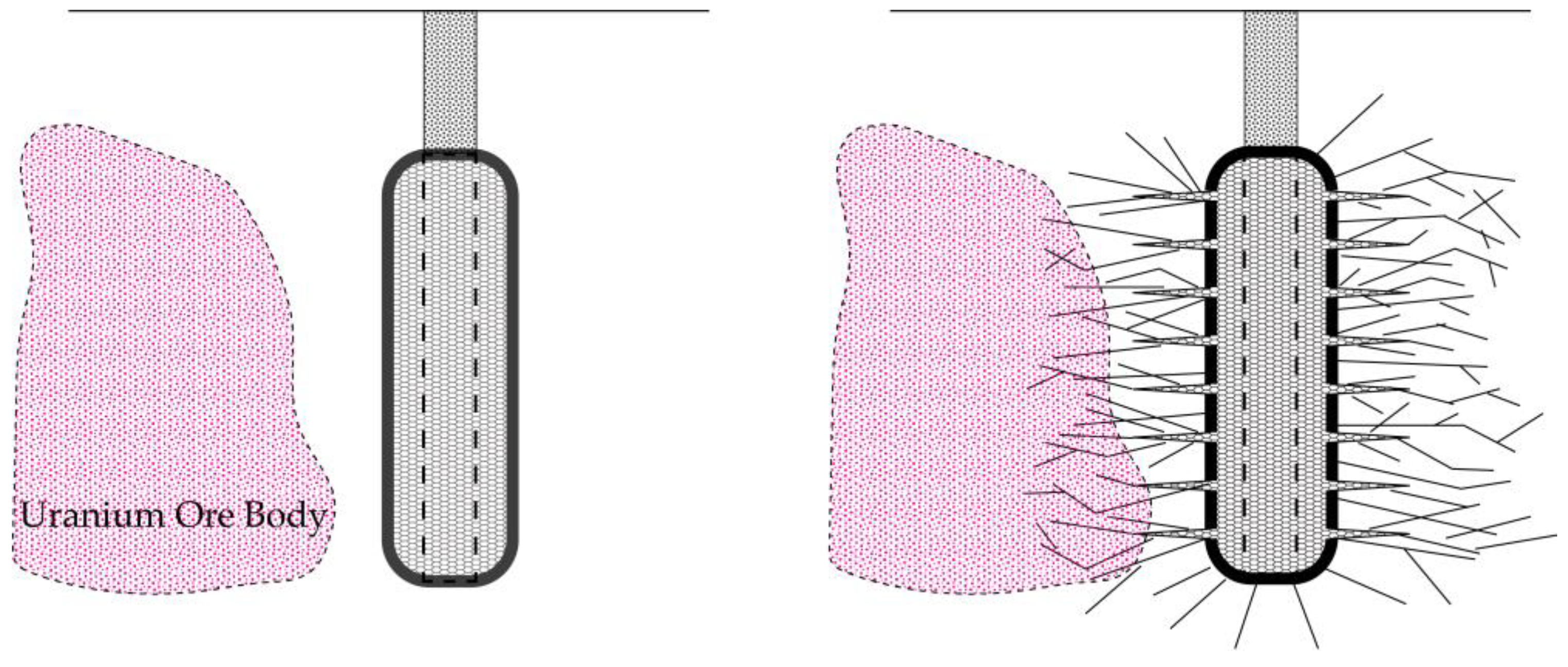
3. In Situ Leaching Techniques
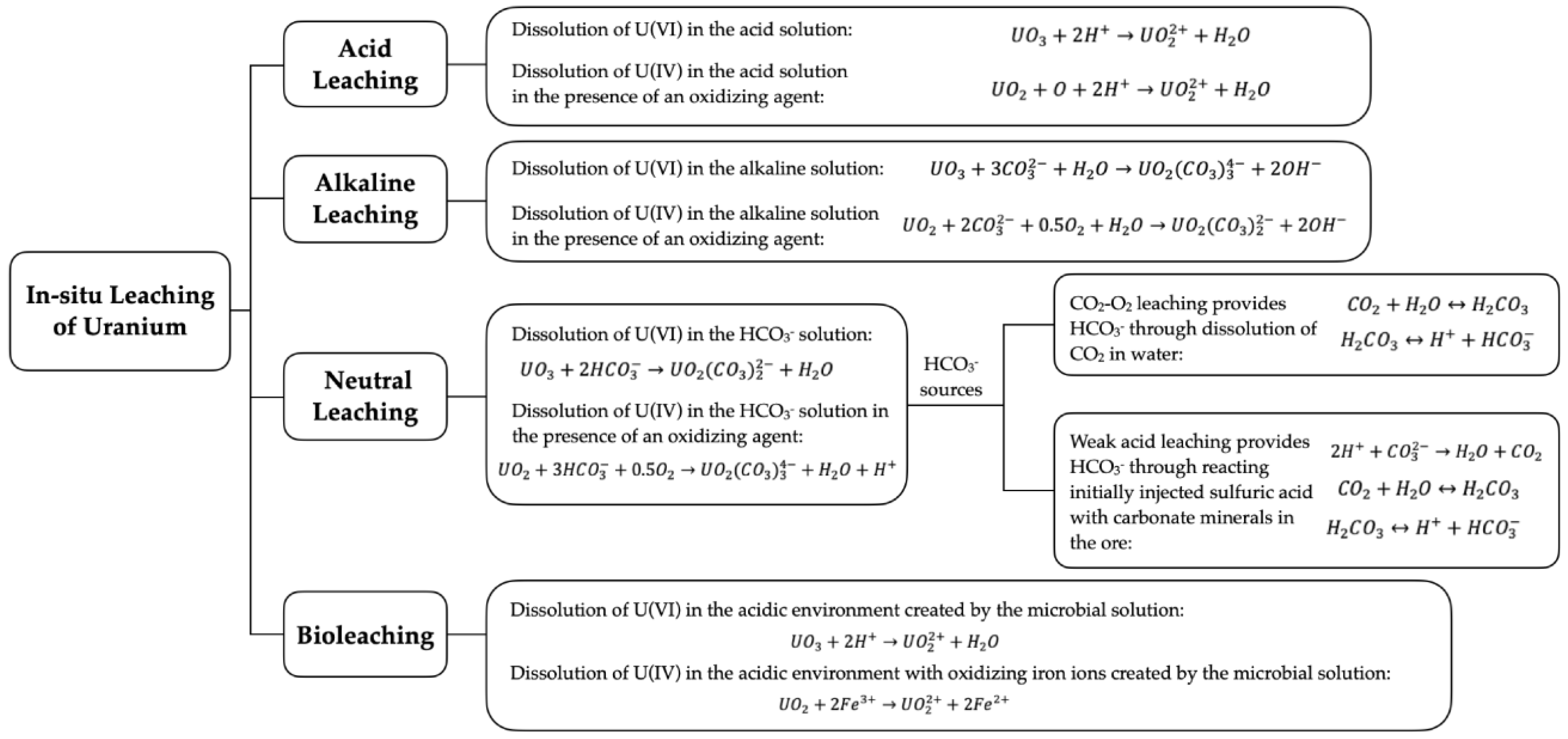
3.1. Acid Leaching
3.2. Alkaline Leaching
3.3. Neutral Leaching
3.3.1. CO2-O2 Leaching
3.3.2. Weak Acid Leaching
3.4. Bioleaching
4. Technological Innovations in In Situ Leaching
4.1. Permeability Modification Technique for In Situ Leaching
4.2. Prediction Technique for Fluid Flow and Geochemical Reaction for In Situ Leaching
4.3. Information Technology for In Situ Leaching
5. Application Status of In Situ Leaching
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Storrs, K.; Lyhne, I.; Drustrup, R. A Comprehensive Framework for Feasibility of CCUS Deployment: A Meta-Review of Literature on Factors Impacting CCUS Deployment. Int. J. Greenh. Gas Control 2023, 125, 103878. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Han, J.; Li, J.; Tang, X.; Wang, L.; Yang, X.; Ge, Z.; Yuan, F. Coal-Fired Power Plant CCUS Project Comprehensive Benefit Evaluation and Forecasting Model Study. J. Clean. Prod. 2023, 385, 135657. [Google Scholar] [CrossRef]
- Bai, X.; Yan, G.; Kong, S.; Yao, J.; Wen, P.; Li, G.; Li, J.; Zhang, J. Suppression of Anthracite Dust by a Composite of Oppositely-Charged Ionic Surfactants with Ultra-High Surface Activity: Theoretical Calculation and Experiments. Fuel 2023, 344, 128075. [Google Scholar] [CrossRef]
- Su, C.-W.; Pang, L.-D.; Qin, M.; Lobonţ, O.-R.; Umar, M. The Spillover Effects among Fossil Fuel, Renewables and Carbon Markets: Evidence under the Dual Dilemma of Climate Change and Energy Crises. Energy 2023, 274, 127304. [Google Scholar] [CrossRef]
- Bai, X.; Yan, G.; Kong, S.; Yang, T.; Yao, J.; Wen, P.; Li, G. Study on the Mechanism of the Influence of Surfactant Alkyl Chain Length on the Wettability of Anthracite Dust Based on EDLVO Theory and Inverse Gas Chromatography. Fuel 2023, 353, 129187. [Google Scholar] [CrossRef]
- Yao, J.; Li, G.; Wu, J. Application of In-Situ Combustion for Heavy Oil Production in China: A Review. J. Oil Gas Petrochem. Sci. 2018, 1, 69–72. [Google Scholar] [CrossRef]
- Fetisov, V.; Gonopolsky, A.M.; Davardoost, H.; Ghanbari, A.R.; Mohammadi, A.H. Regulation and Impact of VOC and CO2 Emissions on Low-carbon Energy Systems Resilient to Climate Change: A Case Study on an Environmental Issue in the Oil and Gas Industry. Energy Sci. Eng. 2023, 11, 1516–1535. [Google Scholar] [CrossRef]
- Yao, J.; Song, Y. Dynamic Analysis Approach to Evaluate In-Situ Combustion Performance for Heavy Oil Production. J. Oil Gas Petrochem. Sci. 2019, 2, 42–47. [Google Scholar] [CrossRef]
- Vieira, L.C.; Longo, M.; Mura, M. From Carbon Dependence to Renewables: The European Oil Majors’ Strategies to Face Climate Change. Bus. Strategy Environ. 2023, 32, 1248–1259. [Google Scholar] [CrossRef]
- Li, G.; Yao, J.; Song, Y.; Tang, J.; Han, H.; Cui, X. A Review of the Metallogenic Mechanisms of Sandstone-Type Uranium Deposits in Hydrocarbon-Bearing Basins in China. Eng 2023, 4, 1723–1741. [Google Scholar] [CrossRef]
- Rehm, T.E. Advanced Nuclear Energy: The Safest and Most Renewable Clean Energy. Curr. Opin. Chem. Eng. 2023, 39, 100878. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, Y.; Liu, X.; Li, Y.; Wang, J.; Chen, Z.; Yang, H.; Hu, B.; Shen, C.; Tang, Z.; et al. Functional Nanomaterials for Selective Uranium Recovery from Seawater: Material Design, Extraction Properties and Mechanisms. Coord. Chem. Rev. 2023, 483, 215097. [Google Scholar] [CrossRef]
- Degueldre, C. Uranium as a Renewable for Nuclear Energy. Prog. Nucl. Energy 2017, 94, 174–186. [Google Scholar] [CrossRef]
- Costa Peluzo, B.M.T.; Kraka, E. Uranium: The Nuclear Fuel Cycle and Beyond. Int. J. Mol. Sci. 2022, 23, 4655. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.K.; Gonzales, A.; Wagner, A.R.; Sooby, E.S.; Jaques, B.J. Challenges and Opportunities to Alloyed and Composite Fuel Architectures to Mitigate High Uranium Density Fuel Oxidation: Uranium Mononitride. J. Nucl. Mater. 2021, 553, 153048. [Google Scholar] [CrossRef]
- Muellner, N.; Arnold, N.; Gufler, K.; Kromp, W.; Renneberg, W.; Liebert, W. Nuclear Energy—The Solution to Climate Change? Energy Policy 2021, 155, 112363. [Google Scholar] [CrossRef]
- Wu, R.; Xu, Z.; Gong, W.; Cai, J.; Ning, J. Genesis of Baixingtui Uranium Deposit in Songliao Basin. Uranium Geol. 2012, 3, 142–147. [Google Scholar]
- Rong, H.; Jiao, Y.; Wu, L.; Ji, D.; Li, H.; Zhu, Q.; Cao, M.; Wang, X.; Li, Q.; Xie, H. Epigenetic Alteration and Its Constraints on Uranium Mineralization from the Qianjiadian Uranium Deposit, Southern Songliao Basin. Earth Sci.-J. China Univ. Geosci. 2016, 41, 2675–2696. [Google Scholar] [CrossRef]
- Cuney, M. The Extreme Diversity of Uranium Deposits. Minim. Depos. 2009, 44, 3–9. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, L.; Rong, H.; Zhang, F. Review of Basin Uranium Resources in China. Earth Sci.-J. China Univ. Geosci. 2021, 46, 2675. [Google Scholar] [CrossRef]
- Jin, R.; Yu, R.; Miao, P. Basin Uranium Mineralization Law; Springer: Singapore, 2023; pp. 325–356. [Google Scholar]
- World Nuclear Association. Available online: https://world-nuclear.org/information-library/nuclear-fuel-cycle/mining-of-uranium/world-uranium-mining-production.aspx (accessed on 21 August 2023).
- OECD. Uranium 2022: Resources, Production and Demand. Available online: https://read.oecd-ilibrary.org/nuclear-energy/uranium-2022_2c4e111b-en (accessed on 11 May 2023).
- Espinoza, D.; Goycoolea, M.; Moreno, E.; Newman, A. MineLib: A Library of Open Pit Mining Problems. Ann. Oper. Res. 2013, 206, 93–114. [Google Scholar] [CrossRef]
- Ben-Awuah, E.; Richter, O.; Elkington, T.; Pourrahimian, Y. Strategic Mining Options Optimization: Open Pit Mining, Underground Mining or Both. Int. J. Min. Sci. Technol. 2016, 26, 1065–1071. [Google Scholar] [CrossRef]
- Petersen, J. Heap Leaching as a Key Technology for Recovery of Values from Low-Grade Ores—A Brief Overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Abzalov, M.Z. Sandstone-Hosted Uranium Deposits Amenable for Exploitation by In Situ Leaching Technologies. Appl. Earth Sci. 2012, 121, 55–64. [Google Scholar] [CrossRef]
