A Methodology for Building a Medical Ontology with a Limited Domain Experts’ Involvement
Abstract
1. Introduction
2. State of the Art
3. Materials and Methods
3.1. Phase 1: Ontology Domain and Scope Definition
- What is the domain that the ontology will cover?
- What is the purpose of creating this ontology?
3.2. Phase 2: Corpus Building and Term Extraction
3.3. Phase 3: Building of Preliminary Ontology
3.3.1. Step 1: Semantic Normalization
3.3.2. Step 2: Knowledge Formalization
3.3.3. Step 3: Toward a Computational Ontology
3.4. Phase 4: Ontology Reuse and Enrichment
3.4.1. Step 1: Finding Ontologies
- Both the natural and formal languages of the candidate ontology are the same as those of the ontology we are building.
- There is a mapping from a non-empty subset of the new ontology requirements (defined in phase 1) to a robust set of concepts of the ontology candidate.
- The candidate ontology is accepted within its user community. This acceptance is assessed by using some metrics such as the number of community members that endorse the ontology, the number of times the ontology has been reused, etc.
- The candidate ontology has been published in a peer-reviewed publication.
3.4.2. Step 2: Choosing Relevant Ontologies
3.4.3. Step 3: Resolving Conflicts
- Does the concept have a definition (in the form of annotation)? If yes, does its definition correspond to an intended model of the ontology?
- Does the concept have a superclass? If yes, is it relevant to the ontology?
- Does the concept have children? If yes, is each of them relevant to the ontology?
- Does the concept have synonyms?
- Does the concept have alternative names?
- Does the concept have related names?
- If the concerned concept is not found in any ontology, then we propose some relevant annotations that can be obtained from UMLS, such as definitions, synonyms, or concept unique identifier (CUI). If the concept is not found in UMLS, no annotation is added.
- If the concept is represented in only one ontology, then we reuse it if relevant, i.e., its representation fits the requirements, with its identifier and annotations. If not, we propose a new identifier. If the concept is found in UMLS, we propose to enrich it with annotations.
- If the concept is found in more than one ontology, then there is a conflict, and we have to choose the ontology that provides the best concept representation for the domain, i.e., the one that provides answers to the CRQs and corresponds to an intended model.
- If the chosen ontology is SNOMED-CT where concepts can have multiple parents and multi-hierarchies [45] that often cause user uncertainty in the selection of concepts and a messy situation in their classification [46], then we check if this is the case for the concerned concept. If it has more than one hierarchy, then we identify the most relevant hierarchy or combine one or more hierarchies to build a new one. If not, we reuse the concept with its hierarchy.
- If the chosen ontology is not SNOMED-CT, reuse the concept as it is represented in the ontology.
3.5. Phase 5: Ontology Evaluation
3.5.1. Consistency
3.5.2. Accuracy and Coverage (Completeness)
3.5.3. Clarity
3.5.4. Conciseness
3.5.5. Computational Efficiency
3.5.6. Adaptability
3.6. Phase 6: Ontology Documentation
3.7. Phase 7: Maintenance and Evolution
4. Results
4.1. Ontology Domain and Scope Definition
- What are the symptoms and clinical signs of pneumonia?
- What are the types of pneumonia?
- How is pneumonia diagnosed?
- What are the pathogens of pneumonia?
- What is the clinical history of the patient?
- What are the laboratory tests to diagnose pneumonia?
- What are the results of the physical examination of the patient?
- What is the result of lung imaging of the patient?
- What are the results of the lab tests of the patient?
- Once the diagnosis of pneumonia has been made, what complications should the physician look for?
4.2. Corpus Building and Term Extraction
4.3. Building of Preliminary Ontology
4.3.1. Semantic Normalization
4.3.2. Knowledge Formalization
4.3.3. Toward a Computational Ontology
4.4. Ontology Reuse and Enrichment
4.4.1. Finding Ontologies
4.4.2. Choosing Relevant Ontologies
4.4.3. Resolving Conflicts
- CRQ 1: “Does hypoxemia have a definition in each ontology?” Answer: Only HPO provides a definition.
- CRQ 2: “Does its definition correspond to the PNADO requirements?” Answer: The definition given in HPO is relevant to PNADO.
- CRQ 3: “Does hypoxemia have children?” Answer: Hypoxemia has children in HPO and SNOMED-CT but none in SYMP.
- CRQ 4: “Is each found child relevant to the PNADO requirements?” Answer: Hypoxemia’s children found in HPO are relevant to the PNADO requirements.
