Polyoxometalates’ Progress for the Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. Alzheimer’s Disease
3. Polyoxometalates
4. Polyoxometalate Studies in Alzheimer’s Disease
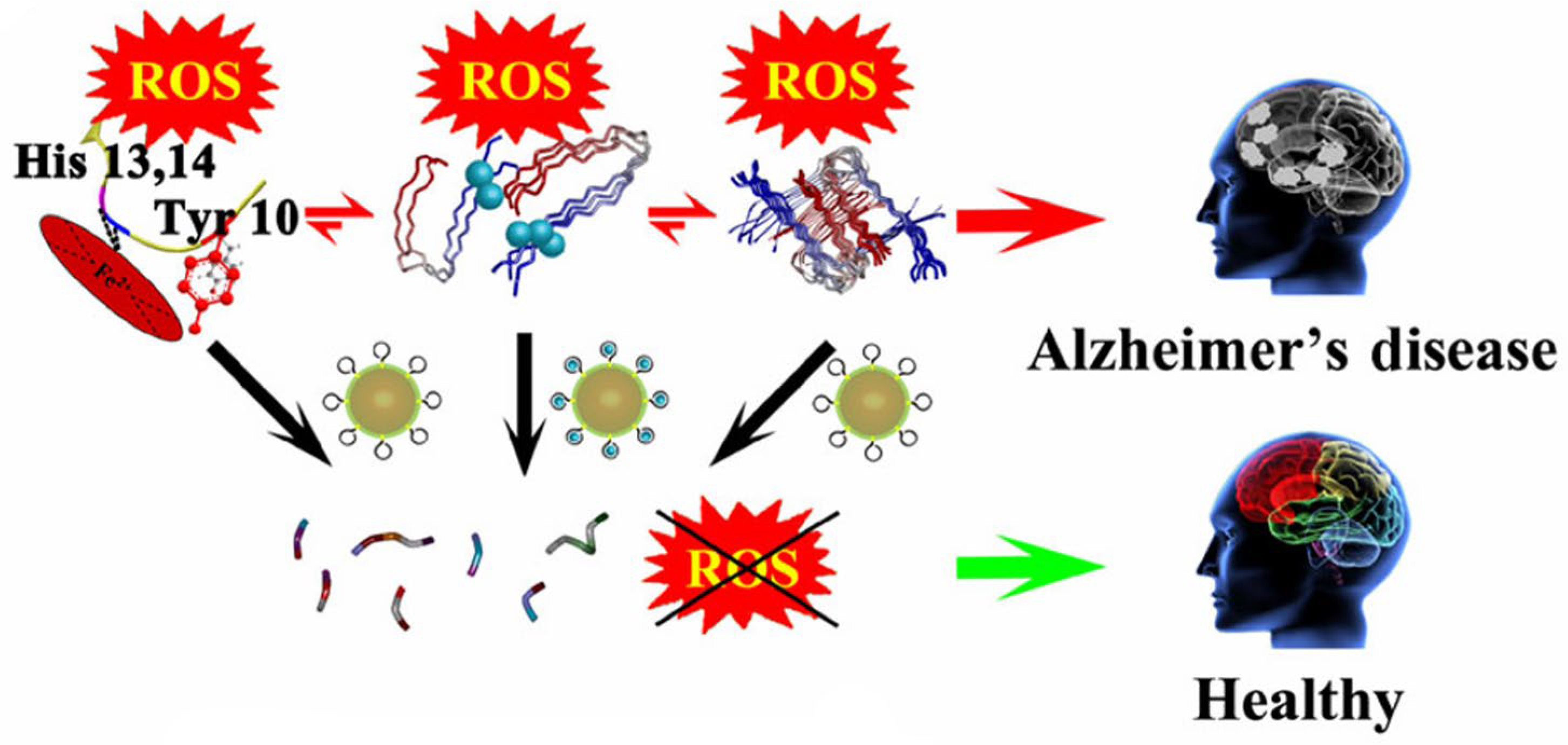
4.1. Pure Polyoxometalate Studies in Alzheimer’s Disease
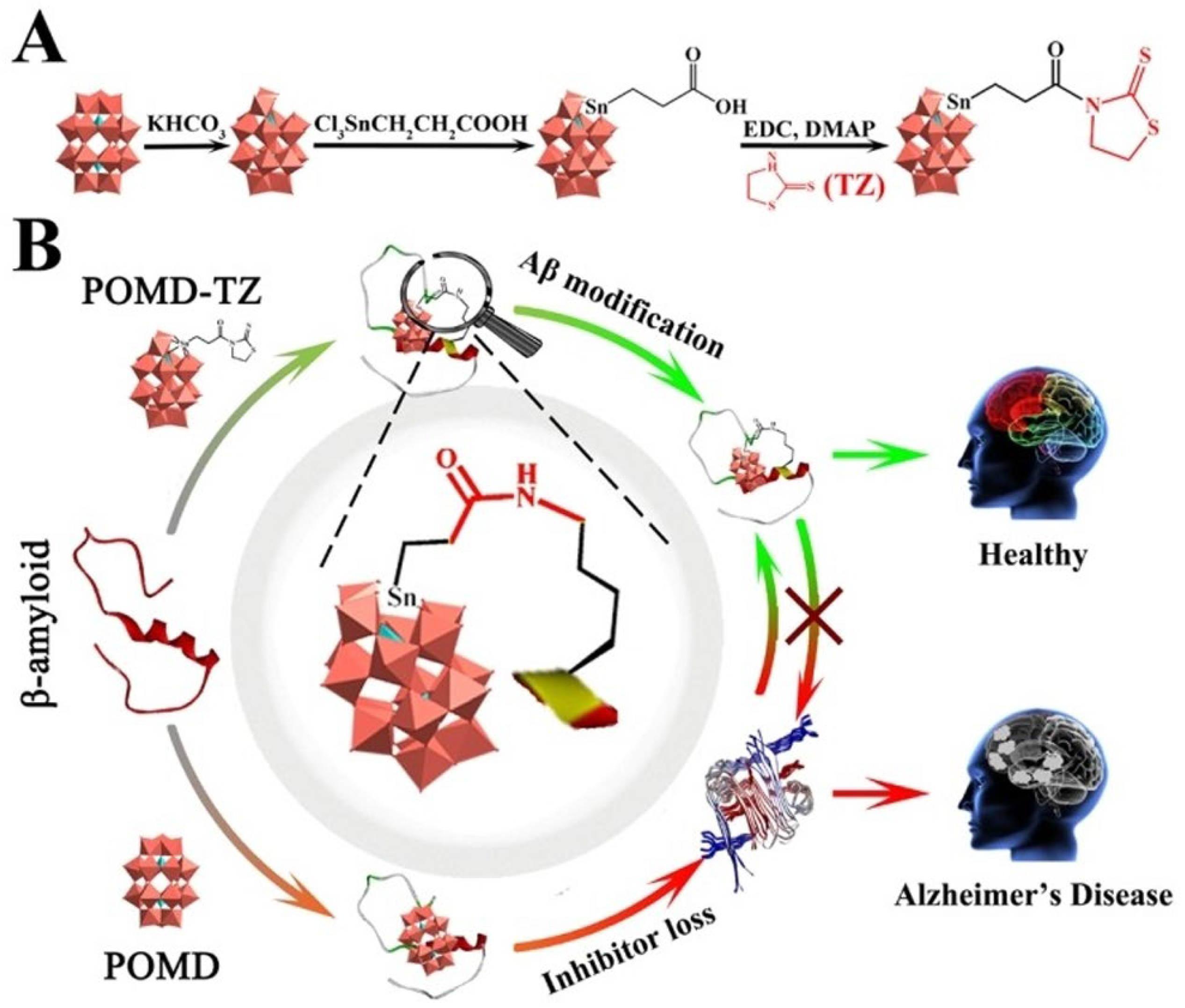
4.2. Hybrid Polyoxometalate Studies in Alzheimer’s Disease
4.3. Nanoparticle-Based Polyoxometalate Studies in Alzheimer’s Disease
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AChE | Achetylcolinesterase |
| AFM | Atomic force microscopy |
| APP | Amyloid precursor protein |
| AuNRs | Gold nanorods |
| Aβ | β-amyloid |
| BBB | Blood–brain barrier |
| BO | Bayesian Optimization |
| CD | Circular dicroism |
| CNS | Central nervous system |
| DFT | Density functional theory |
| DLS | Dynamic light scattering |
| IC50 | Half maximal inhibitory concentration |
| IL6 | Interleukin-6 |
| ITC | Isothermal titration calorimetry |
| LPO | Lipid peroxidation |
| NFT | Neurofibrillary tangles |
| NIR | Near-infrared radiation |
| NMDA | N-methyl-D-aspartate |
| NPs | Nanoparticles |
| Nrf2 | Transcription factor |
| P2 | Purinergic P2 receptors |
| PBS | Phosphate-buffered solution |
| PC12 | Cell line from rat pheochromocytoma |
| PEG | Polyethylene glycol |
| POMo | Polyoxmolybdates |
| POMs | Polyoxometalates |
| POVs | Polyoxovanadates |
| PS1 | Presenilin-1 |
| PS2 | Presenilin-2 |
| PTM | Post-translational modification |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TNF | Tumor necrosis factor |
| TZ | Thiazolidinethione |
| WHO | World Health Organization |
References
- Morris, J.C. Neurodegenerative Disorders of Aging: The Down Side of Rising Longevity. Mo Med. 2013, 110, 393–3944. [Google Scholar]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Heemels, M.T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K. Cellular pathology of neurodegenerative disorders. Clin. Neurol. 2013, 53, 609–617. [Google Scholar][Green Version]