- Abzalov, M.Z.; Drobov, S.R.; Gorbatenko, O.; Vershkov, A.F.; Bertoli, O.; Renard, D.; Beucher, H. Resource Estimation of In Situ Leach Uranium Projects. Appl. Earth Sci. 2014, 123, 71–85. [Google Scholar] [CrossRef]
- Padilla, G.A.; Cisternas, L.A.; Cueto, J.Y. On the Optimization of Heap Leaching. Miner. Eng. 2008, 21, 673–678. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Becker, M.; Mainza, A.; Franzidis, J.-P.; Petersen, J. Large Particle Effects in Chemical/Biochemical Heap Leach Processes—A Review. Miner. Eng. 2011, 24, 1172–1184. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap Leaching Technology—Current State, Innovations and Future Directions: A Review. Miner. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Robertson, S.W.; van Staden, P.J.; Cherkaev, A.; Petersen, J. Properties Governing the Flow of Solution through Crushed Ore for Heap Leaching. Hydrometallurgy 2022, 208, 105811. [Google Scholar] [CrossRef]
- Van Lien, T.; Dinh, T.T.; Dung, N.T.K. Study on Leaching Systems and Recovery for PALUA–PARONG Low Grade Uranium Sandstone Ores. Hydrometallurgy 2020, 191, 105164. [Google Scholar] [CrossRef]
- Collet, A.; Regnault, O.; Ozhogin, A.; Imantayeva, A.; Garnier, L. Three-Dimensional Reactive Transport Simulation of Uranium In Situ Recovery: Large-Scale well Field Applications in Shu Saryssu Bassin, Tortkuduk Deposit (Kazakhstan). Hydrometallurgy 2022, 211, 105873. [Google Scholar] [CrossRef]
- Seredkin, M.; Zabolotsky, A.; Jeffress, G. In Situ Recovery, an Alternative to Conventional Methods of Mining: Exploration, Resource Estimation, Environmental Issues, Project Evaluation and Economics. Ore Geol. Rev. 2016, 79, 500–514. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Ram, R.; Pownceby, M.; Grocott, S.; Ring, B.; Tardio, J.; Jones, L. A Review of Acid Leaching of Uraninite. Hydrometallurgy 2015, 151, 10–24. [Google Scholar] [CrossRef]
- Mudd, G.M. Acid In Situ Leach Uranium Mining—1. USA and Australia. In Tailings and Mine Waste 2000; CRC Press: Boca Raton, FL, USA, 2022; pp. 517–526. [Google Scholar]
- Mudd, G. Critical Review of Acid In Situ Leach Uranium Mining: 2. Soviet Block and Asia. Environ. Geol. 2001, 41, 404–416. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Xu, Y.; Zhang, C. Mineral Alteration and Pore-Plugging Caused by Acid In Situ Leaching: A Case Study of the Wuyier Uranium Deposit, Xinjiang, NW China. Arab. J. Geosci. 2018, 11, 707. [Google Scholar] [CrossRef]
- Su, X.; Liu, Z.; Yao, Y.; Du, Z. Petrology, Mineralogy, and Ore Leaching of Sandstone-Hosted Uranium Deposits in the Ordos Basin, North China. Ore Geol. Rev. 2020, 127, 103768. [Google Scholar] [CrossRef]
- Zheng, F.; Teng, Y.; Zhai, Y.; Hu, J.; Dou, J.; Zuo, R. Geo-Environmental Models of In-Situ Leaching Sandstone-Type Uranium Deposits in North China: A Review and Perspective. Water 2023, 15, 1244. [Google Scholar] [CrossRef]
- Mukherjee, S.; Goswami, S.; Zakaulla, S. Geological Relationship between Hydrocarbon and Uranium: Review on Two Different Sources of Energy and the Indian Scenario. Geoenergy Sci. Eng. 2023, 221, 111255. [Google Scholar] [CrossRef]
- Herring, J.S. Uranium and Thorium Resources. In Nuclear Energy; Springer: New York, NY, USA, 2013; pp. 463–490. [Google Scholar]
- Jia, J.; Rong, H.; Jiao, Y.; Wu, L.; Guo, X.; Cao, M.; Cui, Z.; Tao, Z. Occurrence of Carbonate Cements and Relationship between Carbonate Cementation and Uranium Mineralization of Qianjiadian Uranium Deposit. Earth Sci. 2018, 2, 149–161. [Google Scholar] [CrossRef]
- Guthrie, V.A.; Kleeman, J.D. Changing Uranium Distributions during Weathering of Granite. Chem. Geol. 1986, 54, 113–126. [Google Scholar] [CrossRef]
- Cuney, M.; Mercadier, J.; Bonnetti, C. Classification of Sandstone-Related Uranium Deposits. J. Earth Sci. 2022, 33, 236–256. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, G.; Xu, L.; Liu, J.; Sun, Z.; Shi, W. Uranium Recovery from Sandstone-Type Uranium Deposit by Acid In-Situ Leaching—An Example from the Kujieertai. Hydrometallurgy 2020, 191, 105209. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Jiao, Y.; Wu, L.; Rong, H. Trapping of Uranium by Organic Matter within Sandstones during Mineralization Process: A Case Study from the Shuanglong Uranium Deposit, China. Ore Geol. Rev. 2021, 138, 104296. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P.; Lang, C. Comparative Life-Cycle Assessment of Uranium Extraction Processes. J. Clean. Prod. 2018, 202, 666–683. [Google Scholar] [CrossRef]
- Babaeian, M.; Sereshki, F.; Ataei, M.; Nehring, M.; Mohammadi, S. Application of Soft Computing, Statistical and Multi-Criteria Decision-Making Methods to Develop a Predictive Equation for Prediction of Flyrock Distance in Open-Pit Mining. Mining 2023, 3, 304–333. [Google Scholar] [CrossRef]
- El Hiouile, L.; Errami, A.; Azami, N. Toward Automatic Monitoring for Anomaly Detection in Open-Pit Phosphate Mines Using Artificial Vision: A Case Study of the Screening Unit. Mining 2023, 3, 645–658. [Google Scholar] [CrossRef]
- Brown, S.H.; Chambers, D.B. Uranium Mining and Norm in North America—Some Perspectives on Occupational Radiation Exposure. Health Phys. 2017, 113, 13–22. [Google Scholar] [CrossRef]
- Woods, P.H. Uranium Mining (Open Cut and Underground) and Milling. In Uranium for Nuclear Power; Elsevier: Amsterdam, The Netherlands, 2016; pp. 125–156. [Google Scholar]
- Cardozo, F.A.C.; Campos, H.J.S.; Petter, C.O.; Ambrós, W.M. Application of Monte Carlo Analytic Hierarchy Process (MAHP) in Underground Mining Access Selection. Mining 2023, 3, 773–785. [Google Scholar] [CrossRef]
- Ngwaku, S.R.; Pascoe, J.; Pelser, W.A.; Vosloo, J.C.; van Laar, J.H. A Data-Driven Framework to Reduce Diesel Spillages in Underground Mines. Mining 2023, 3, 683–695. [Google Scholar] [CrossRef]
- Oryngozhin, E.S.; Fedorov, E.V.; Alisheva, Z.N.; Mitishova, N.A. In-Situ Leaching Technology for Uranium Deposits. Eurasian Min. 2021, 2, 31–35. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Jiao, T.; Xiang, D.; Yan, X.; Tang, Z.; Yang, J. Using Clustering, Geochemical Modeling, and a Decision Tree for the Hydrogeochemical Characterization of Groundwater in an In Situ Leaching Uranium Deposit in Bayan-Uul, Northern China. Water 2023, 15, 4234. [Google Scholar] [CrossRef]