- Analyze: Hypoxemia presented in HPO seems to have the best representation for PNADO since it provides a relevant definition and relevant children that correspond to the PNADO requirements.
- CRQ 1: “Does patient have a definition in each ontology?” Answer: No ontology provides a definition.
- CRQ 2: “What is the parent concept of patient?” Answer: In CPRO, the parent concept is organism that is provided by OGMS. In SNOMED-CT, the parent is social context that does not fit the PNADO requirements.
- CRQ 3: “Is any person a patient?” Answer in CPRO is the axiom “Human/Person and (“Plays Role” some Patient role) and (“Participates_in” some Clinical act).” No answer is found in SNOMED-CT.
- Analyze: CPRO provides a better representation for patient than SNOMED-CT. It specifies with an axiom in which case a person can be a patient, and it is a subclass of organism defined by OGMS.
4.5. Ontology Evaluation
4.5.1. Consistency
4.5.2. Accuracy and Coverage (Completeness)
4.5.3. Clarity
4.5.4. Conciseness
4.5.5. Computational Efficiency
4.5.6. Adaptability
4.5.7. Characteristics of PNADO
4.6. Ontology Documentation
4.7. Maintenance and Evolution
5. Discussion
5.1. Generality of the Methodology
5.2. Ontology Reuse
- Choosing relevant ontologies to reuse is very important and determinant for the rest of the reuse process. Some candidate ontologies can be easily excluded while others require careful analysis.
- Even if the chosen ontology is of good quality, the representation of a concept may not be relevant to the requirements of the ontology that is being built. We also found out during the PNADO building that some ontologies have bad representations for some concepts. For example, SYMP represents diseases as symptoms. An example of a relation found in RO that is not relevant for PNADO is discussed in Section 5.3.
- Each ontology is chosen according to a significant topic, as discussed in Section 3.4.2, but in the process of reuse, the chosen ontology may contain concepts that deal with other topics. For example, DOID was chosen for the topic of diseases, but it was involved in conflicts regarding concepts related to symptoms and clinical signs.
- A concept can be differently presented in different ontologies, and choosing the best representation to reuse requires reflection about the relevant CRQs.
5.3. Upper Domain Ontologies
5.4. PNADO New Concepts
- Complication of
- Differential Diagnosis of
- Has Symptom
- Symptom
- Pathogen
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Patel, A.; Debnath, N.C. A Comprehensive Overview of Ontology: Fundamental and Research Directions. Curr. Mater. Sci. Former. Recent. Pat. Mater. Sci. 2024, 17, 2–20. [Google Scholar] [CrossRef]
- Amith, M.; He, Z.; Bian, J.; Lossio-Ventura, J.A.; Tao, C. Assessing the practice of biomedical ontology evaluation: Gaps and opportunities. J. Biomed. Inf. 2018, 80, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amith, M.; Manion, F.; Liang, C.; Harris, M.; Wang, D.; He, Y.; Tao, C. Architecture and usability of OntoKeeper, an ontology evaluation tool. BMC Med. Inform. Decis. Mak. 2019, 19, 152. [Google Scholar] [CrossRef]
