Design of Multi-Stage Solvent Extraction Process for Separation of Rare Earth Elements
Abstract
:1. Introduction
1.1. Aqueous Organic Equilibrium
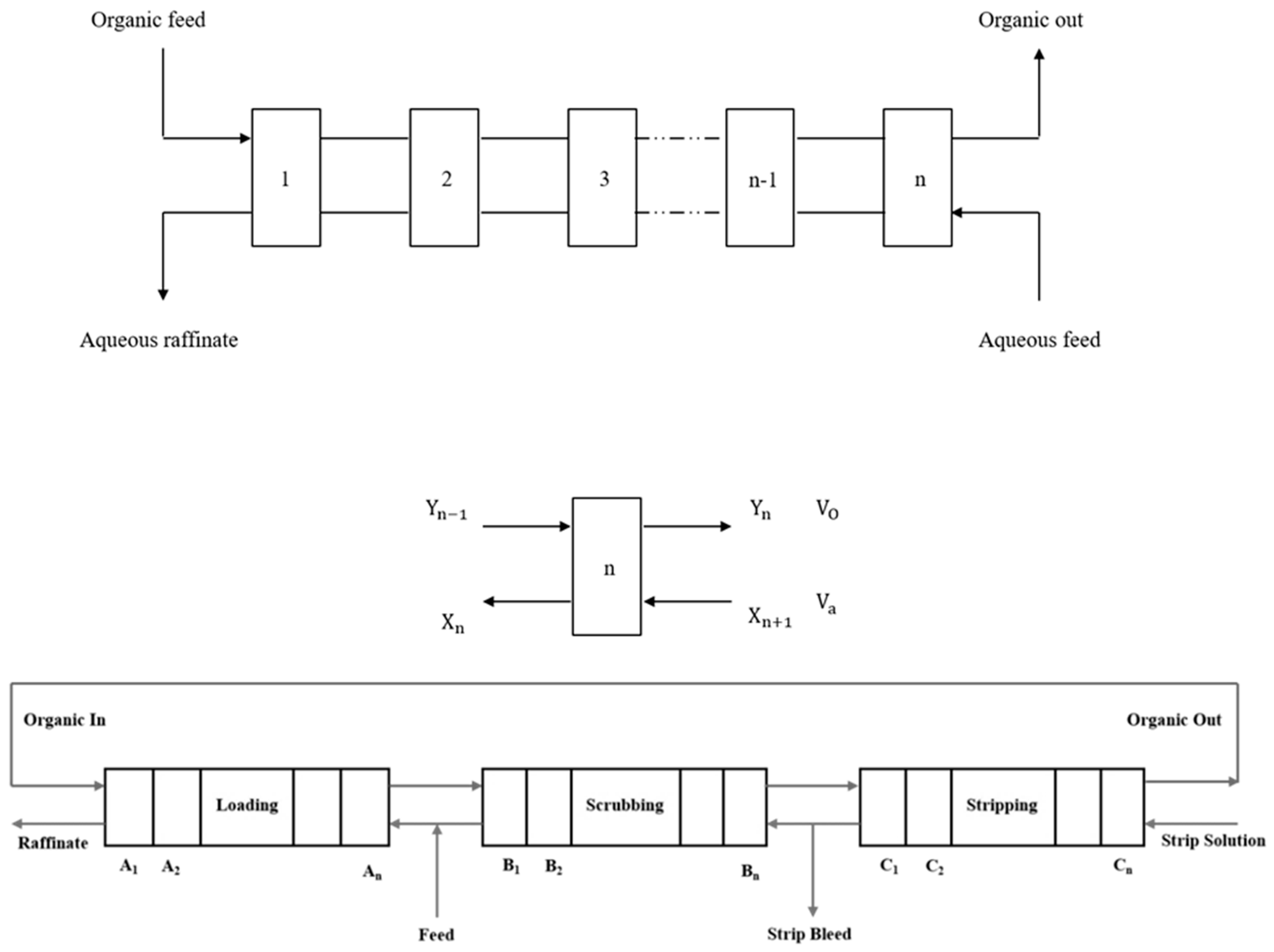
1.2. Counter-Flow SX Configuration and Design
1.3. Application of SX Modeling in Design
1.4. Gaps and Approach
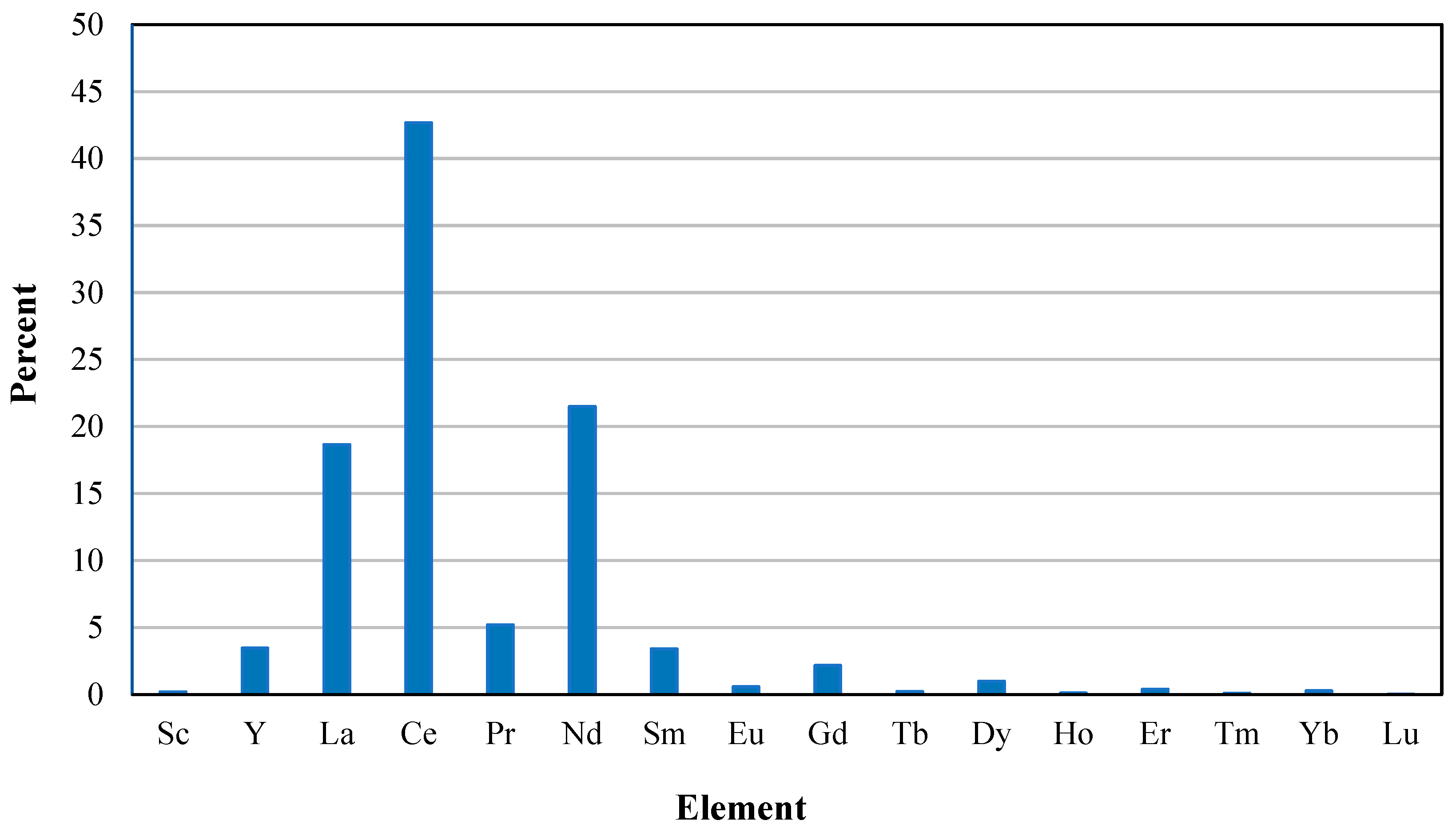
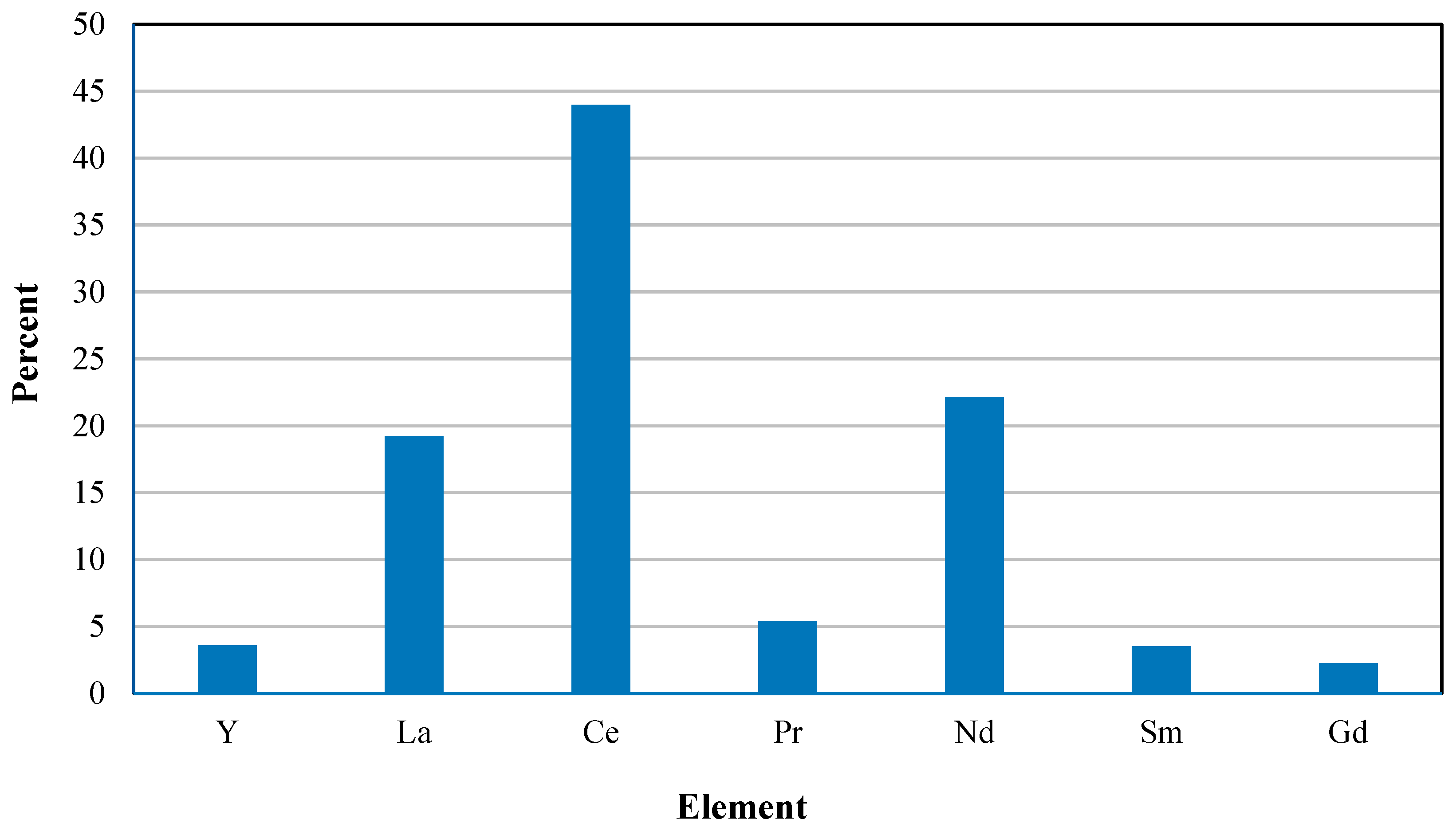
- Identify the initial SX feed characteristics considered for design (REE concentration in this case);
- Determine the ideal pH to achieve separation using bench-scale equilibrium experiments;
- Perform a circuit layout composed of specific SX “trains” defined as loading, scrubbing, and stripping;
- Determine the ideal phase ratio for improved separation, thereby holding the phase flow parameters constant, using bench-scale equilibrium experiments as the basis for developing equilibrium regression models for these variables;