- Saunders, J.A.; Pivetz, B.E.; Voorhies, N.; Wilkin, R.T. Potential Aquifer Vulnerability in Regions Down-Gradient from Uranium In Situ Recovery (ISR) Sites. J. Environ. Manag. 2016, 183, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, L.; Thompson, J. In Situ Leaching of Copper: Challenges and Future Prospects. Hydrometallurgy 2015, 157, 306–324. [Google Scholar] [CrossRef]
- Alipour, A.; Khodaiari, A.A.; Jafari, A.; Tavakkoli-Moghaddam, R. An Integrated Approach to Open-Pit Mines Production Scheduling. Resour. Policy 2022, 75, 102459. [Google Scholar] [CrossRef]
- Neiva, A.M.R.; Carvalho, P.C.S.; Antunes, I.M.H.R.; Albuquerque, M.T.D.; Santos, A.C.S.; Cunha, P.P.; Henriques, S.B.A. Assessment of Metal and Metalloid Contamination in the Waters and Stream Sediments around the Abandoned Uranium Mine Area from Mortórios, Central Portugal. J. Geochem. Explor. 2019, 202, 35–48. [Google Scholar] [CrossRef]
- Zhang, Z. In-Situ Uranium Mining Technology and Its Application. Resour. Surv. Environ. 2002, 23, 200–204. [Google Scholar]
- Skripchenko, S.Y.; Nalivaiko, K.A.; Titova, S.M.; Rychkov, V.N.; Semenishchev, V.S. Recovery of Uranium from Conversion Production Sludge by Leaching with Nitric Acid and Subsequent Ion-Exchange Concentration. Hydrometallurgy 2024, 224, 106255. [Google Scholar] [CrossRef]
- Baigenzhenov, O.; Khabiyev, A.; Mishra, B.; Turan, M.D.; Akbarov, M.; Chepushtanova, T. Uranium (VI) Recovery from Black Shale Leaching Solutions Using Ion Exchange: Kinetics and Equilibrium Studies. Minerals 2020, 10, 689. [Google Scholar] [CrossRef]
- Mudd, G.M. The Future of Yellowcake: A Global Assessment of Uranium Resources and Mining. Sci. Total Environ. 2014, 472, 590–607. [Google Scholar] [CrossRef]
- Wohlers, A.; Wood, B.J. Uranium, Thorium and REE Partitioning into Sulfide Liquids: Implications for Reduced S-Rich Bodies. Geochim. Cosmochim. Acta 2017, 205, 226–244. [Google Scholar] [CrossRef]
- Feng, L.; Wang, H.; Feng, T.; Yan, B.; Yu, Q.; Zhang, J.; Guo, Z.; Yuan, Y.; Ma, C.; Liu, T.; et al. In Situ Synthesis of Uranyl-Imprinted Nanocage for Selective Uranium Recovery from Seawater. Angew. Chem. 2022, 134, e202101015. [Google Scholar] [CrossRef]
- Keener, M.; Shivaraam, R.A.K.; Rajeshkumar, T.; Tricoire, M.; Scopelliti, R.; Zivkovic, I.; Chauvin, A.-S.; Maron, L.; Mazzanti, M. Multielectron Redox Chemistry of Uranium by Accessing the +II Oxidation State and Enabling Reduction to a U(I) Synthon. J. Am. Chem. Soc. 2023, 145, 16271–16283. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.C.; Ewing, R.C.; Miller, M.L. Incorporation Mechanisms of Actinide Elements into the Structures of U6+ Phases Formed during the Oxidation of Spent Nuclear Fuel. J. Nucl. Mater. 1997, 245, 1–9. [Google Scholar] [CrossRef]
- Schindler, M.; Hawthorne, F.C.; Freund, M.S.; Burns, P.C. XPS Spectra of Uranyl Minerals and Synthetic Uranyl Compounds. I: The U 4f Spectrum. Geochim. Cosmochim. Acta 2009, 73, 2471–2487. [Google Scholar] [CrossRef]
- Li, T.; Wang, Q.; Zhu, C.; Huang, X.; Yang, G. Uranium(VI) Reduction at Mineral Surfaces by Fe(II): Periodic DFT Studies of Mechanistic Aspects and Combined Action of Fe(II) with Humic Acid. Surf. Interfaces 2021, 26, 101391. [Google Scholar] [CrossRef]
- Xie, T.; Qian, T.; Lian, B.; Chen, C.; Liang, P.; Liu, X.; Li, T.; Wang, T.; Chen, K.; Zhang, A.; et al. Research on Leaching Behavior of Uranium from a Uranium Tailing and Its Adsorption Behavior in Geotechnical Media. J. Environ. Manag. 2024, 353, 120207. [Google Scholar] [CrossRef]
- Gallegos, T.J.; Campbell, K.M.; Zielinski, R.A.; Reimus, P.W.; Clay, J.T.; Janot, N.; Bargar, J.R.; Benzel, W.M. Persistent U(IV) and U(VI) Following In-Situ Recovery (ISR) Mining of a Sandstone Uranium Deposit, Wyoming, USA. Appl. Geochem. 2015, 63, 222–234. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Lakaniemi, A.-M.; Tuovinen, O.H. Acid and Ferric Sulfate Bioleaching of Uranium Ores: A Review. J. Clean. Prod. 2020, 264, 121586. [Google Scholar] [CrossRef]
- Kurmanseiit, M.B.; Tungatarova, M.S.; Kaltayev, A.; Royer, J.-J. Reactive Transport Modeling during Uranium In Situ Leaching (ISL): The Effects of Ore Composition on Mining Recovery. Minerals 2022, 12, 1340. [Google Scholar] [CrossRef]
- Avasarala, S.; Torres, C.; Ali, A.-M.S.; Thomson, B.M.; Spilde, M.N.; Peterson, E.J.; Artyushkova, K.; Dobrica, E.; Lezama-Pacheco, J.S.; Cerrato, J.M. Effect of Bicarbonate and Oxidizing Conditions on U(IV) and U(VI) Reactivity in Mineralized Deposits of New Mexico. Chem. Geol. 2019, 524, 345–355. [Google Scholar] [CrossRef]
- Taylor, G.; Farrington, V.; Woods, P.; Ring, R.; Molloy, R. Review of Environmental Impacts of the Acid In-Situ Leaching Uranium Mining Process; CSIRO Clayton: Clayton, VIC, Australia, 2004. [Google Scholar]
- Kvashnina, K.O.; Butorin, S.M.; Martin, P.; Glatzel, P. Chemical State of Complex Uranium Oxides. Phys. Rev. Lett. 2013, 111, 253002. [Google Scholar] [CrossRef]
- Bank, T.L.; Kukkadapu, R.K.; Madden, A.S.; Ginder-Vogel, M.A.; Baldwin, M.E.; Jardine, P.M. Effects of Gamma-Sterilization on the Physico-Chemical Properties of Natural Sediments. Chem. Geol. 2008, 251, 1–7. [Google Scholar] [CrossRef]
- Zakrzewska-Koltuniewicz, G.; Herdzik-Koniecko, I.; Cojocaru, C.; Chajduk, E. Experimental Design and Optimization of Leaching Process for Recovery of Valuable Chemical Elements (U, La, V, Mo, Yb and Th) from Low-Grade Uranium Ore. J. Hazard. Mater. 2014, 275, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Tan, Y.; Wang, L.; Li, J. Oxygen Used as an Oxidizer in Acid In-Situ Leach Uranium: From Theory to Practice. In Proceedings of the International Conference on Nuclear Engineering, Charlotte, NC, USA, 26–30 June 2016; American Society of Mechanical Engineers: Charlotte, NC, USA, 2016. [Google Scholar]
- Habashi, F. Dissolution of Uraninite. Hydrometallurgy 2020, 194, 105329. [Google Scholar] [CrossRef]
- Abd El-Hamid, A.M.; Zahran, M.A.; Khalid, F.M.; Mahmoud, A.H. Leaching of Hafnium, Zirconium, Uranium and Other Nuclear Economic Elements from Petroleum Ash. RSC Adv. 2014, 4, 12506. [Google Scholar] [CrossRef]
- Olsson, D.; Li, J.; Jonsson, M. Kinetic Effects of H2O2 Speciation on the Overall Peroxide Consumption at UO2–Water Interfaces. ACS Omega 2022, 7, 15929–15935. [Google Scholar] [CrossRef] [PubMed]