- Fung, K.W.; Bodenreider, O. Knowledge Representation and Ontologies. In Clinical Research Informatics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 367–388. [Google Scholar]
- Katsumi, M.; Grüninger, M. What is Ontology Reuse? FOIS: Kalyan, India, 2016. [Google Scholar]
- Pathak, J.; Johnson, T.M.; Chute, C.G. Survey of modular ontology techniques and their applications in the biomedical domain. Integr. Comput. Aided Eng. 2009, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Simperl, E. Reusing ontologies on the Semantic Web: A feasibility study. Data Knowl. Eng. 2009, 68, 905–925. [Google Scholar] [CrossRef]
- Zulkarnain, N.Z.; Meziane, F.; Crofts, G. A Methodology for Biomedical Ontology Reuse. In Proceedings of the International Conference on Applications of Natural Language to Information Systems, Salford, UK, 22–24 June 2016; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ochs, C.; Perl, Y.; Geller, J.; Arabandi, S.; Tudorache, T.; Musen, M.A. An empirical analysis of ontology reuse in BioPortal. J. Biomed. Inform. 2017, 71, 165–177. [Google Scholar] [CrossRef]
- Alharbi, R.; Tamma, V.; Grasso, F. Requirement-Based Methodological Steps to Identify Ontologies for Reuse. In Proceedings of the International Conference on Advanced Information Systems Engineering, Limassol, Cyprus, 3–7 June 2024; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Fernández-López, M.; Gómez-Pérez, A. Overview and analysis of methodologies for building ontologies. Knowl. Eng. Rev. 2002, 17, 129. [Google Scholar] [CrossRef]
- Brendish, N.J.; Malachira, A.K.; Beard, K.R.; Armstrong, L.; Lillie, P.J.; Clark, T.W. Hospitalised adults with pneumonia are frequently misclassified as another diagnosis. Respir. Med. 2019, 150, 81–84. [Google Scholar] [CrossRef]
- Kanwal, K.; Asif, M.; Khalid, S.G.; Liu, H.; Qurashi, A.G.; Abdullah, S. Current Diagnostic Techniques for Pneumonia: A Scoping Review. Sensors 2024, 24, 4291. [Google Scholar] [CrossRef]
- Uschold, M.; King, M. Towards a Methodology for Building Ontologies. In Proceedings of the IJCAI’95 Workshop on Basic Ontological Issues in Knowledge Sharing, Montreal, Canada, 13 April 1995. [Google Scholar]
- Uschold, M.; King, M.; Moralee, S.; Zorgios, Y. The enterprise ontology. Knowl. Eng. Rev. 1998, 13, 31–89. [Google Scholar] [CrossRef]
- Grüninger, M.; Fox, M.S. Methodology for the Design and Evaluation of Ontologies. In Proceedings of the IJCAI’95 Workshop on Basic Ontological Issues in Knowledge Sharing, Montreal, Canada, 13 April 1995. [Google Scholar]
- Uschold, M.; Gruninger, M. Ontologies: Principles, methods and applications. Knowl. Eng. Rev. 1996, 11, 93–136. [Google Scholar] [CrossRef]