- Develop stagewise arithmetic determination of an equilibrium utilizing MATLAB Simulink as blocks corresponding to discrete functions such as loading, scrubbing, and stripping;
- Perform by training a particle swarm optimization method to design and simulate a flowsheet and determine the number of stages needed for separation on the basis of recovery and/or purity.
2. Materials and Methods
2.1. Part 1: Development of Modeling Theory and Methods
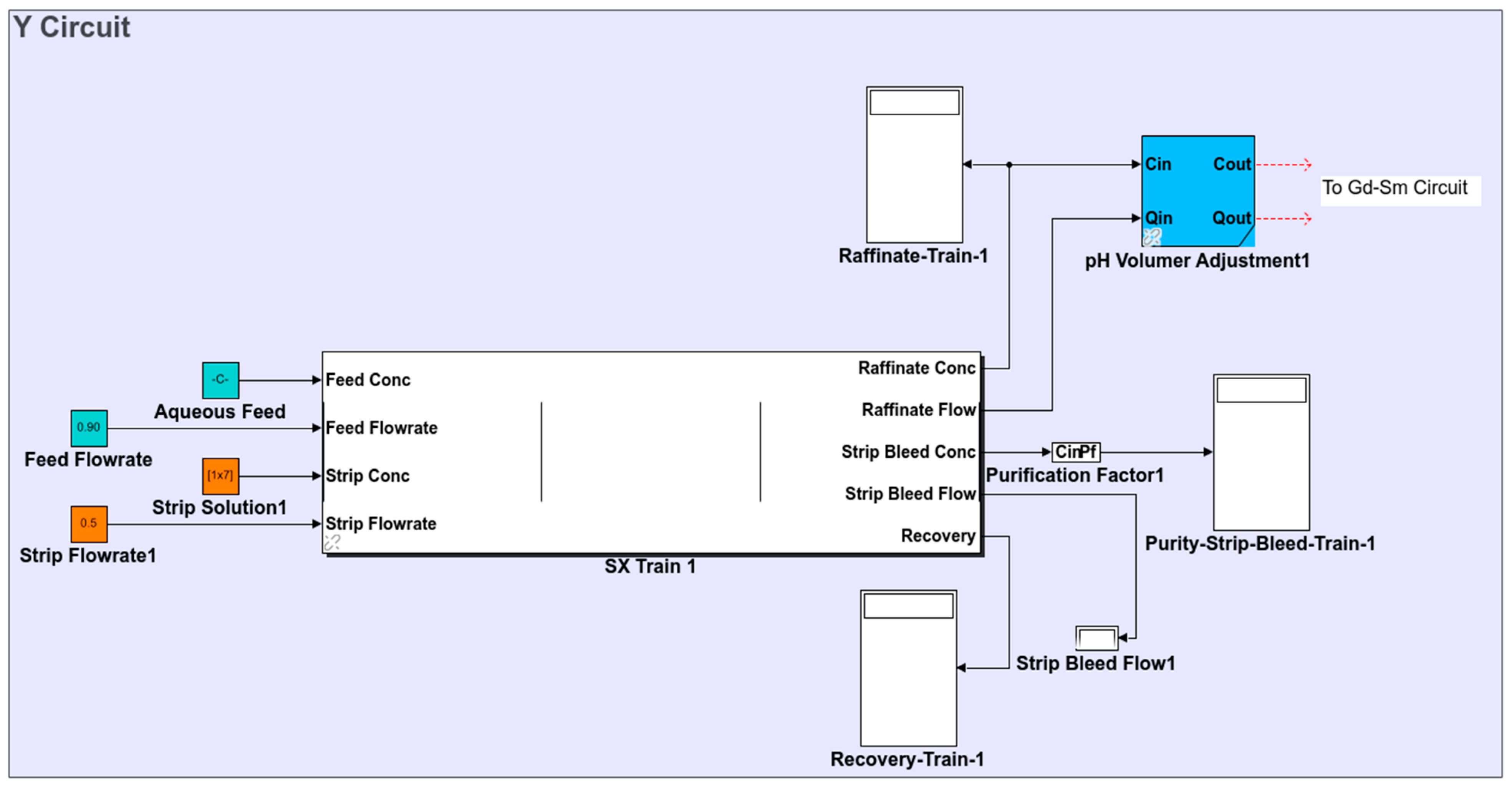
2.1.1. Circuit Definition
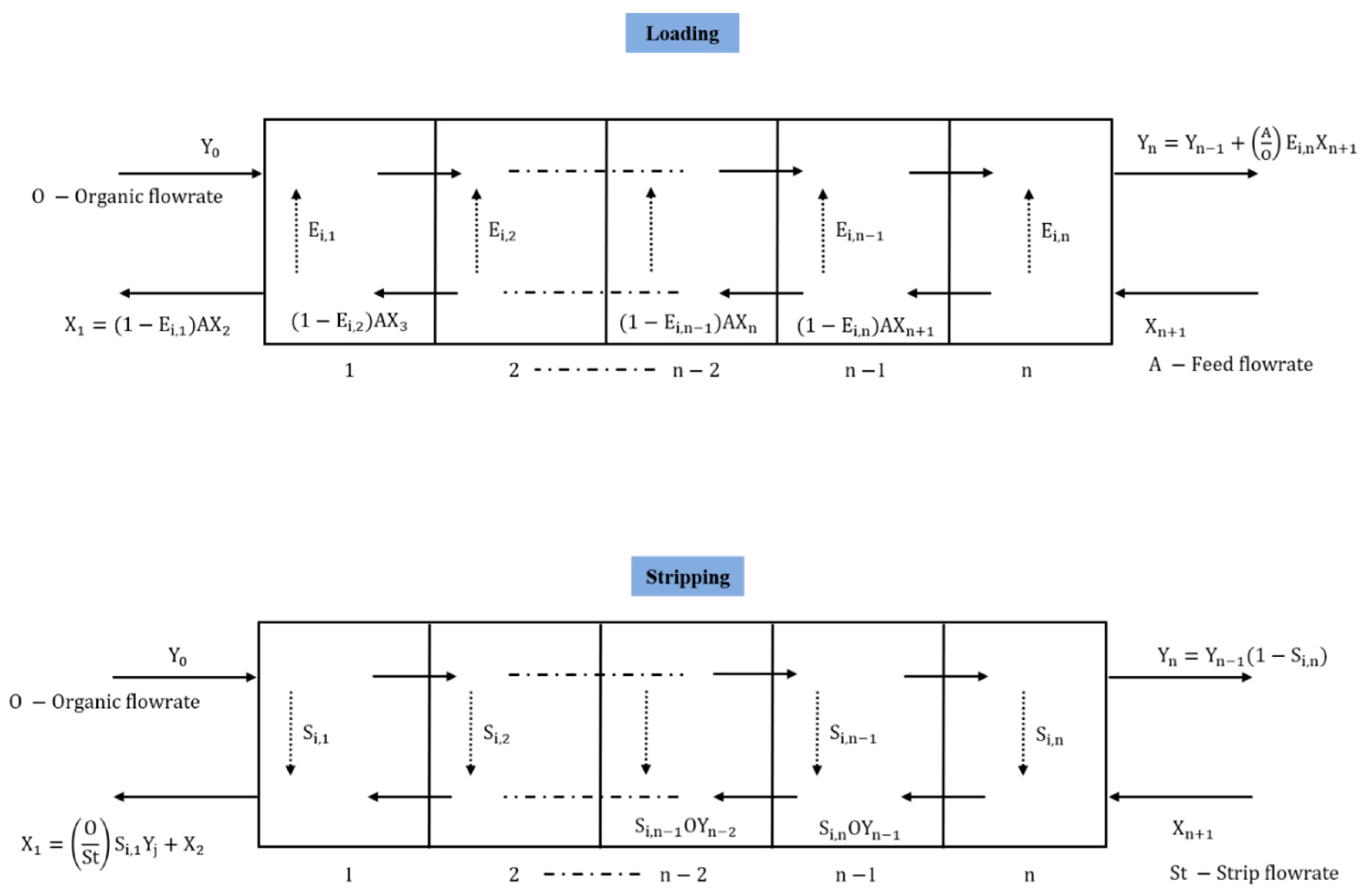
2.1.2. Algebraic Mass-Transfer Definition
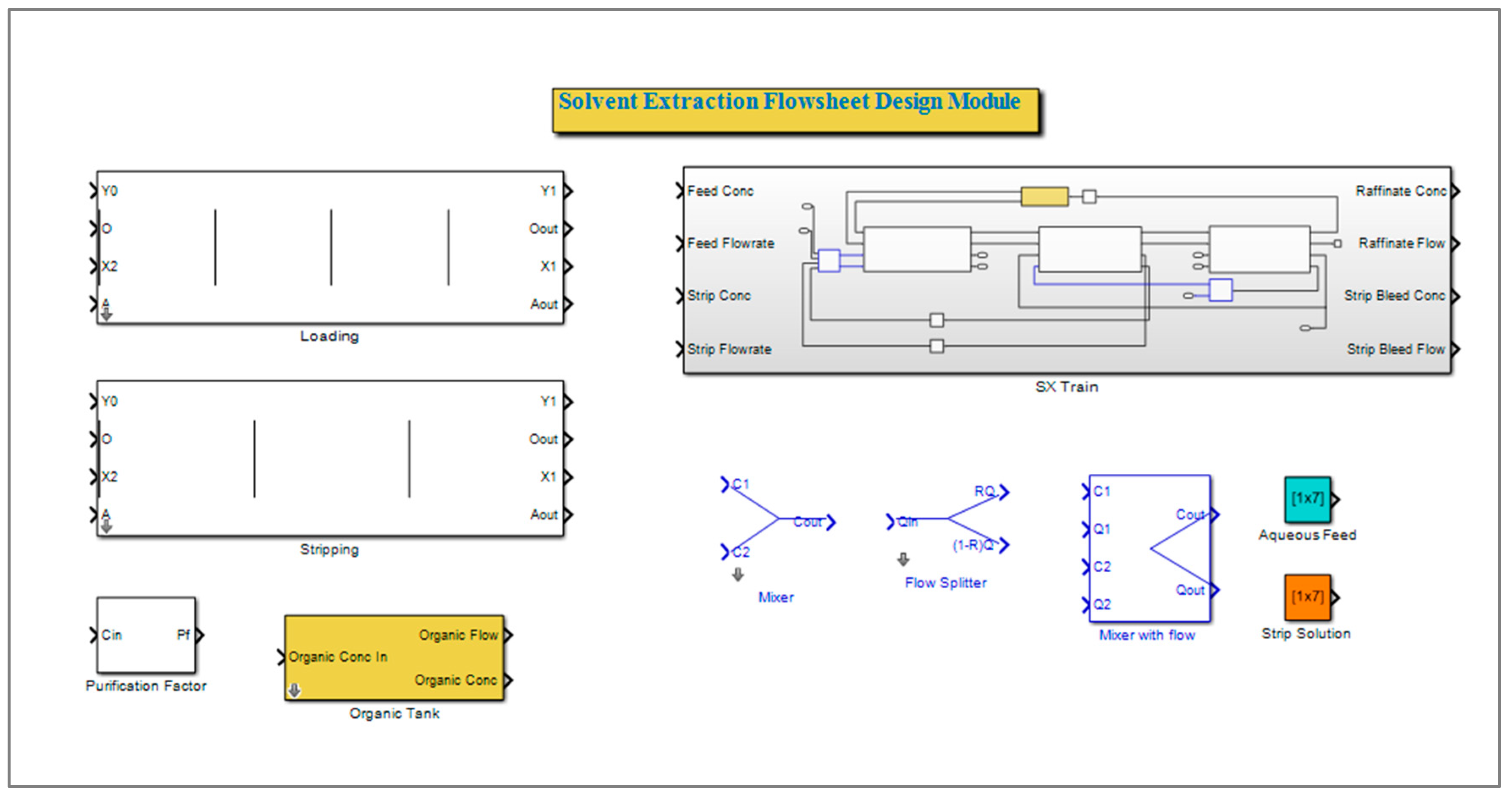
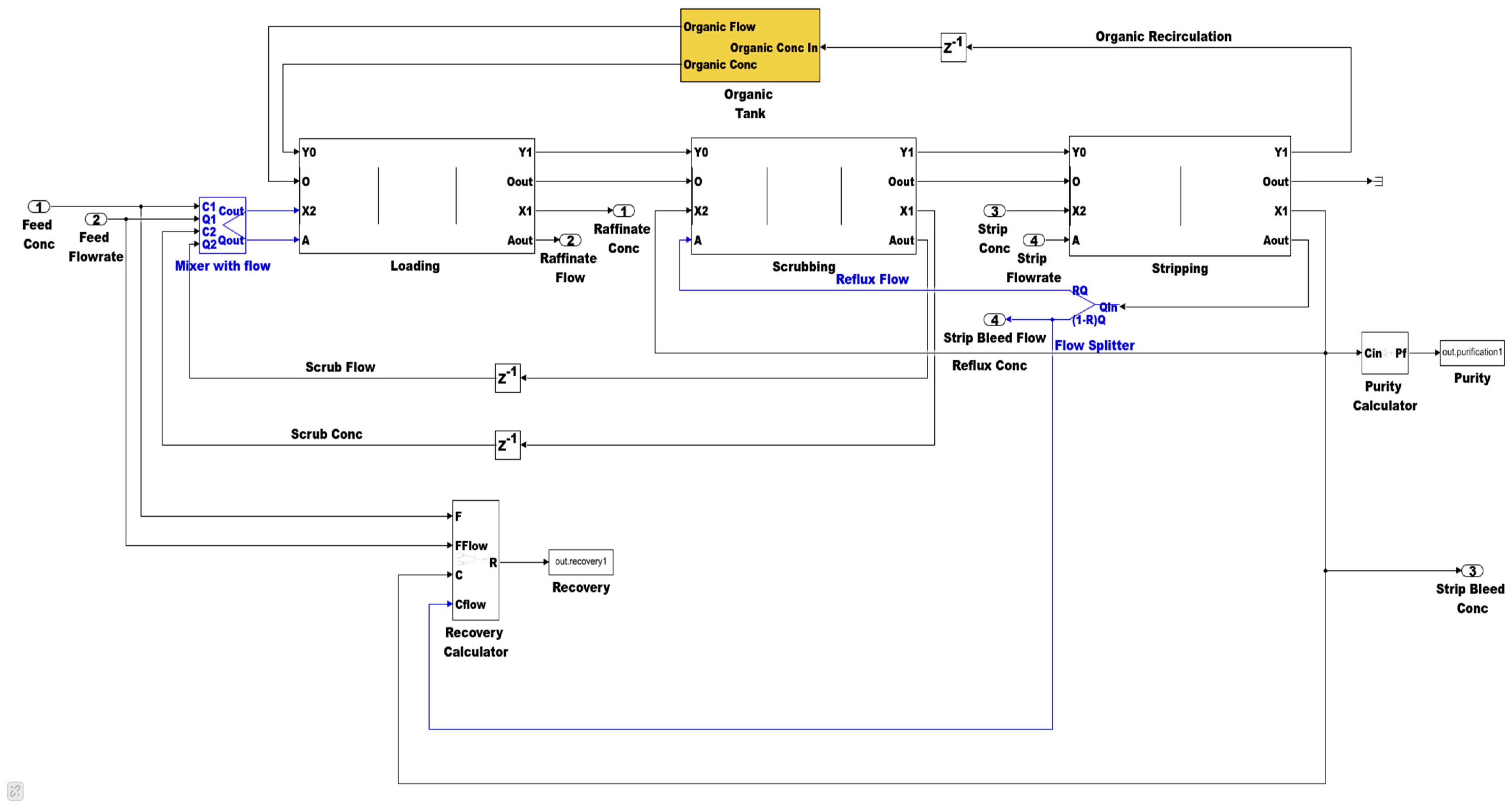
2.1.3. Simulink Library Development
2.1.4. Particle Swarm Optimization Algorithm
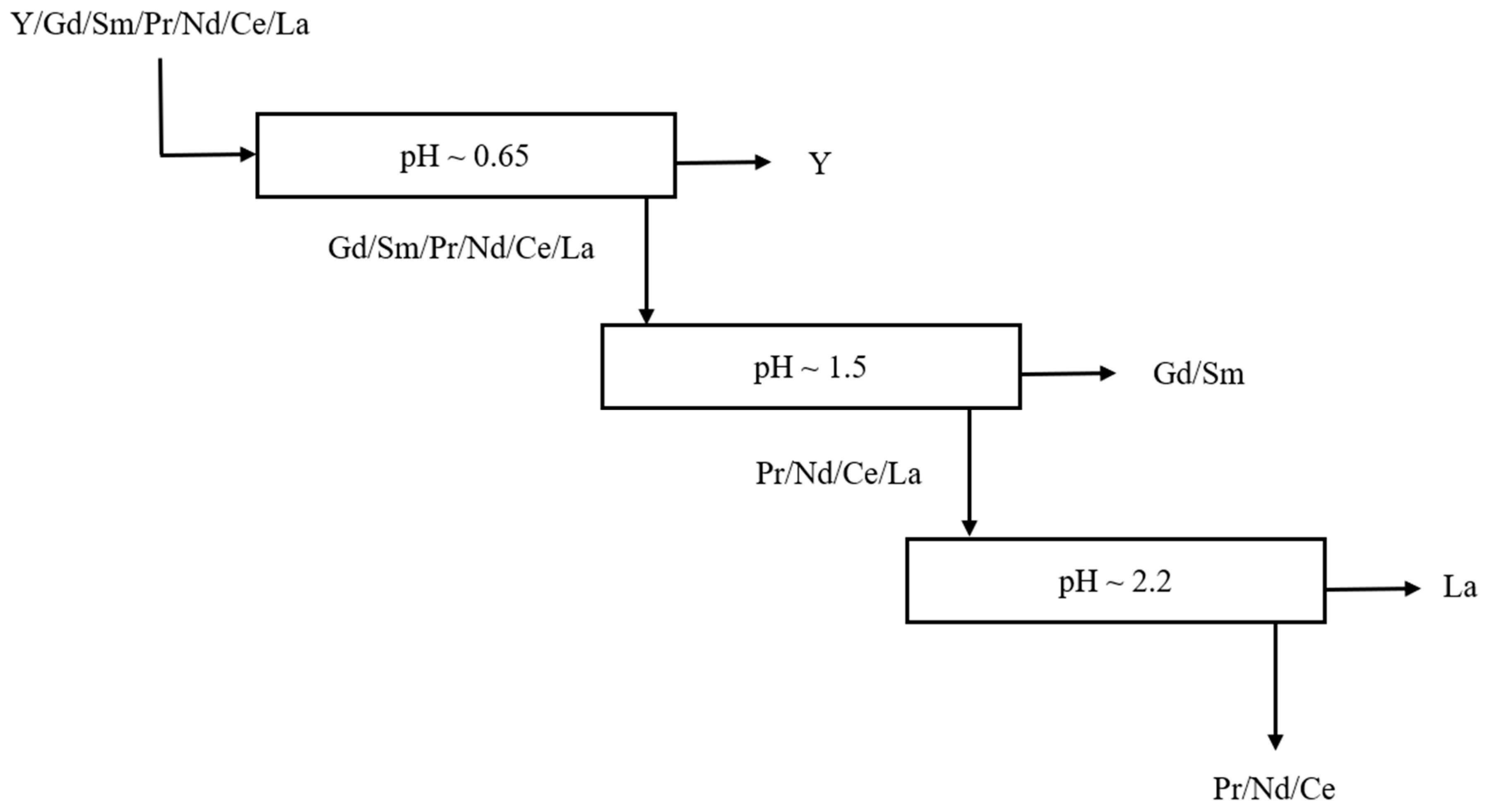
2.2. Part 2: Conceptual Flowsheet and pH Determination
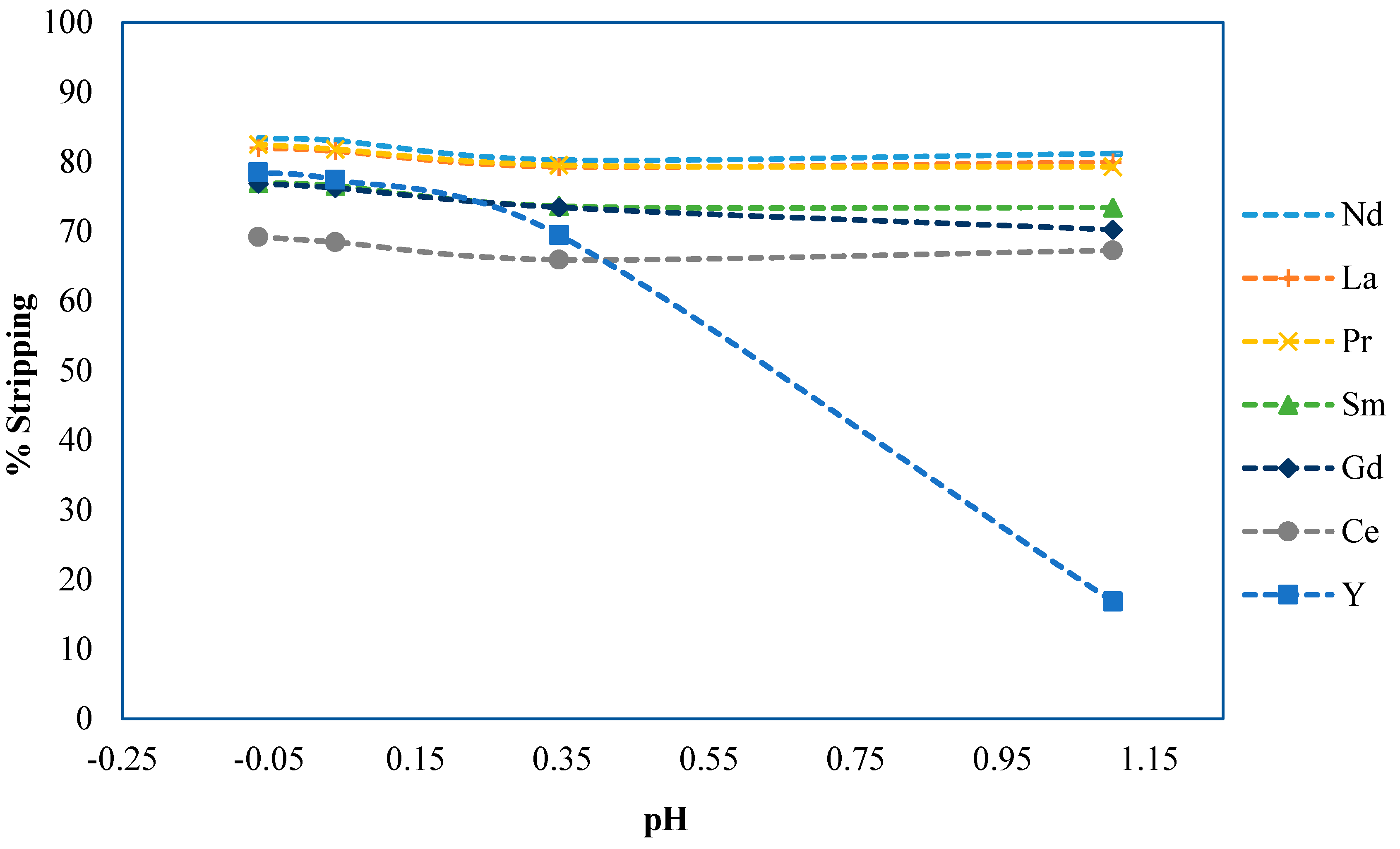
pH Selection
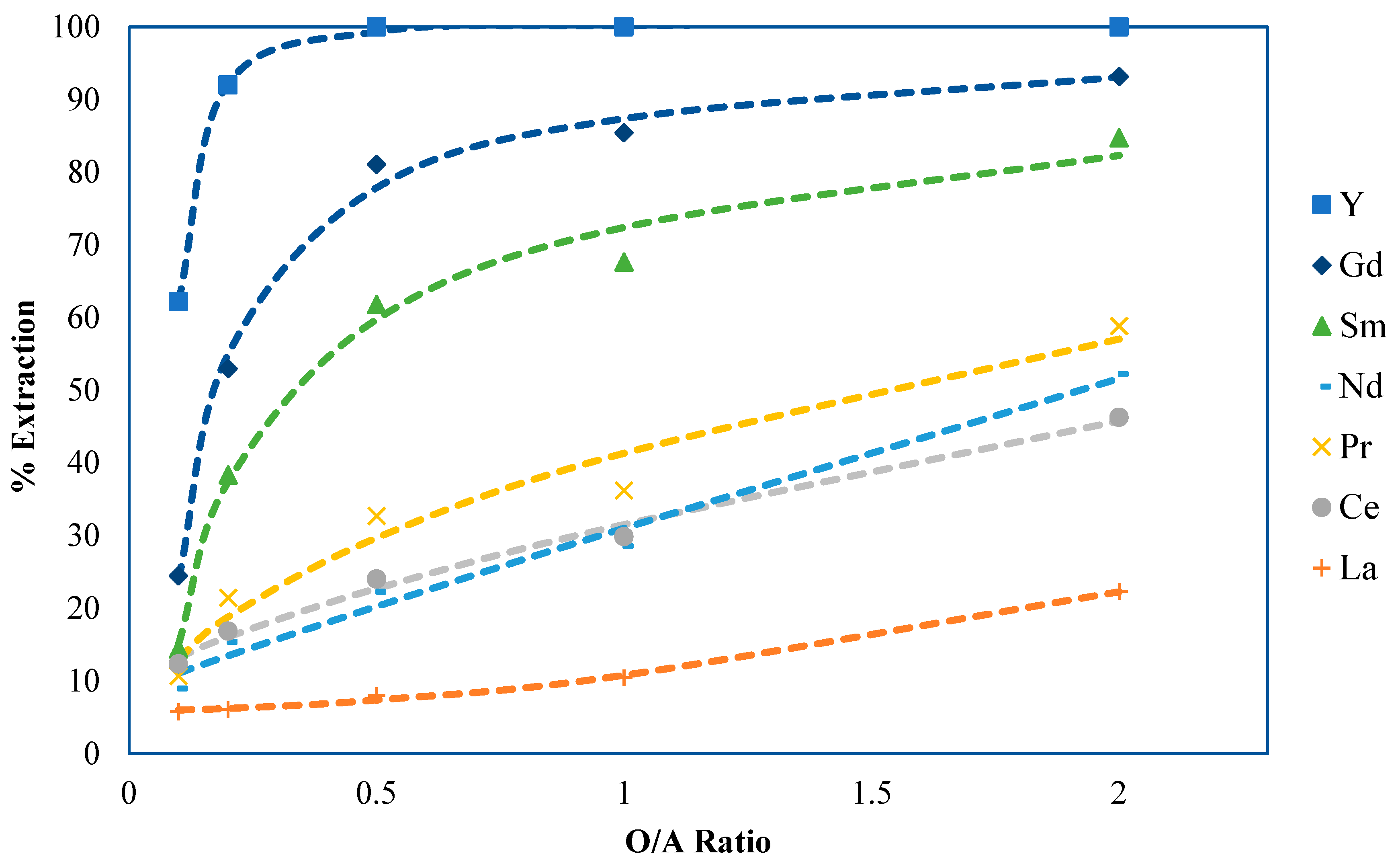
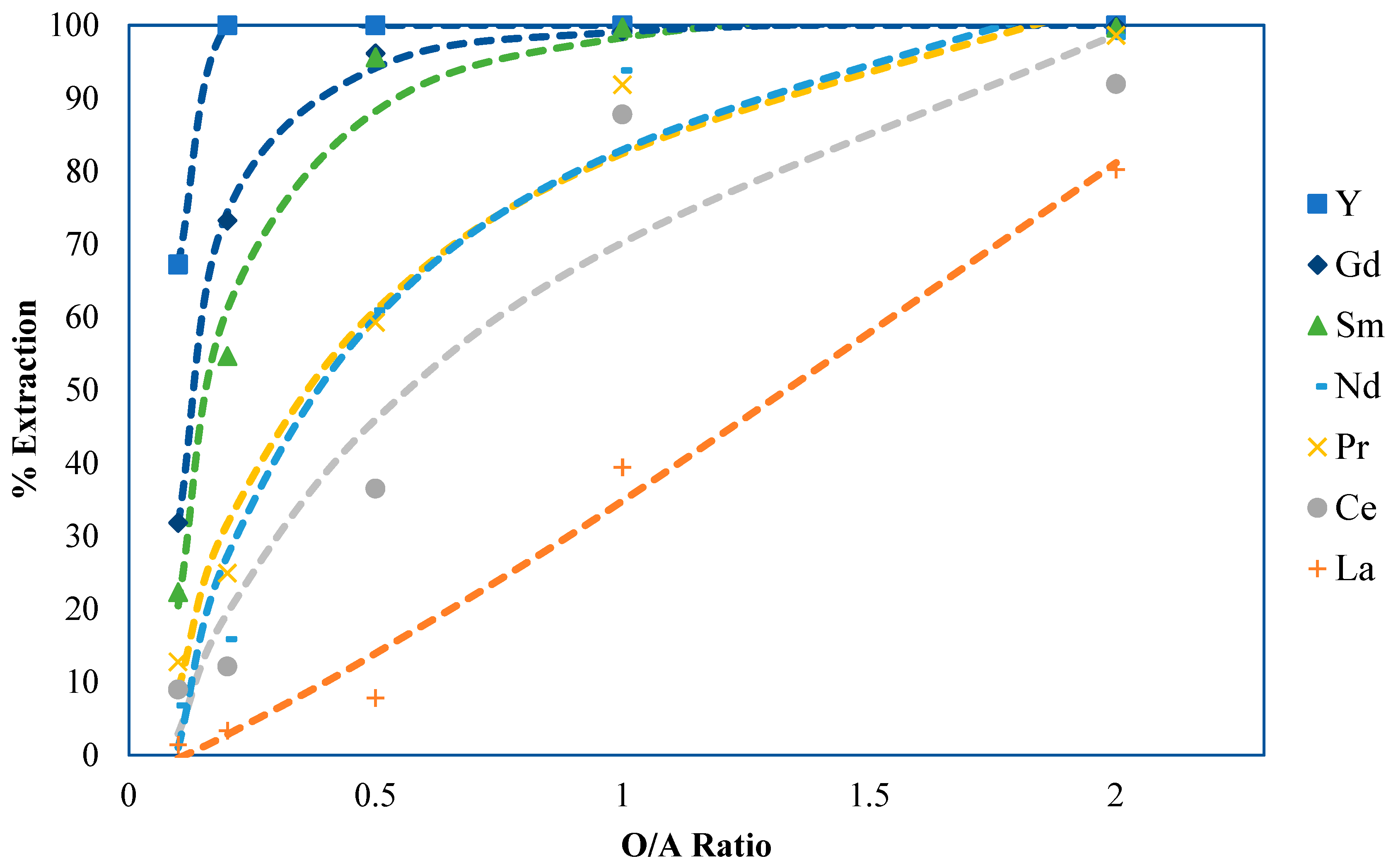
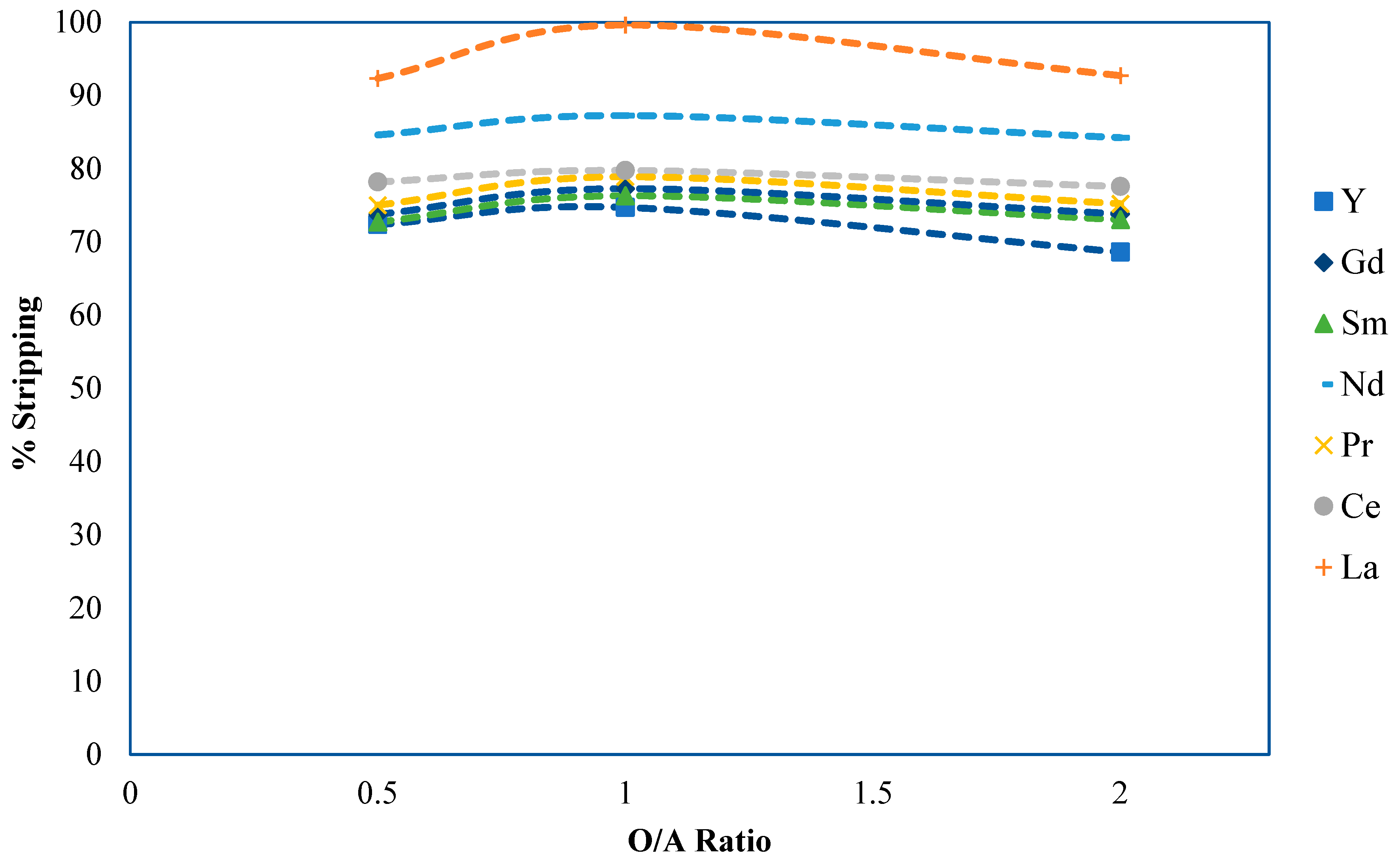