- Davis, A.A.; Leyns, C.E.G.; Holtzman, D.M. Intercellular Spread of Protein Aggregates in Neurodegenerative Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 545–568. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376. [Google Scholar]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Frozza, R.L.; Lourenco, M.V.; de Felice, F.G. Challenges for Alzheimer’s Disease Therapy: Insights from Novel Mechanisms Beyond Memory Defects. Front. Neurosci. 2018, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood–Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Aureliano, M.; Mitchell, S.G.; Yin, P. Editorial: Emerging polyoxometalates with biological, biomedical, and health applications. Front. Chem. 2022, 10, 977317. [Google Scholar] [CrossRef]
- Čolović, M.B.; Lacković, M.; Lalatović, J.; Mougharbel, A.S.; Kortz, U.; Krstić, D.Z. Polyoxometalates in Biomedicine: Update and Overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Budych, M.J.; Staszak, K.; Bajek, A.; Pniewski, F.; Jastrząb, R.; Staszak, M.; Tylkowski, B.; Wieszczycka, K. The future of polyoxymetalates for biological and chemical apllications. Coord. Chem. Rev. 2023, 493, 215306. [Google Scholar] [CrossRef]
- Georges, J.; Bintener, C.; Miller, O. Dementia in Europe Yearbook 2019: Estimating the prevalence of dementia in Europe; Alzheimer Europe: Sandweiler, Luxembourg, 2020; pp. 1–108. [Google Scholar]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Igarashi, K.M. Entorhinal cortex dysfunction in Alzheimer’s disease. Trends Neurosci. 2023, 46, 124. [Google Scholar] [CrossRef]
- Hippius, H.; Neundörfer, G. The discovery of Alzheimer’s disease. Dialogues Clin. Neurosci. 2003, 5, 101–108. [Google Scholar] [CrossRef]
- Madnani, R.S. Alzheimer’s disease: A mini-review for the clinician. Front. Neurol. 2023, 14, 1178588. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; Loures, C.d.M.G.; Alves, L.C.V.; De Souza, L.C.; Borges, K.B.; Carvalho, M.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Coronel, R.; Bernabeu-Zornoza, A.; Palmer, C.; Muñiz-Moreno, M.; Zambrano, A.; Cano, E.; Liste, I. Role of Amyloid Precursor Protein (APP) and Its Derivatives in the Biology and Cell Fate Specification of Neural Stem Cells. Mol. Neurobiol. 2018, 55, 7107–7117. [Google Scholar] [CrossRef]
- Griffiths, J.; Grant, S.G.N. Synapse pathology in Alzheimer’s disease. Semin. Cell. Dev. Biol. 2023, 139, 13–23. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Joshi, S.; Khambete, M.; Degani, M. Role of calcium dysregulation in Alzheimer’s disease and its therapeutic implications. Chem. Biol. Drug Des. 2023, 101, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Tatulian, S.A. Challenges and hopes for Alzheimer’s disease. Drug Discov. Today 2022, 27, 1027–1043. [Google Scholar] [CrossRef]