- Fernández-López, M.; Gómez-Pérez, A.; Juristo, N. Methontology: From Ontological Art Towards Ontological Engineering. In Proceedings of the Ontological Engineering AAAI-97 Spring Symposium Series, Stanford, CA, USA, 24–26 March 1997. [Google Scholar]
- Bachimont, B.; Isaac, A.; Troncy, R. Semantic Commitment for Designing Ontologies: A Proposal. In Proceedings of the International Conference on Knowledge Engineering and Knowledge Management, Siguenza, Spain, 1–4 October 2002; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Schultz, D.J. IEEE Standard for Developing Software Life Cycle Processes; IEEE: Piscataway, NJ, USA, 1997; pp. 1074–1997. [Google Scholar]
- Noy, N.F.; McGuinness, D.L. Ontology Development 101: A Guide to Creating Your First Ontology; Stanford Knowledge Systems Laboratory Technical Report KSL-01-05 and Stanford Medical Informatics Technical Report SMI-2001-0880; Stanford University: Stanford, CA, USA, 2001. [Google Scholar]
- Al-Aswadi, F.N.; Chan, H.Y.; Gan, K.H. Automatic ontology construction from text: A review from shallow to deep learning trend. Artif. Intell. Rev. 2020, 53, 3901–3928. [Google Scholar] [CrossRef]
- Faure, D.; Nédellec, C. A Corpus-Based Conceptual Clustering Method for Verb Frames and Ontology Acquisition. In Proceedings of the LREC Workshop on Adapting Lexical and Corpus Resources to Sublanguages and Applications, Granada, Spain, 26 May 1998. [Google Scholar]
- Maedche, A.; Staab, S. Ontology Learning. In Handbook on Ontologies; Springer: Berlin/Heidelberg, Germany, 2004; pp. 173–190. [Google Scholar]
- Cimiano, P.; Völker, J. text2onto. In Proceedings of the International Conference on Application of Natural Language to Information Systems, Alicante, Spain, 15–17 June 2005; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Shamsfard, M.; Barforoush, A.A. Learning ontologies from natural language texts. Int. J. Hum. Comput. Stud. 2004, 60, 17–63. [Google Scholar] [CrossRef]
- Hahn, U.; Romacker, M. The SYNDIKATE Text Knowledge Base Generator. In Proceedings of the First International Conference on Human Language Technology Research, San Diego, California, 18–21 March 2001. [Google Scholar]
- Gillani Andleeb, S. From Text Mining to Knowledge Mining: An Integrated Framework of Concept Extraction and Categorization for Domain Ontology. Ph.D. Thesis, Budapesti Corvinus Egyetem, Budapest, Hungary, 2015. [Google Scholar]
- Navigli, R.; Velardi, P.; Gangemi, A. Ontology learning and its application to automated terminology translation. IEEE Intell. Syst. 2003, 18, 22–31. [Google Scholar] [CrossRef]
- Buitelaar, P.; Olejnik, D.; Sintek, M. A Protégé Plug-In for Ontology Extraction from Text Based on Linguistic Analysis. In European Semantic Web Symposium; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Fortuna, B.; Grobelnik, M.; Mladenic, D. Semi-Automatic Data-Driven Ontology Construction System. In Proceedings of the 9th International Multi-Conference Information Society IS-2006, Ljubljana, Slovenia, 9 October 2006. [Google Scholar]