- Lasheen, T.A.; El-Ahmady, M.E.; Hassib, H.B.; Helal, A.S. Oxidative Leaching Kinetics of Molybdenum-Uranium Ore in H2SO4 Using H2O2 as an Oxidizing Agent. Front. Chem. Sci. Eng. 2013, 7, 95–102. [Google Scholar] [CrossRef]
- Wang, P.; Tan, K.; Li, Y.; Liu, Z.; Li, C.; Tan, W.; Tian, Y.; Huang, W. Effect of Pyrite on the Leaching Kinetics of Pitchblende in the Process of Acid In Situ Leaching of Uranium. Minerals 2022, 12, 570. [Google Scholar] [CrossRef]
- You, W.; Peng, W.; Tian, Z.; Zheng, M. Uranium Bioremediation with U(VI)-Reducing Bacteria. Sci. Total Environ. 2021, 798, 149107. [Google Scholar] [CrossRef]
- Ali, H.N.; Atekwana, E.A. The Effect of Sulfuric Acid Neutralization on Carbonate and Stable Carbon Isotope Evolution of Shallow Groundwater. Chem. Geol. 2011, 284, 217–228. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. Alkaline Leaching of Brannerite. Part 1: Kinetics, Reaction Mechanisms and Mineralogical Transformations. Hydrometallurgy 2017, 169, 399–410. [Google Scholar] [CrossRef]
- Shen, N.; Li, J.; Guo, Y.; Li, X. Thermodynamic Modeling of In Situ Leaching of Sandstone-Type Uranium Minerals. J. Chem. Eng. Data 2020, 65, 2017–2031. [Google Scholar] [CrossRef]
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D. Silicate, Phosphate and Carbonate Mineral Dissolution Behaviour in the Presence of Organic Acids: A Review. Miner. Eng. 2017, 100, 115–123. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Montenegro, M.R. Leaching Behaviour and the Solution Consumption of Uranium–Vanadium Ore in Alkali Carbonate–Bicarbonate Column Leaching. Hydrometallurgy 2016, 161, 127–137. [Google Scholar] [CrossRef]
- Jana, A.; Unni, A.; Ravuru, S.S.; Das, A.; Das, D.; Biswas, S.; Sheshadri, H.; De, S. In-Situ Polymerization into the Basal Spacing of LDH for Selective and Enhanced Uranium Adsorption: A Case Study with Real Life Uranium Alkaline Leach Liquor. Chem. Eng. J. 2022, 428, 131180. [Google Scholar] [CrossRef]
- Osiensky, J.L.; Williams, R.E. Factors Affecting Efficient Aquifer Restoration at In Situ Uranium Mine Sites. Groundw. Monit. Remediat. 1990, 10, 107–112. [Google Scholar] [CrossRef]
- Cecal, A.; Humelnicu, D.; Popa, K.; Rudic, V.; Gulea, A.; Palamaru, I.; Nemtoi, G. Bioleaching of UO22+ Ions from Poor Uranium Ores by Means of Cyanobacteria. J. Radioanal. Nucl. Chem. 2000, 245, 427–429. [Google Scholar] [CrossRef]
- Caldeira, C.L.; Ciminelli, V.S.T.; Osseo-Asare, K. The Role of Carbonate Ions in Pyrite Oxidation in Aqueous Systems. Geochim. Cosmochim. Acta 2010, 74, 1777–1789. [Google Scholar] [CrossRef]
- Yang, Y.; Zuo, J.; Qiu, W.; Wu, J.; Que, W.; Zhou, G.; Liu, Z.; Wu, J. Assessment of the Greenhouse Gas Footprint and Environmental Impact of CO2 and O2 In Situ Uranium Leaching. Acta Geol. Sin.-Engl. Ed. 2023, 97, 986–994. [Google Scholar] [CrossRef]
- Qiu, W.; Yang, Y.; Song, J.; Que, W.; Liu, Z.; Weng, H.; Wu, J.; Wu, J. What Chemical Reaction Dominates the CO2 and O2 In-Situ Uranium Leaching? Insights from a Three-Dimensional Multicomponent Reactive Transport Model at the Field Scale. Appl. Geochem. 2023, 148, 105522. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, H.; Min, T.; Han, B.; Lim, S.; Park, J. Carbon Dioxide Utilization in Lithium Carbonate Precipitation: A Short Review. Environ. Eng. Res. 2023, 29, 230553. [Google Scholar] [CrossRef]
- Lu, C.; Xiu, W.; Guo, H.; Lian, G.; Yang, B.; Zhang, T.; Bi, E.; Shi, Z. Multi-Isotope Based Identification and Quantification of Oxygen Consuming Processes in Uranium Hosting Aquifers with CO2 + O2 In Situ Leaching. Water Resour. Res. 2023, 59, e2022WR033980. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Wang, W.; Niu, Q.; Zhuo, J.; Su, X.; Zhou, G.; Zhao, L.; Li, P.; Yuan, W.; et al. Mineral Composition and Full-Scale Pore Structure of Qianjiadian Sandstone-Type Uranium Deposits: Application for In Situ Leaching Mining. Geofluids 2022, 2022, 2860737. [Google Scholar] [CrossRef]
- Dong, W.; Brooks, S.C. Determination of the Formation Constants of Ternary Complexes of Uranyl and Carbonate with Alkaline Earth Metals (Mg2+, Ca2+, Sr2+, and Ba2+) Using Anion Exchange Method. Environ. Sci. Technol. 2006, 40, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Iglauer, S.; Cai, J.; Amooie, M.A.; Qin, C. Local Instabilities during Capillary-Dominated Immiscible Displacement in Porous Media. Capillarity 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Yao, J.; Oakey, J. Geometrically-Mediated Snap-off of Water-in-Oil Emulsion Droplets in Microfluidic Flow Focusing Devices. J. Oil Gas Petrochem. Sci. 2018, 1, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yao, J. Snap-Off during Imbibition in Porous Media: Mechanisms, Influencing Factors, and Impacts. Eng 2023, 4, 2896–2925. [Google Scholar] [CrossRef]
- Kanakiya, S.; Adam, L.; Esteban, L.; Rowe, M.C.; Shane, P. Dissolution and Secondary Mineral Precipitation in Basalts Due to Reactions with Carbonic Acid. J. Geophys. Res. Solid Earth 2017, 122, 4312–4327. [Google Scholar] [CrossRef]
- Kaszuba, J.; Yardley, B.; Andreani, M. Experimental Perspectives of Mineral Dissolution and Precipitation Due to Carbon Dioxide-Water-Rock Interactions. Rev. Miner. Geochem. 2013, 77, 153–188. [Google Scholar] [CrossRef]
- Chen, S.; Wei, X.; Liu, J.; Sun, Z.; Chen, G.; Yang, M.; Liu, Y.; Wang, D.; Ma, C.; Kong, D. Weak Acid Leaching of Uranium Ore from a High Carbonate Uranium Deposit. J. Radioanal. Nucl. Chem. 2022, 331, 2583–2596. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. The Microbiology of Biomining: Development and Optimization of Mineral-Oxidizing Microbial Consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef]
- Laurent, G.; Izart, C.; Lechenard, B.; Golfier, F.; Marion, P.; Collon, P.; Truche, L.; Royer, J.-J.; Filippov, L. Numerical Modelling of Column Experiments to Investigate In-Situ Bioleaching as an Alternative Mining Technology. Hydrometallurgy 2019, 188, 272–290. [Google Scholar] [CrossRef]
- Ram, R.; Charalambous, F.; Tardio, J.; Bhargava, A.H.S. Investigation of the Dissolution Kinetics of Synthetic Uraninite (UO2/UO22+). In Proceedings of the Conference: Engineering Our Future: Are We up to the Challenge? (Chemeca 2009), Burswood, Australia, 27–30 September 2009; Engineers Australia: Barton, ACT, Austalia, 2009. [Google Scholar]