2.3. Part 3: Phase Ratio Determination and Extraction Equilibrium Development
2.3.1. Phase Ratio Development Methods and Materials
2.3.2. Experimental Results and Discussion
2.3.3. Development of Continuous Mathematical Expressions of Equilibrium for Model Input
2.4. Part 4: Flowsheet Development and Optimization
2.4.1. Gadolinium and Samarium Separation
2.4.2. Lanthanum Separation
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Du, X.; Graedel, T.E. Global rare earth in-use stocks in NdFeB permanent magnets. J. Ind. Ecol. 2011, 15, 836–843. [Google Scholar] [CrossRef]
- Ganguli, R.; Cook, D.R. Rare earths: A review of the landscape. MRS Energy Sustain. 2018, 5, E9. [Google Scholar] [CrossRef]
- Pavel, C.C.; Lacal-Arantegui, R.; Marmier, A.; Schuler, D.; Tzimas, E.; Buchert, M.; Jenseit, W.; Blagoeva, D. Substitution strategies for reducing the use of rare earths in wind turbines. Resour. Policy 2017, 52, 349–357. [Google Scholar] [CrossRef]
- Schmid, M. Mitigating supply risks through involvement in rare earth projects: Japan’s strategies and what the US can learn. Resour. Policy 2019, 63, 101457. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Rydberg, J. Solvent Extraction Principles and Practice, Revised and Expanded; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Turgeon, K.; Boulanger, J.-F.; Bazin, C. Simulation of Solvent Extraction Circuits for the Separation of Rare Earth Elements. Minerals 2023, 13, 714. [Google Scholar] [CrossRef]
- Mohammadi, M.; Forsberg, K.; Kloo, L.; de la Cruz, J.M.; Rasmuson, Å. Separation of Nd (III), Dy (III) and Y (III) by solvent extraction using D2EHPA and EHEHPA. Hydrometallurgy 2015, 156, 215–224. [Google Scholar] [CrossRef]
- Basualto, C.; Valenzuela, F.; Molina, L.; Munoz, J.; Fuentes, E.; Sapag, J. Study of the solvent extraction of the lighter lanthanide metal ions by means of organophosphorus extractants. J. Chil. Chem. Soc. 2013, 58, 1785–1789. [Google Scholar] [CrossRef]