- Biébow, B.; Szulman, S.; Clement, A.J.B. TERMINAE: A Linguistics-Based Tool for the Building of a Domain Ontology. In Proceedings of the International Conference on Knowledge Engineering and Knowledge Management, Sigüenza, Spain, 1–4 October 2002; pp. 49–66. [Google Scholar]
- Trokanas, N.; Koo, L.; Cecelja, F. Towards a Methodology for Reusable Ontology Engineering: Application to the Process Engineering Domain. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 471–476. [Google Scholar]
- Dramé, K.; Diallo, G.; Delva, F.; Dartigues, J.F.; Mouillet, E.; Salamon, R.; Mougin, F. Reuse of termino-ontological resources and text corpora for building a multilingual domain ontology: An application to Alzheimer’s disease. J. Biomed. Inform. 2014, 48, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Bright, T.J.; Furuya, E.Y.; Kuperman, G.J.; Cimino, J.J.; Bakken, S. Development and evaluation of an ontology for guiding appropriate antibiotic prescribing. J. Biomed. Inform. 2012, 45, 120–128. [Google Scholar] [CrossRef]
- Nachabe, L.; Jahan, N. A Proposed Ontology Evaluation Tool to Assist Ontology Engineers in Selecting Ontologies During the Reuse Phase. In Proceedings of the International Knowledge Graph and Semantic Web Conference, Leipzig, Germany, 26–28November 2024; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Hulshof, C.T. 1710d Systematic Reviews and Evidence-Based Guidelines, Two of a Different Kind? BMJ Publishing Group Ltd.: London, UK, 2018. [Google Scholar]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef]
- Gillois, P.; Chatellier, G.; Jaulent, M.C.; Colombet, I.; Fieschi, M.; Degoulet, P. From paper-based to electronic guidelines: Application to French guidelines. Stud. Health Technol. Inform. 2001, 84 Pt 1, 196–200. [Google Scholar]
- Guarino, N.; Oberle, D.; Staab, S. What is an Ontology? In Handbook on Ontologies; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–17. [Google Scholar]
- Grenon, P.; Smith, B.; Goldberg, L. Biodynamic ontology: Applying BFO in the biomedical domain. Stud. Health Technol. Inform. 2004, 102, 20–38. [Google Scholar]
- Arp, R.; Smith, B. Function, role and disposition in basic formal ontology. Nat. Preced. 2008, 1941, 1. [Google Scholar] [CrossRef]
- Aronson, A.R. Metamap: Mapping Text to the Umls Metathesaurus; NLM; NIH; DHHS: Bethesda, MD, USA, 2006; pp. 1–26. [Google Scholar]
- Katsumi, M.; Grüninger, M. Choosing ontologies for reuse. Appl. Ontol. 2017, 12, 195–221. [Google Scholar] [CrossRef]
- Cui, L.; Zhu, W.; Tao, S.; Case, J.T.; Bodenreider, O.; Zhang, G.-Q. Mining non-lattice subgraphs for detecting missing hierarchical relations and concepts in SNOMED CT. J. Am. Med. Inform. Assoc. 2017, 24, 788–798. [Google Scholar] [CrossRef]
- Yamagata, Y.; Kozaki, K.; Imai, T.; Ohe, K.; Mizoguchi, R. An ontological modeling approach for abnormal states and its application in the medical domain. J. Biomed. Semant. 2014, 5, 23. [Google Scholar] [CrossRef]