- Janzen, M.P.; Nicholson, R.V.; Scharer, J.M. Pyrrhotite Reaction Kinetics: Reaction Rates for Oxidation by Oxygen, Ferric Iron, and for Nonoxidative Dissolution. Geochim. Cosmochim. Acta 2000, 64, 1511–1522. [Google Scholar] [CrossRef]
- Meruane, G.; Vargas, T. Bacterial Oxidation of Ferrous Iron by Acidithiobacillus Ferrooxidans in the PH Range 2.5–7.0. Hydrometallurgy 2003, 71, 149–158. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y. Remediation Technology for the Uranium Contaminated Environment: A Review. Procedia Environ. Sci. 2012, 13, 1609–1615. [Google Scholar] [CrossRef]
- Banala, U.K.; Das, N.P.I.; Toleti, S.R. Microbial Interactions with Uranium: Towards an Effective Bioremediation Approach. Environ. Technol. Innov. 2021, 21, 101254. [Google Scholar] [CrossRef]
- Chung, A.P.; Sousa, T.; Pereira, A.; Morais, P.V. Microorganisms—Tools for Bioremediation of Uranium Contaminated Environments. Procedia Earth Planet. Sci. 2014, 8, 53–58. [Google Scholar] [CrossRef]
- Yu, Q.; Cui, O. Enrichment and Remediation of Uranium by Microorganisms: A Review. Open J. Environ. Biol. 2023, 8, 20–38. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, K.; Li, C.; Li, Y.; Zhang, C.; Song, J.; Liu, L. Geochemical and S Isotopic Studies of Pollutant Evolution in Groundwater after Acid In Situ Leaching in A Uranium Mine Area in Xinjiang. Nucl. Eng. Technol. 2023, 55, 1476–1484. [Google Scholar] [CrossRef]
- Yang, Y.; Ram, R.; McMaster, S.A.; Pownceby, M.I.; Chen, M. A Comparative Bio-Oxidative Leaching Study of Synthetic U-Bearing Minerals: Implications for Mobility and Retention. J. Hazard. Mater. 2021, 403, 123914. [Google Scholar] [CrossRef]
- Zeng, S.; Song, J.; Sun, B.; Wang, F.; Ye, W.; Shen, Y.; Li, H. Seepage Characteristics of the Leaching Solution during In Situ Leaching of Uranium. Nucl. Eng. Technol. 2023, 55, 566–574. [Google Scholar] [CrossRef]
- Yin, S.; Yang, X.; Chen, W.; Wang, L.; Chen, X. Permeability Characteristics of Sandstone-Type Uranium Deposits under Different Temperature and Confining Pressure. J. Cent. South Univ. 2023, 30, 2302–2312. [Google Scholar] [CrossRef]
- Wang, W.; Liang, X.; Niu, Q.; Wang, Q.; Zhuo, J.; Su, X.; Zhou, G.; Zhao, L.; Yuan, W.; Chang, J.; et al. Reformability Evaluation of Blasting-Enhanced Permeability in In Situ Leaching Mining of Low-Permeability Sandstone-Type Uranium Deposits. Nucl. Eng. Technol. 2023, 55, 2773–2784. [Google Scholar] [CrossRef]
- De Silva, V.R.S.; Ranjith, P.G.; Perera, M.S.A.; Wu, B.; Wanniarachchi, W.A.M. A Low Energy Rock Fragmentation Technique for In-Situ Leaching. J. Clean. Prod. 2018, 204, 586–606. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, W.; Su, X.; Wen, L.; Chang, J. Experimental and Numerical Study on the Effect of Water-Decoupling Charge Structure on the Attenuation of Blasting Stress. Int. J. Rock Mech. Min. Sci. 2019, 124, 104133. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; He, G.; Hu, N.; Zhou, S.; Yu, Q.; Ding, D. Damage and Stability Analysis of Sandstone-Type Uranium Ore Body under Physical and Chemical Action of Leaching Solution. Arch. Min. Sci. 2023, 68, 409–423. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, C.; Wang, K.; Zhang, W.; Moghanloo, R.G.; Sun, X.; Wang, Y.; Zhou, T. Enhance Horizontal Well Performance by Optimising Multistage Hydraulic Fracture and Water Flooding. Int. J. Oil Gas Coal Technol. 2017, 15, 25–46. [Google Scholar] [CrossRef]
- Yuan, B.; Wood, D.A.; Yu, W. Stimulation and Hydraulic Fracturing Technology in Natural Gas Reservoirs: Theory and Case Studies (2012–2015). J. Nat. Gas Sci. Eng. 2015, 26, 1414–1421. [Google Scholar] [CrossRef]
- Yang, J.; Tan, K.; Huang, X. Evaluation and Analysis of Geological Condition of In-Situ Fragmentation Leaching Uranium. China Nucl. Sci. Technol. Rep. 2003, 2, 157–172. [Google Scholar]
- Himanshu, V.K.; Mishra, A.K.; Roy, M.P.; Singh, P.K. Overview of Underground Metalliferous Mining. In Blasting Technology for Underground Hard Rock Mining; Springer Nature: Singapore, 2023; pp. 9–24. [Google Scholar]
- Cai, Z.; Li, X.; Lei, B.; Yuan, J.; Hong, C.; Wang, H. Laboratory Experimental Laws for the Radon Exhalation of Similar Uranium Samples with Low-Frequency Vibrations. Sustainability 2018, 10, 2937. [Google Scholar] [CrossRef]
- Debashish, M.; Rajesh, K. Advanced Vibration Management to Improve Productivity and Optimize Cost for Excavation of Iron Ore: A Case Study. In Proceedings of the Conference on Recent Advances in Rock Engineering (RARE 2016), Bengaluru, India, 16–18 November 2016; Atlantis Press: Paris, France, 2016. [Google Scholar]
- Niu, Q.; Hu, M.; He, J.; Zhang, B.; Su, X.; Zhao, L.; Pan, J.; Wang, Z.; Du, Z.; Wei, Y. The Chemical Damage of Sandstone after Sulfuric Acid-Rock Reactions with Different Duration Times and Its Influence on the Impact Mechanical Behaviour. Heliyon 2023, 9, e22346. [Google Scholar] [CrossRef]
- Yun, M.; Yu, B.; Cai, J. Analysis of Seepage Characters in Fractal Porous Media. Int. J. Heat Mass Transf. 2009, 52, 3272–3278. [Google Scholar] [CrossRef]
- Qu, Z.; Yao, J.; Yang, Y.; Wang, B.; He, L. Laboratory Experiments of a Microfluidic Model for Imbibition. J. Petrochem. Univ. 2014, 37, 64–66. [Google Scholar]
- Yao, J. Microfluidic Studies on Snap-Off during Imbibition Process. Master Thesis, China University of Petroleum (East China), Qingdao, China, 2014. [Google Scholar]
- Yao, J. A Microfluidic Model for Visualizing Snap-Off During Imbibition. Ph.D. Dissertation, University of Wyoming, Laramie, WY, USA, 2017. [Google Scholar]
- Yao, J. Microfluidic Studies of Geometrically-Mediated Snap-Off. Master Thesis, University of Wyoming, Laramie, WY, USA, 2014. [Google Scholar]
- Roy, M.P.; Himanshu, V.K.; Kaushik, A.P.; Singh, P.K. Influence of Ring Blasting Pattern on the Safety of Nearby Underground Structures. Sādhanā 2022, 47, 192. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Sanchidrián, J.A.; Ouchterlony, F.; Luukkanen, S. Reduction of Fragment Size from Mining to Mineral Processing: A Review. Rock Mech. Rock. Eng. 2023, 56, 747–778. [Google Scholar] [CrossRef]