- Free, M. Hydrometallurgy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zhang, J.; Zhao, B.; Schreiner, B. Separation Hydrometallurgy of Rare Earth Elements; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Thakur, N. Separation of rare earths by solvent extraction. Miner. Process. Extr. Metullargy Rev. 2000, 21, 277–306. [Google Scholar] [CrossRef]
- Anitha, M.; Singh, H. Artificial neural network simulation of rare earths solvent extraction equilibrium data. Desalination 2008, 232, 59–70. [Google Scholar] [CrossRef]
- Giles, A.; Aldrich, C. Modelling of rare earth solvent extraction with artificial neural nets. Hydrometallurgy 1996, 43, 241–255. [Google Scholar] [CrossRef]
- Sharp, B.M.; Smutz, M. Stagewise Calculation for Solvent Extraction System Monazite Rare Earth Nitrates-Nitric Acid-Tributyl Phosphate-Water. Ind. Eng. Chem. Process. Des. Dev. 1965, 4, 49–54. [Google Scholar] [CrossRef]
- Sebenik, R.F.; Sharp, B.M.; Smutz, M. Computer Solution of Solvent-Extraction-Cascade Calculations for the Monazite Rare-Earth Nitrates-Nitric Acid-Tributyl Phosphate-Water System. Sep. Sci. 1966, 1, 375–386. [Google Scholar] [CrossRef]
- Voit, D.O. Computer simulation of rare earth solvent extraction circuits. In Rare Earths, Extraction, Preparation and Applications; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1988; pp. 213–226. [Google Scholar]
- Reddy, M.L.P.; Ramamohan, T.; Bhat, C.C.S.; Shanthi, P.; Damodaran, A. Mathematical Modelling of the Liquid-Liquid Extraction Separation of Rare Earths. Miner. Process. Extr. Metall. Rev. 1992, 9, 273–282. [Google Scholar] [CrossRef]
- Wenli, L.; D’Ascenzo, G.; Curini, E.; Chunhua, Y.; Jianfang, W.; Tao, J.J.; Minwen, W. Simulation of the development automatization control system for rare earth extraction process: Combination of ESRECE simulation software and EDXRF analysis technique. Anal. Chim. Acta 2000, 417, 111–118. [Google Scholar] [CrossRef]
- Bourget, C.; Soderstrom, M.; Jakovljevic, B.; Morrison, J. Optimization of the Design Parameters of a CYANEX® 272 Circuit for Recovery of Nickel and Cobalt. Solvent Extr. Ion Exch. 2011, 29, 823–836. [Google Scholar] [CrossRef]
- Soderstrom, M.; Bourget, C.; Jakovljevic, B.; Bednarski, T. Development of process modeling for CYANEX® 272. In ALTA 2010 Nickel/Cobalt, Copper and Uranium Proceedings; ALTA Metallurgical Services: Melbourne, Australia, 2010; pp. 1–17. [Google Scholar]
- Evans, H.A.; Bahri, P.A.; Vu, L.T.; Barnard, K.R. Modelling cobalt solvent extraction using Aspen Custom Modeler. In Computer Aided Chemical Engineering; Klemeš, P.S.V.J.J., Liew, P.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33, pp. 505–510. [Google Scholar]
- Lyon, K.; Greenhalgh, M.; Herbst, R.S.; Garn, T.; Welty, A.; Soderstrom, M.D.; Jakovljevic, B. Enhanced Separation of Rare Earth Elements. 2016. Available online: https://www.osti.gov/biblio/1363891 (accessed on 1 September 2016).