- Pardridge, W.M. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimer’s Dement. 2009, 5, 427. [Google Scholar] [CrossRef]
- Gao, Y.; Choudhari, M.; Such, G.K.; Ritchie, C. Polyoxometalates as chemically and structurally versatile components in self-assembled materials. Chem. Sci. 2022, 13, 2510–2527. [Google Scholar] [CrossRef]
- Hill, C.L. Introduction: Polyoxometalates Multicomponent Molecular Vehicles To Probe Fundamental Issues and Practical Problems. Chem. Rev. 1998, 98, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gumerova, N.I.; Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018, 2, 112. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Li, S.; Zhang, L.; Sun, M. A review of application and prospect for polyoxometalate-based composites in electrochemical sensor. Inorg. Chem. Commun. 2022, 135, 109084. [Google Scholar] [CrossRef]
- Liu, R.; Streb, C. Polyoxometalate-Single Atom Catalysts (POM-SACs) in Energy Research and Catalysis. Adv. Energy Mater. 2021, 11, 2101120. [Google Scholar] [CrossRef]
- Samaraj, E.; Balaraman, E.; Manickam, S. Functional POM-catalyst for selective oxidative dehydrogenative couplings under aerobic conditions. Mol. Catal. 2021, 502, 111396. [Google Scholar] [CrossRef]
- Asif, H.M.; Shakoor, Z.; Ibraheem, S.; Salama, A.M.; Khan, M.A.; Nguyen, T.A.; Yasin, G. Chapter 12—Polyoxometalate-based metal organic frameworks (POMOFs) for lithium-ion batteries. In Micro and Nano Technologies; Metal-Organic Framework-Based Nanomaterials for Energy Conversion and Storage; Gupta, R.K., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 245–268. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Guo, H.; Guo, Y.; Song, R. Recent advances in polyoxometalate-based materials and their derivatives for electrocatalysis and energy storage. Mater. Chem. Front. 2024, 8, 732–768. [Google Scholar] [CrossRef]
- Wang, X.; Wei, S.; Zhao, C.; Li, X.; Jin, J.; Shi, X.; Su, Z.; Li, J.; Wang, J. Promising application of polyoxometalates in the treatment of cancer, infectious diseases and Alzheimer’s disease. J. Biol. Inorg. Chem. 2022, 27, 405–419. [Google Scholar] [CrossRef]