- Vennes, I.; Mitri, H.; Chinnasane, D.R.; Yao, M. Effect of Stress Anisotropy on the Efficiency of Large-Scale Destress Blasting. Rock Mech. Rock. Eng. 2021, 54, 31–46. [Google Scholar] [CrossRef]
- Kan, J.; Dou, L.; Li, J.; Li, X.; Bai, J.; Wang, M. Characteristics of Microseismic Waveforms Induced by Underground Destress Blasting: Comparison with Those Induced by Ground Blasting and Coal Mining. Front. Earth Sci. 2022, 10, 797358. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, M. Characteristics of Peak Load on a Borehole Wall in Water-Coupling Blasting. J. Eng. Mech. 2023, 149, 04022087. [Google Scholar] [CrossRef]
- Huo, X.; Qiu, X.; Shi, X.; Chen, H.; Gou, Y.; Yu, Z. Experimental and Numerical Investigation on the Peak Value and Loading Rate of Borehole Wall Pressure in Decoupled Charge Blasting. Int. J. Rock Mech. Min. Sci. 2023, 170, 105535. [Google Scholar] [CrossRef]
- Johnson, R.H.; Paradis, C.J.; Kent, R.D.; Tigar, A.D.; Reimus, P.W. Single-Well Push–Pull Tracer Test Analyses to Determine Aquifer Reactive Transport Parameters at a Former Uranium Mill Site (Grand Junction, Colorado). Minerals 2023, 13, 228. [Google Scholar] [CrossRef]
- Cheng, Y.; Arora, B.; Şengör, S.S.; Druhan, J.L.; Wanner, C.; van Breukelen, B.M.; Steefel, C.I. Microbially Mediated Kinetic Sulfur Isotope Fractionation: Reactive Transport Modeling Benchmark. Comput. Geosci. 2021, 25, 1379–1391. [Google Scholar] [CrossRef]
- Deng, H.; Navarre-Sitchler, A.; Heil, E.; Peters, C. Addressing Water and Energy Challenges with Reactive Transport Modeling. Environ. Eng. Sci. 2021, 38, 109–114. [Google Scholar] [CrossRef]
- Schabernack, J.; Fischer, C. Improved Kinetics for Mineral Dissolution Reactions in Pore-Scale Reactive Transport Modeling. Geochim. Cosmochim. Acta 2022, 334, 99–118. [Google Scholar] [CrossRef]
- Chen, J.; Dai, Z.; Yang, Z.; Pan, Y.; Zhang, X.; Wu, J.; Reza Soltanian, M. An Improved Tandem Neural Network Architecture for Inverse Modeling of Multicomponent Reactive Transport in Porous Media. Water Resour. Res. 2021, 57, e2021WR030595. [Google Scholar] [CrossRef]
- Xiong, Y.; Hou, Z.; Tan, X.; Luo, J.; Yue, Y.; Wu, K. Constraining Fluid-Rock Interactions during Eogenetic Karst and Their Impacts on Carbonate Reservoirs: Insights from Reactive Transport Modeling. Appl. Geochem. 2021, 131, 105050. [Google Scholar] [CrossRef]
- Harrison, A.L.; Tutolo, B.M.; DePaolo, D.J. The Role of Reactive Transport Modeling in Geologic Carbon Storage. Elements 2019, 15, 93–98. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Wissmeier, L. PhreeqcRM: A Reaction Module for Transport Simulators Based on the Geochemical Model PHREEQC. Adv. Water Resour. 2015, 83, 176–189. [Google Scholar] [CrossRef]
- Kempka, T.; Steding, S.; Kühn, M. Verification of TRANSPORT Simulation Environment Coupling with PHREEQC for Reactive Transport Modelling. Adv. Geosci. 2022, 58, 19–29. [Google Scholar] [CrossRef]
- Prommer, H.; Barry, D.A.; Zheng, C. MODFLOW/MT3DMS-Based Reactive Multicomponent Transport Modeling. Groundwater 2003, 41, 247–257. [Google Scholar] [CrossRef]
- Xu, T.; Spycher, N.; Sonnenthal, E.; Zhang, G.; Zheng, L.; Pruess, K. TOUGHREACT Version 2.0: A Simulator for Subsurface Reactive Transport under Non-Isothermal Multiphase Flow Conditions. Comput. Geosci. 2011, 37, 763–774. [Google Scholar] [CrossRef]
- Kim, B.-J.; Ko, M.-S. Two-Dimensional Reactive Transport Model as a New Approach for Identifying the Origins and Contribution of Arsenic in a Soil and Water System. Sci. Total Environ. 2023, 898, 165468. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, A.C.Q.; Morais, C.A. Uranium Recovery from Industrial Effluent by Ion Exchange—Column Experiments. Miner. Eng. 2005, 18, 1337–1340. [Google Scholar] [CrossRef]
- Dangelmayr, M.A.; Reimus, P.W.; Wasserman, N.L.; Punsal, J.J.; Johnson, R.H.; Clay, J.T.; Stone, J.J. Laboratory Column Experiments and Transport Modeling to Evaluate Retardation of Uranium in an Aquifer Downgradient of a Uranium In-Situ Recovery Site. Appl. Geochem. 2017, 80, 1–13. [Google Scholar] [CrossRef]
- Lagneau, V.; Regnault, O.; Descostes, M. Industrial Deployment of Reactive Transport Simulation: An Application to Uranium In Situ Recovery. Rev. Miner. Geochem. 2019, 85, 499–528. [Google Scholar] [CrossRef]
- Lagneau, V.; van der Lee, J. HYTEC Results of the MoMas Reactive Transport Benchmark. Comput. Geosci. 2010, 14, 435–449. [Google Scholar] [CrossRef]
- van der Lee, J.; De Windt, L.; Lagneau, V.; Goblet, P. Module-Oriented Modeling of Reactive Transport with HYTEC. Comput. Geosci. 2003, 29, 265–275. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Z.; Feng, G.; Chen, M.; Du, Z. Improving the Efficiency of In Situ Uranium Leaching (Remediation) with Periodic Injection and Extraction: Insight from Reactive Transport Modeling and Field Test. J. Hydrol. 2024, 630, 130767. [Google Scholar] [CrossRef]
- Sprocati, R.; Rolle, M. Integrating Process-Based Reactive Transport Modeling and Machine Learning for Electrokinetic Remediation of Contaminated Groundwater. Water Resour. Res. 2021, 57, e2021WR029959. [Google Scholar] [CrossRef]
- Noskov, M.; Istomin, A.; Kesler, A.; Cheglokov, A. Innovative Intellectual Management Technology of Uranium Mining by the ISL Method. In Proceedings of the International Symposium on Uranium Raw Material for the Nuclear Fuel Cycle: Exploration, Mining, Production, Supply and Demand, Economics and Environmental Issues (URAM-2018), Vienna, Austria, 25–29 June 2018; International Atomic Energy Agency: Vienna, Austria, 2018; p. 157. [Google Scholar]
- Karami, E.; Kuhar, L.; Bona, A.; Nikoloski, A.N. A Review of Electrokinetic, Ultrasonic and Solution Pulsing Methods for Mass Transfer Enhancement in In-Situ Processing. Miner. Eng. 2021, 170, 107029. [Google Scholar] [CrossRef]
- Denison Mines Corp. Denison Announces Successful Completion of Recovered Solution Management Phase of Phoenix ISR Feasibility Field Test. Available online: https://www.prnewswire.com/news-releases/denison-announces-successful-completion-of-recovered-solution-management-phase-of-phoenix-isr-feasibility-field-test-301975387.html (accessed on 2 November 2023).