- Brank, J.; Grobelnik, M.; Mladenic, D. A Survey of Ontology Evaluation Techniques. In Proceedings of the Conference on Data Mining and Data Warehouses (SiKDD 2005), Ljubljana, Slovenia, 17 October 2005. [Google Scholar]
- Porzel, R.; Malaka, R. A Task-Based Approach for Ontology Evaluation. In Proceedings of the ECAI Workshop on Ontology Learning and Population, Valencia, Spain, 22–24 August 2004; Citeseer: Princeton, NJ, USA, 2004. [Google Scholar]
- Maedche, A.; Staab, S. Measuring Similarity Between Ontologies. In Proceedings of the International Conference on Knowledge Engineering and Knowledge Management, Siguenza, Spain, 1–4 October 2002; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Brewster, C.; Alani, H.; Dasmahapatra, S.; Wilks, Y. Data Driven Ontology Evaluation. In Proceedings of the International Conference on Language Resources and Evaluation, Lisbon, Portugal, 24–30 May 2004. [Google Scholar]
- Lozano-Tello, A.; Gómez-Pérez, A. Ontometric: A method to choose the appropriate ontology. J. Database Manag. (JDM) 2004, 15, 1–18. [Google Scholar] [CrossRef]
- Gómez-Pérez, A. Evaluation of ontologies. Int. J. Intell. Syst. 2001, 16, 391–409. [Google Scholar] [CrossRef]
- Vrandečić, D. Ontology Evaluation. In Handbook on Ontologies; Springer: Berlin/Heidelberg, Germany, 2009; pp. 293–313. [Google Scholar]
- Zhu, X.; Fan, J.-W.; Baorto, D.M.; Weng, C.; Cimino, J.J. A review of auditing methods applied to the content of controlled biomedical terminologies. J. Biomed. Inform. 2009, 42, 413–425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burton-Jones, A.; Storey, V.C.; Sugumaran, V.; Ahluwalia, P. A semiotic metrics suite for assessing the quality of ontologies. Data Knowl. Eng. 2005, 55, 84–102. [Google Scholar] [CrossRef]
- Aruna, T.; Saranya, K.; Bhandari, C. A Survey on Ontology Evaluation Tools. In Proceedings of the 2011 International Conference on Process Automation, Control and Computing, Coimbatore, India, 20–22 July 2011. [Google Scholar]
- Corbridge, C.; Rugg, G.; Major, N.; Shadbolt, N.; Burton, A. Laddering: Technique and tool use in knowledge acquisition. Knowl. Acquis. 1994, 6, 315–341. [Google Scholar] [CrossRef]
- Poveda-Villalón, M.; Gómez-Pérez, A.; Suárez-Figueroa, M.C. Oops! (ontology pitfall scanner!): An on-line tool for ontology evaluation. Int. J. Semant. Web Inf. Syst. (IJSWIS) 2014, 10, 7–34. [Google Scholar] [CrossRef]
- Doran, P.; Tamma, V.; Iannone, L. Ontology Module Extraction for Ontology Reuse: An Ontology Engineering Perspective. In Proceedings of the Sixteenth ACM Conference on Conference on Information and Knowledge Management, Lisbon, Portugal, 6–10 November 2007. [Google Scholar]
- Kumar, S.; Baliyan, N. Quality Evaluation of Ontologies. In Semantic Web-Based Systems; Springer: Berlin/Heidelberg, Germany, 2018; pp. 19–50. [Google Scholar]
- Halland, K.; Britz, K. Investigations into the use of SNOMED CT to enhance an OpenMRS health information system. S. Afr. Comput. J. 2011, 47, 33–45. [Google Scholar] [CrossRef][Green Version]