- Li, B.; Xu, X.; Lv, Y.; Wu, Z.; He, L.; Song, Y.F. Polyoxometalates as Potential Artificial Enzymes toward Biological Applications. Small 2024, 20, e2305539. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with Emerging Biomedical Activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Fraqueza, G.; Aureliano, M. Repurposing Therapeutic Drugs Complexed to Vanadium in Cancer. Pharmaceuticals 2024, 17, 12. [Google Scholar] [CrossRef]
- Gonzalez-Cano, S.I.; Flores, G.; Guevara, J.; Morales-Medina, J.C.; Treviño, S.; Diaz, A. Polyoxidovanadates a new therapeutic alternative for neurodegenerative and aging diseases. Neural Regen. Res. 2024, 19, 571–577. [Google Scholar] [CrossRef]
- Aureliano, M.; Fraqueza, G.; Berrocal, M.; Cordoba-Granados, J.J.; Gumerova, N.I.; Rompel, A.; Gutierrez-Merino, C.; Mata, A.M. Inhibition of SERCA and PMCA Ca2+-ATPase Activities by Polyoxotungstates. J. Inorg. Biochem. 2022, 236, 111952. [Google Scholar] [CrossRef] [PubMed]
- Poejo, J.; Gumerova, N.I.; Rompel, A.; Mata, A.M.; Aureliano, M.; Gutierrez-Merino, C. Unveiling the Agonistic Properties of Preyssler-Type Polyoxotungstates on Purinergic P2 Receptors. J. Inorg. Biochem. 2024, 259, 112640. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, M.; Ren, J.; Wang, E.; Qu, X. Polyoxometalates as Inhibitors of the Aggregation of Amyloid β Peptides Associated with Alzheimer’s Disease. Angew. Chem. Int. Ed. 2011, 50, 4184–4188. [Google Scholar] [CrossRef]
- Li, M.; Xu, C.; Wu, L.; Ren, J.; Wang, E.; Qu, X. Self-assembled peptide-polyoxometalate hybrid nanospheres: Two in one enhances targeted inhibition of amyloid B-peptide aggregation associated with Alzheimer’s disease. Small 2013, 9, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Duan, T.; Xu, C.; Qu, X. Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for Alzheimer’s disease. Nat Commun. 2014, 5, 3422. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef]
- Li, M.; Guan, Y.; Zhao, A.; Ren, J.; Qu, X. Using Multifunctional Peptide Conjugated Au Nanorods for Monitoring β-amyloid Aggregation and Chemo-Photothermal Treatment of Alzheimer’s Disease. Theranostics 2017, 7, 2996–3006. [Google Scholar] [CrossRef]
- Ma, X.; Hua, J.; Wang, K.; Zhang, H.; Zhang, C.; He, Y.; Guo, Z.; Wang, X. Modulating Conformation of Aβ-Peptide: An Effective Way to Prevent Protein-Misfolding Disease. Inorg. Chem. 2018, 57, 13533–13543. [Google Scholar] [CrossRef]