- World Nuclear News History-in-the-Making for Canadian Uranium Mining. Available online: https://world-nuclear-news.org/Articles/History-in-the-making-for-Canadian-uranium-mining (accessed on 18 October 2022).
- Dolchinkov, N.T. Development of Uranium Mining and Uranium Processing in the Russian Federation. Sci. Bus. Soc. 2019, 4, 108–114. [Google Scholar]
- Dooley, J.R.; Harshman, E.N.; Rosholt, J.N. Uranium-Lead Ages of the Uranium Deposits of the Gas Hills and Shirley Basin, Wyoming. Econ. Geol. 1974, 69, 527–531. [Google Scholar] [CrossRef]
- Asnani, C.K. Development of Alkali Leaching Technology: Key to Self-Sufficiency in Uranium Production in India. In Proceedings of the URAM-2018: International Symposium on Uranium Raw Material for the Nuclear Fuel Cycle: Exploration, Mining, Production, Supply and Demand, Economics and Environmental Issues (1), Vienna, Austria, 25–29 June 2018; International Atomic Energy Agency (IAEA): Vienna, Austria, 2018; p. IAEACN261. [Google Scholar]
- Roberto, F.F.; Schippers, A. Progress in Bioleaching: Part B, Applications of Microbial Processes by the Minerals Industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef] [PubMed]
- Bampton, K.F.; Haines, J.B.; Randell, M.H. Geology of the Honeymoon Uranium Project. AusIMM Proc. 2001, 306, 17–27. [Google Scholar]
- Abiru, T. The Tokyo-Moscow-Astana Triangle: Strategic Partnership in Nuclear Energy Is Inevitable. Secur. Index A Russ. J. Int. Secur. 2008, 14, 117–122. [Google Scholar] [CrossRef]
- Zine, H.; El Mansour, A.; Hakkou, R.; Papazoglou, E.G.; Benzaazoua, M. Advancements in Mine Closure and Ecological Reclamation: A Comprehensive Bibliometric Overview (1980–2023). Mining 2023, 3, 798–813. [Google Scholar] [CrossRef]
- Yin, M.; Sun, J.; He, H.; Liu, J.; Zhong, Q.; Zeng, Q.; Huang, X.; Wang, J.; Wu, Y.; Chen, D. Uranium Re-Adsorption on Uranium Mill Tailings and Environmental Implications. J. Hazard. Mater. 2021, 416, 126153. [Google Scholar] [CrossRef]
- Seigneur, N.; De Windt, L.; Déjeant, A.; Lagneau, V.; Descostes, M. Long-Term Evolution of Uranium Mobility within Sulfated Mill Tailings in Arid Regions: A Reactive Transport Study. Minerals 2021, 11, 1201. [Google Scholar] [CrossRef]
- Hamza, M.F.; El-Aassy, I.E.; Guibal, E. Integrated Treatment of Tailing Material for the Selective Recovery of Uranium, Rare Earth Elements and Heavy Metals. Miner. Eng. 2019, 133, 138–148. [Google Scholar] [CrossRef]
- Reynier, N.; Gagné-Turcotte, R.; Coudert, L.; Costis, S.; Cameron, R.; Blais, J.-F. Bioleaching of Uranium Tailings as Secondary Sources for Rare Earth Elements Production. Minerals 2021, 11, 302. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Hou, D.-F.; Aladejare, A.; Ozoji, T.; Qiao, Y. World Mineral Loss and Possibility to Increase Ore Recovery Ratio in Mining Production. Int. J. Min. Reclam. Environ. 2021, 35, 670–691. [Google Scholar] [CrossRef]
| Method | Applicable Condition | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Open-pit Mining | Shallow burial depths of main ore bodies | Short construction period; Large mining space and high labor productivity; Safe working conditions | Subject to climate conditions; High infrastructure and equipment investment; Large land footprint and environmental damage | [50,61] |
| Underground Mining | Significant burial depths or surface conditions unsuitable for open-pit mining | Limited climate disruption; Minimal impact on surface ecosystems; High mining efficiency | High extraction costs; Complex and challenging construction and maintenance of underground mining facilities; Potential impact on underground geological environment; Elevated safety risks | [26,53,54] |
| In Situ Leaching | Situated in aquifers with favorable permeabilities | Safe and simple mining process; Short construction period and minimal infrastructure investment; Low labor intensity and high automation level; Less environmental pollution due to avoidance of waste rocks; Capability to process low-grade ore deposits | Requirements for geological and hydrogeological conditions; Slow extraction rate; Underground water management challenges | [58,59,60,62] |