- El-Sappagh, S.; Franda, F.; Ali, F.; Kwak, K.-S. SNOMED CT standard ontology based on the ontology for general medical science. BMC Med. Inform. Decis. Mak. 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Rabhi, F.; Ray, P.; Taylor, K. A guiding framework for ontology reuse in the biomedical domain. In Proceedings of the 2014 47th Hawaii International Conference on System Sciences, Waikoloa, HI, USA, 6–9 January 2014. [Google Scholar]
- Johnson, A.E.; Pollard, T.J.; Shen, L.; Lehman, L.-W.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Celi, L.A.; Mark, R.G. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 160035. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]


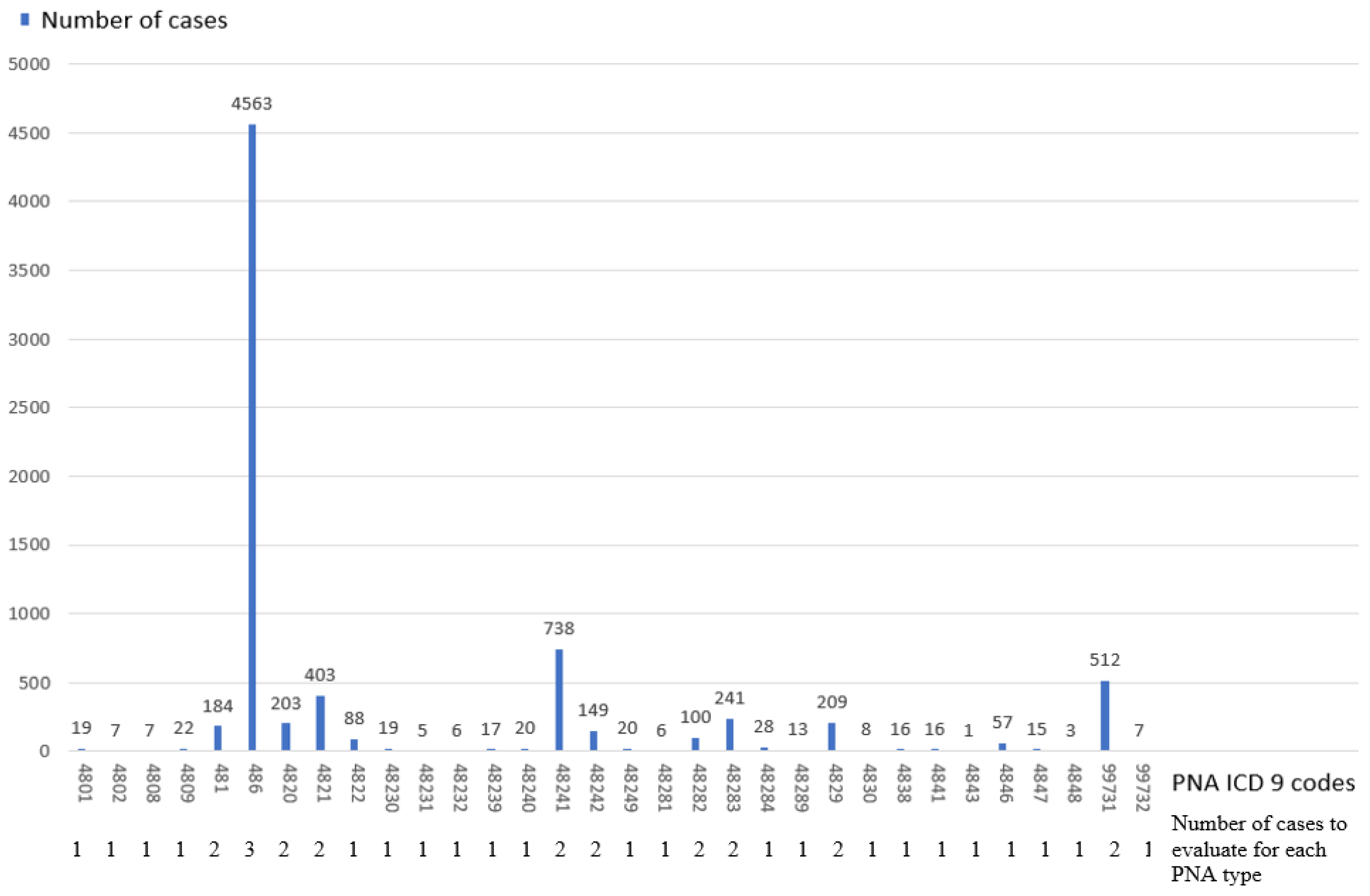

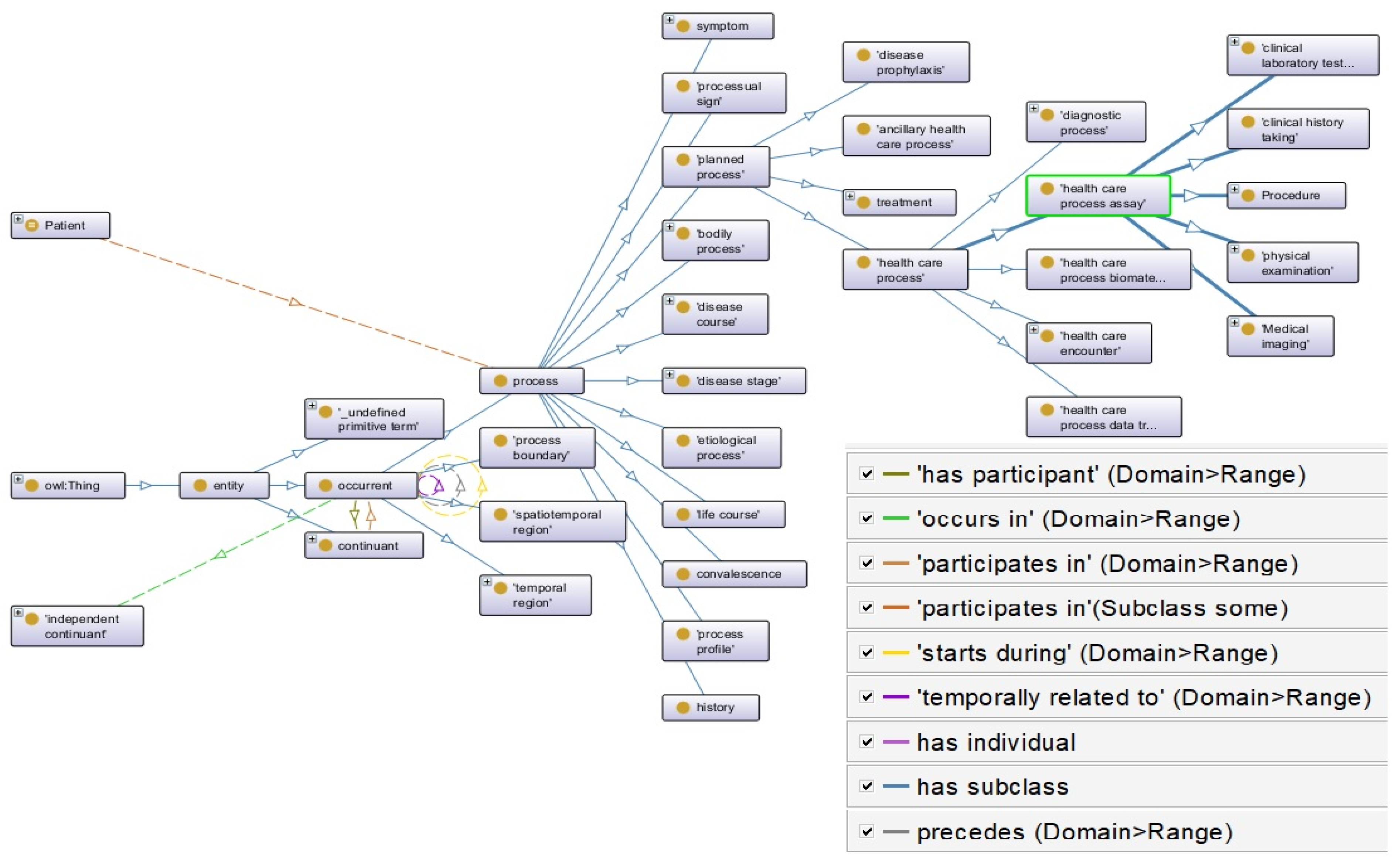
| Criteria | Definition |
|---|---|
| Accuracy | Does the asserted knowledge in the ontology agree with the expert’s knowledge, which is often measured in terms of precision and recall? |
| Completeness | Is the domain of interest appropriately covered? |
| Conciseness | Does the ontology include irrelevant or redundant axioms? |
| Consistency | Does the ontology include or allow for contradictions? |
| Computational efficiency | How fast can the reasoners work with the ontology? |
| Adaptability | How easy or difficult is it to use the ontology in different contexts? |
| Clarity | Does the ontology communicate effectively the intended meaning of the defined terms? |
| Concept | Ontologies | CRQs | CRQs Responses |
|---|---|---|---|
| Hypotension | HPO SNOMED-CT SYMP | Is hypotension a symptom or a vital sign? | - HPO: Hypotension is a phenotypic abnormality. - SNOMED-CT: Hypotension (low blood pressure) is considered a disorder of the cardiovascular system. - SYMP: Hypotension is considered a hemic system symptom. |
| What is the synonym of hypotension? | - Hypotension has two synonyms in HPO, three synonyms in SNOMED-CT, and no synonym in SYMP. | ||
| Are there any hypotension’s children? | - Hypotension has three children in HPO, twelve children in SNOMED-CT, and one child in SYMP. | ||
| Is each child relevant to the diagnosis of pneumonia? | - Only hypotension’s children provided by HPO and SYMP are relevant for the diagnosis of pneumonia. | ||
| Analyze: Blood pressure is a measurement of the pressure in arteries. We consider it in PNADO as a vital sign. Hypotension’s children in HPO combined with SYMP’s child correspond to PNADO intended model. We reuse hypotension’s synonyms of SNOMED-CT. | |||
| Respiratory rates | SNOMED-CT LOINC | Is respiratory rate a symptom or a vital sign? | - In both SNOMED-CT and LOINC, it is mentioned that the scale type of respiratory rate is quantitative. |
| Are there any respiratory rate’s children? | - SNOMED-CT: The respiratory rate has two children. - LOINC: The respiratory rate has no children. | ||