- Gao, N.; Du, Z.; Guan, Y.; Dong, K.; Ren, J.; Qu, X. Chirality-Selected Chemical Modulation of Amyloid Aggregation. J. Am. Chem. Soc. 2019, 141, 6915–6921. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.; Wan, K.; Sun, T.; Zheng, N.; Zhu, F.; Ma, J.; Jiao, J.; Li, T.; Ni, J.; et al. Organoplatinum-Substituted Polyoxometalate Inhibits β-amyloid Aggregation for Alzheimer’s Therapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 18032–18039. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Lazarević-Pašti, T.D.; Leskovac, A.R.; Petrović, S.Ž.; Čolović, M.B.; Parac-Vogt, T.N.; Janjić, G.V. A new acetylcholinesterase allosteric site responsible for binding voluminous negatively charged molecules—The role in the mechanism of AChE inhibition. Eur. J. Pharm. Sci. 2020, 151, 105376. [Google Scholar] [CrossRef]
- Perxés Perich, M.; Palma-Florez, S.; Solé, C.; Goberna-Ferrón, S.; Samitier, J.; Gómez-Romero, P.; Mir, M.; Lagunas, A. Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a µphysiological Model. Nanomaterials 2023, 13, 2697. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, L.; Han, F.; Zhang, G.; Ma, Y.; Yao, J.; Keita, B.; Oliveira, P.; Nadjo, L. Inhibition of amyloid-β protein fibrillization upon interaction with polyoxometalates nanoclusters, Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 97–101. [Google Scholar] [CrossRef]
- Li, M.; Xu, C.; Ren, J.; Wang, E.; Qu, X. Photodegradation of β-sheet amyloid fibrils associated with Alzheimer’s disease by using polyoxometalates as photocatalysts. Chem. Commun. 2013, 49, 11394–11396. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Barsukova-Stuckart, M.; Ibrahim, M.; Ali, S.U.; Khan, A.A.; Kortz, U. Polyoxometalates as potent inhibitors for acetyl and butyrylcholinesterases and as potential drugs for the treatment of Alzheimer’s disease. Med. Chem. Res. 2013, 22, 1224–1228. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, L.; Zheng, C.; Zheng, W.; Zhang, J.; Zhou, Y.; Liu, J. Mo polyoxometalate nanoclusters capable of inhibiting the aggregation of Aβ-peptide associated with Alzheimer’s disease. Nanoscale 2014, 6, 6886–6897. [Google Scholar] [CrossRef]