| Technique | Applicable Condition | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Acid Leaching | Applicable to uranium deposits with low carbonate content | Low risk of groundwater contamination outside the wellfield; High leaching efficiency and short leaching cycles | Obligatory use of corrosion-resistant instruments and pipelines; Significant impact on groundwater within the wellfield; Possible formation of sulfate precipitates, causing blockage and permeability deterioration in the ore body | [37,48,64,78,87,119] |
| Alkaline Leaching | Widely applicable to uranium deposits with high carbonate content; Not suitable for uranium deposits with high pyrite content | Utilization of common equipment and pipelines | Low leaching efficiency and long leaching cycles; High risk of groundwater contamination outside the wellfield; Formation of carbonate or sulfate precipitates, potentially causing deposit clogging | [90] |
| Neutral Leaching | Wide applicability with no apparent restrictions | Leaching solution with gentler components for enhanced environmental friendliness; Simultaneous uranium mining with CO2 utilization and storage for CO2-O2 leaching | Possibility of gangue mineral dissolution and carbonate precipitation leading to deposit clogging due to pH drop | [2,99,109] |
| Bioleaching | Wide applicability, especially suitable for uranium deposits rich in pyrite and sulfides | High leaching rate and high overall leaching efficiency; Sustainable and environmentally friendly | Initial acid consumption must be considered until microbial oxidation of reducible sulfur compounds initiates acid production; | [75,96,111,120] |
| Potential clogging due to gangue mineral dissolution and secondary precipitation resulting from the generation of H+ and SO42− |
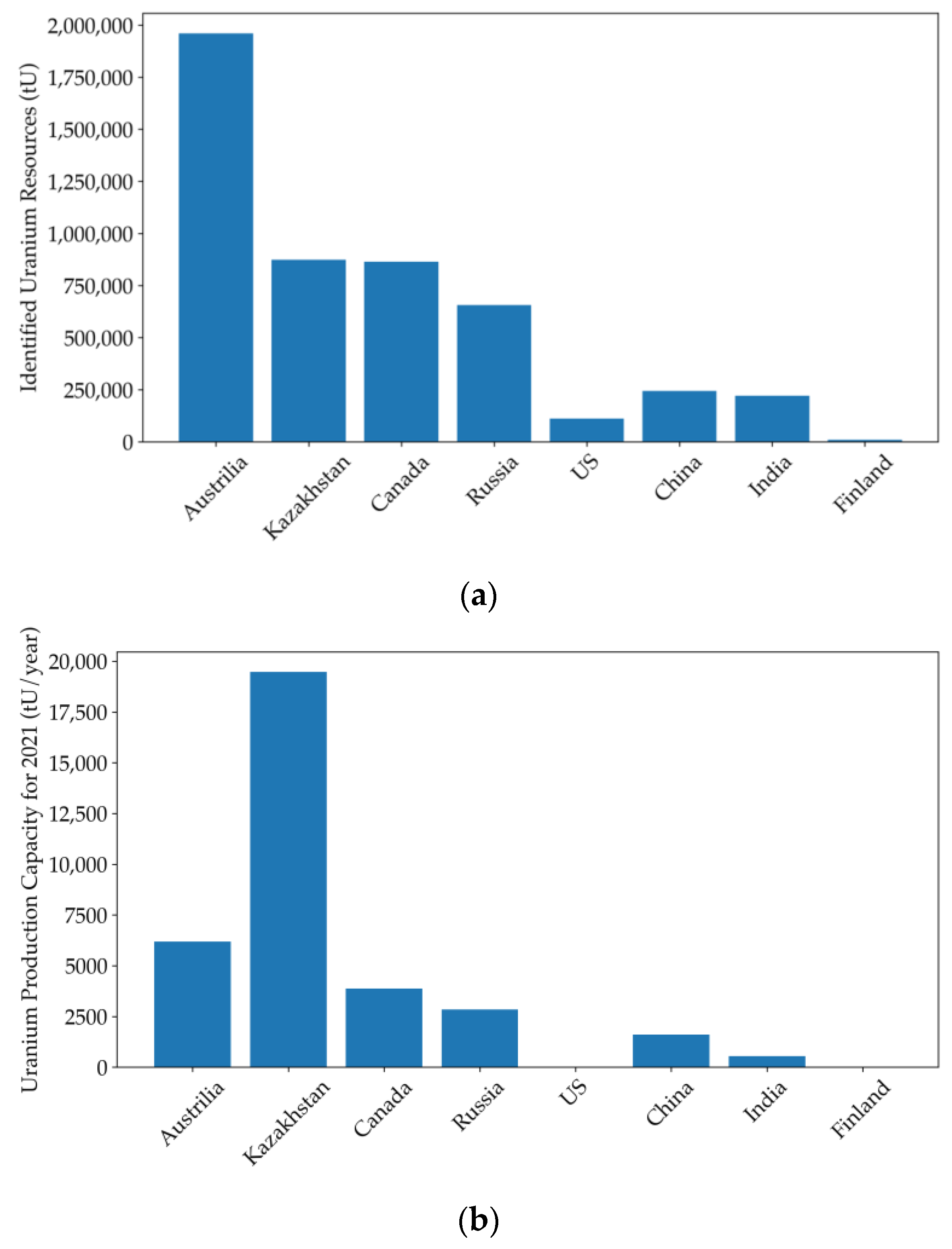
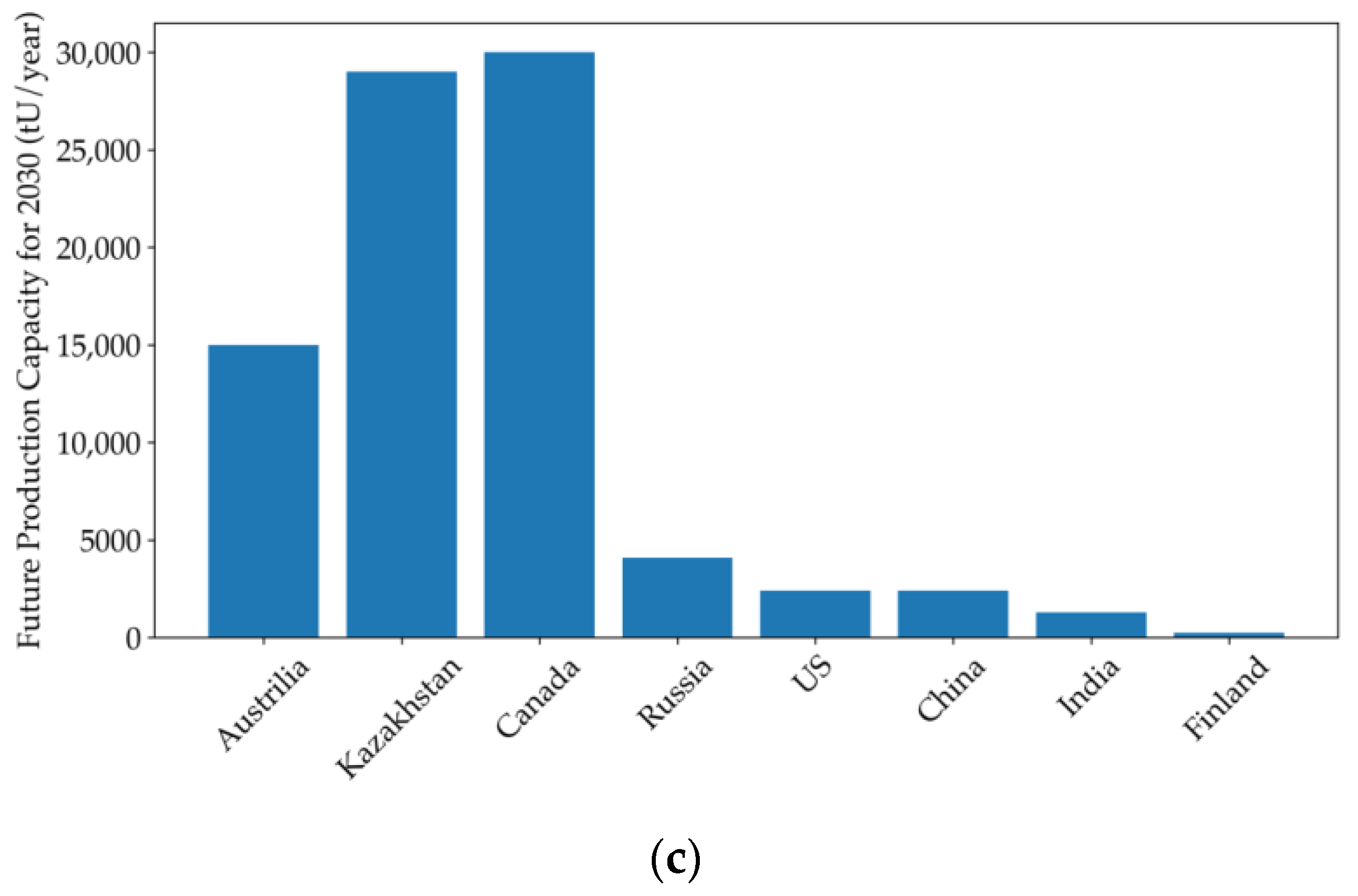
| Country | Uranium Mine | Production Capacity (tU/Year) | Start Date | Technique | Reference |
|---|---|---|---|---|---|
| Australia | Beverley and Beverley North Uranium Deposit (Four Mile Uranium Mine) | Approximately 1200 | 2001/2014 | Acid leaching/weak acid leaching | [24,38,78] |
| Honeymoon Uranium Mine | Approximately 312 (average production for three years) | 2019 (resumed production) | Acid leaching | ||
| Kazakhstan | Katco Mine (Tortkuduk and Muyunkum Deposits) | 3000–4000 | 2009 | Acid leaching assisted by RTM simulation | [24,35] |
| Zarechnoye Deposit | Approximately 1000 | 2020 | Acid leaching with valuable by-product production | ||
| Canada | Phoenix Uranium Deposit | Approximately 2300 (expected average production for ten years) | 2023 | Acid leaching | [166,167] |
| Russia | Dular Mine (Dobrovolnoye Deposit) | 700 | 2020 | Acid leaching assisted by Smart ISL site digital mining system | [24,168] |
| Khiagda ISL Operation Plant | 1000 | 2020 | Acid leaching assisted by Smart ISL site digital mining system | ||
| USA | Smith Ranch-Highland Operation | Collectively 2900 | 2000 | CO2-O2 leaching | [24,74,169] |
| Lost Creek project | 2013 | CO2-O2 leaching | |||
| China | Erdos Sandstone-hosted Uranium Deposit | Unknown | 2020 (trial test) | CO2-O2 leaching | [24] |
| Songliao Sandstone-hosted Uranium Deposit | Unknown | 2023 (trial test) | CO2-O2 leaching assisted by RTM simulation | ||
| India | Tummalapalle Mine | Unknown | 2017 | Alkaline leaching | [24,170] |
| Finland | Terrafame Mine (formerly Talvivaara Mine) | Unknown | 2024 (trial test) | Bioleaching | [24,75,171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yao, J. A Review of In Situ Leaching (ISL) for Uranium Mining. Mining 2024, 4, 120-148. https://doi.org/10.3390/mining4010009
Li G, Yao J. A Review of In Situ Leaching (ISL) for Uranium Mining. Mining. 2024; 4(1):120-148. https://doi.org/10.3390/mining4010009
Chicago/Turabian StyleLi, Guihe, and Jia Yao. 2024. "A Review of In Situ Leaching (ISL) for Uranium Mining" Mining 4, no. 1: 120-148. https://doi.org/10.3390/mining4010009
APA StyleLi, G., & Yao, J. (2024). A Review of In Situ Leaching (ISL) for Uranium Mining. Mining, 4(1), 120-148. https://doi.org/10.3390/mining4010009