| Is each child relevant to the diagnosis of pneumonia? | - Yes, the respiratory rate’s children provided by SNOMED-CT are relevant for the diagnosis of pneumonia. | ||
| Is there any measurement unit? | - No measurement for respiratory rate is given in SNOMED-CT. - LOINC provides “[4]/min” as an example of the respiratory rate measurement unit. | ||
| Are there any synonyms? Are there any related names? | - SNOMED-CT provides two synonyms and no related names. - LOINC doesn’t provide any synonym for respiratory rate but provides 13 related names. | ||
| Analyze: The respiratory rate is represented as a vital sign in PNADO. We reuse SNOMED-CT’s children because they respond to the PNADO scope and its intended model. We reuse the unit measurement of LOINC and some of the related names. We also reuse the synonyms of SNOMED-CT. | |||
| Concepts from the Text | Equivalences in the PNADO | |||
|---|---|---|---|---|
| Class | Object Property | ID | Parent Entity in PNADO | |
| Chest radiograph | Chest radiography | PNADO:0000783 | Imaging of lung | |
| Patients likely to have pneumonia | Has a diagnosis (patients, pneumonia) | PNADO:0001291 | ||
| Diagnosis | Diagnosis | OGMS:0000073 | Data item | |
| Differentiating CAP from … acute bronchitis | Differential diagnosis of (CAP, Acute bronchitis) | PNADO:0001157 | ||
| CAP | Community-acquired pneumonia | SNOMEDCT_US:385093006 | Pneumonia | |
| Cough | Cough | SNOMEDCT_US:49727002 | Respiratory system and chest symptoms | |
| Fever | Fever | SYMP:0000613 | Neurological and physiological symptoms | |
| Ontology | Usage in PNADO | Classes | Relations | Total | Conflicts | |
|---|---|---|---|---|---|---|
| Hard reuse | ||||||
| 1 | BFO | Upper ontology | 35 | 5 | 40 | 0 |
| 2 | OGMS | Upper domain ontology | 149 | 0 | 149 | 0 |
| Soft reuse | ||||||
| 1 | SYMP | Symptoms | 57 | 0 | 57 | 22 |
| 2 | NCBITAXON | Virus and bacteria | 184 | 0 | 184 | 0 |
| 3 | RO | Relations | 0 | 22 | 22 | 0 |
| 4 | CPRO | Roles | 4 | 0 | 4 | 2 |
| 5 | LOINC | Laboratory | 26 | 0 | 26 | 1 |
| 6 | SNOMED-CT | Diseases, symptoms, and clinical signs | 796 | 0 | 796 | 115 |
| 7 | RADLEX | Imaging | 55 | 0 | 55 | 5 |
| 8 | DOID | Disease | 35 | 0 | 35 | 13 |
| 9 | HPO | Phenotype | 96 | 0 | 96 | 73 |
| 10 | IDO | Pathogens | 11 | 0 | 11 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzi, S. A Methodology for Building a Medical Ontology with a Limited Domain Experts’ Involvement. Digital 2025, 5, 18. https://doi.org/10.3390/digital5020018
Azzi S. A Methodology for Building a Medical Ontology with a Limited Domain Experts’ Involvement. Digital. 2025; 5(2):18. https://doi.org/10.3390/digital5020018
Chicago/Turabian StyleAzzi, Sabrina. 2025. "A Methodology for Building a Medical Ontology with a Limited Domain Experts’ Involvement" Digital 5, no. 2: 18. https://doi.org/10.3390/digital5020018
APA StyleAzzi, S. (2025). A Methodology for Building a Medical Ontology with a Limited Domain Experts’ Involvement. Digital, 5(2), 18. https://doi.org/10.3390/digital5020018