- Liu, Y.; Spencer, S. Dynamic simulation of grinding circuits. Miner. Eng. 2004, 17, 1189–1198. [Google Scholar] [CrossRef]
- Srivastava, V.K. Modeling of Rare Earth Solvent Extraction Process for Flowsheet Design and Optimization. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2021. Volume 62. [Google Scholar]
- Kennedy, J.; Eberhart, R. Particle swarm optimization. In Proceedings of the ICNN’95-International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; IEEE: New York, NY, USA, 1995; Volume 4, pp. 1942–1948. [Google Scholar]
- He, Y.; Ma, W.J.; Zhang, J.P. The parameters selection of PSO algorithm influencing on performance of fault diagnosis. In Proceedings of the MATEC Web of Conferences, Cape Town, South Africa, 1–3 February 2016. [Google Scholar]
- Honaker, R.; Zhang, W.; Yang, X.; Rezaee, M. Conception of an integrated flowsheet for rare earth elements recovery from coal coarse refuse. Miner. Eng. 2018, 122, 233–240. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Process development for the recovery of rare earth elements and critical metals from an acid mine leachate. Miner. Eng. 2020, 153, 106382. [Google Scholar] [CrossRef]
- Srivastava, V.; Werner, J. Solvent Extraction Flowsheet Design for the Separation of Rare Earth Elements: Tools, Methods and Application. In Proceedings of the Conference of Metallurgists, COM 2020, Virtual Event, 14–15 October 2020. [Google Scholar]







| Metal | Year | Method | Tool | Name |
|---|---|---|---|---|
| REEs | 1965 | Separation Factor | Stage-wise iterative calculation | [16] |
| REEs | 1966 | Separation Factor | Stage-wise iterative calculation | [17] |
| Nd | 1989 | Kremser | Solution of Kremser Method | [19] |
| REEs | 1992 | Kremser | Solution of Kremser Method | [20] |
| REEs | 2000 | Online Analytical Measurement | ESRECE simulation system | [21] |
| Cobalt/Nickel/Copper | 2010 | Equilibrium Type | by Cytec (no name) | [22] |
| Cobalt/Nickel | 2011 | Equilibrium Type | MINCHEM (Cytec) | [23] |
| Cobalt | 2014 | Equilibrium Type | Aspen custom modeler | [24] |
| REEs | 2023 | Equilibrium Constants | Algebraic Mass Balance | [8] |
| Targeted Equilibrium pH | Average Equilibrium pH Measured | Standard Deviation in Measured pH |
|---|---|---|
| 0.65 | 0.659 | 0.003 |
| 1.5 | 1.523 | 0.014 |
| 2.2 | 2.234 | 0.028 |
| pH | O/A Ratio | Y/Gd | Gd/Sm | Sm/Nd | Nd/Pr | Pr/Ce | Ce/La |
|---|---|---|---|---|---|---|---|
| 1.5 | 0.1 | 2.54 | 1.69 | 1.61 | 0.84 | 0.87 | 2.13 |
| 0.2 | 1.74 | 1.38 | 2.49 | 0.72 | 1.27 | 2.76 | |
| 0.5 | 1.23 | 1.31 | 2.78 | 0.68 | 1.36 | 2.99 | |
| 1 | 1.17 | 1.26 | 2.37 | 0.79 | 1.21 | 2.85 | |
| 2 | 1.07 | 1.10 | 1.62 | 0.89 | 1.27 | 2.07 | |
| 2.2 | 0.1 | 2.11 | 1.43 | 3.27 | 0.53 | 1.42 | 6.29 |
| 0.2 | 1.36 | 1.34 | 3.43 | 0.64 | 2.05 | 3.59 | |
| 0.5 | 1.04 | 1.01 | 1.57 | 1.03 | 1.62 | 4.64 | |
| 1 | 1.01 | 0.99 | 1.06 | 1.02 | 1.05 | 2.22 | |
| 2 | 1.00 | 1.00 | 1.01 | 1.00 | 1.07 | 1.15 |
| Elements | a | b | c | R2 | Equilibrium pH |
|---|---|---|---|---|---|
| Y | 1141.29 | 0.02 | −1068.81 | 0.993 | 0.65 |
| Y | −0.23 | −2.22 | 100.40 | 0.999 | 1.5 |
| La | 4.87 | 1.75 | 5.94 | 0.997 | |
| Ce | 22.82 | 0.70 | 8.70 | 0.992 | |
| Pr | 44.70 | 0.44 | −3.36 | 0.961 | |
| Nd | 22.57 | 0.94 | 8.46 | 0.983 | |
| Sm | −45.70 | −0.35 | 118.10 | 0.989 | |
| Gd | −14.30 | −0.74 | 101.67 | 0.994 | |
| Y | 0.00 | −15.07 | 100.00 | 1.000 | 2.2 |
| La | 37.94 | 1.15 | −3.11 | 0.986 | |
| Ce | 160.41 | 0.24 | −90.20 | 0.917 | |
| Pr | −862.16 | −0.04 | 944.61 | 0.971 | |
| Nd | −397.83 | −0.08 | 480.77 | 0.956 | |
| Sm | −12.29 | −0.87 | 110.61 | 0.975 | |
| Gd | −3.21 | −1.34 | 102.30 | 0.998 |
| Operating Parameters | Value |
|---|---|
| Feed flow rate (lpm) | 0.9 |
| Organic flow rate (lpm) | 1 |
| Strip flow rate (lpm) | 0.5 |
| Reflux ratio | 0.2 |
| Loading equilibrium pH | 0.65 |
| Strip equilibrium pH | 0.15 (0.70 M) |
| Condition | Value |
|---|---|
| Number of particles | 10 |
| Maximum iterations | 20 |
| w | 0.8 |
| c1 | 2 |
| c2 | 2 |
| Boundary conditions for stages |
| Train | Objective Function | Element Separated | Purity | Stage Combination (Loading–Scrubbing–Stripping) |
|---|---|---|---|---|
| Train-1 | Recovery and Purity of Y | Y | 99.52 | 8-12-3 |
| Train-2 | Purity of Sm | Gd-Sm combined | 46.00/34.65 (80.65) | 7-9-6 |
| Train-3 | Purity of Gd | Gd/Sm | 72.59 | 14-8-5 |
| Train-4 | Purity of Ce | Nd, Pr, Ce combined/La | 26.74/7.94/54.60 (89.28) | 10-3-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, V.; Werner, J.; Honaker, R. Design of Multi-Stage Solvent Extraction Process for Separation of Rare Earth Elements. Mining 2023, 3, 552-578. https://doi.org/10.3390/mining3030031
Srivastava V, Werner J, Honaker R. Design of Multi-Stage Solvent Extraction Process for Separation of Rare Earth Elements. Mining. 2023; 3(3):552-578. https://doi.org/10.3390/mining3030031
Chicago/Turabian StyleSrivastava, Vaibhav, Joshua Werner, and Rick Honaker. 2023. "Design of Multi-Stage Solvent Extraction Process for Separation of Rare Earth Elements" Mining 3, no. 3: 552-578. https://doi.org/10.3390/mining3030031
APA StyleSrivastava, V., Werner, J., & Honaker, R. (2023). Design of Multi-Stage Solvent Extraction Process for Separation of Rare Earth Elements. Mining, 3(3), 552-578. https://doi.org/10.3390/mining3030031