- Čolović, M.B.; Medić, B.; Ćetković, M.; Kravić Stevović, T.; Stojanović, M.; Ayass, W.W.; Mougharbel, A.S.; Radenković, M.; Prostran, M.; Kortz, U.; et al. Toxicity evaluation of two polyoxotungstates with anti-acetylcholinesterase activity. Toxicol. Appl. Pharmacol. 2017, 333, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, D.; Ehlerding, E.B.; Rosenkrans, Z.T.; Engle, J.W.; Wang, Y.; Liu, H.; Ni, D.; Cai, W. Intrathecal Administration of Nanoclusters for Protecting Neurons against Oxidative Stress in Cerebral Ischemia/Reperfusion Injury. ACS Nano. 2019, 13, 13382–13389. [Google Scholar] [CrossRef]
- Aureliano, M.; De Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ Interactions with Proteins: An Overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in Solution: Speciation under Spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Speciation atlas of polyoxometalates in aqueous solutions. Sci Adv. 2023, 9, eadi0814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaudhary, H.; Iashchishyn, I.A.; Romanova, N.V.; Rambaran, M.A.; Musteikyte, G.; Smirnovas, V.; Holmboe, M.; Ohlin, C.A.; Svedružić, Z.M.; Morozova-Roche, L.A. Polyoxometalates as effective nano-inhibitors of amyloid aggregation of pro-inflammatory S100A9 protein involved in neurodegenerative diseases. ACS Appl. Mater. Interfaces 2021, 13, 26721–26734. [Google Scholar] [CrossRef]
- Sciortino, G.; Aureliano, M.; Garribba, E. Rationalizing the Decavanadate(V) and Oxidovanadium(IV) Binding to G-Actin and the Competition with Decaniobate(V) and ATP. Inorg. Chem. 2021, 60, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Atrian-Blasco, E.; de Cremoux, L.; Lin, X.; Mitchell-Heggs, R.; Sabater, L.; Blanchard, S.; Hureau, C. Keggin-type polyoxometalates as Cu(II) chelators in the context of Alzheimer’s disease. Chem. Commun. 2022, 58, 2367–2370. [Google Scholar] [CrossRef]
- Wang, M.; Hua, J.; Zheng, P.; Tian, Y.; Kang, S.; Chen, J.; Duan, Y.; Ma, X. A Nanoscale Cobalt Functionalized Strandberg-Type Phosphomolybdate with b-Sheet Conformation Modulation Ability in Anti-Amyloid Protein Misfolding. Inorganics 2023, 11, 442. [Google Scholar] [CrossRef]
- Lei, S.; Yang, H.; Li, J.X.; Li, Y.; Wang, L.; Chen, B.N.; Li, J. A study of the antioxidant properties of Keggin-type polyoxometalates. Dalton Trans. 2023, 52, 9673–9683. [Google Scholar] [CrossRef]
- Ben Zaken, K.; Bouhnik, R.; Omer, N.; Bloch, N.; Samson, A.O. Polyoxometalates bind multiple targets involved in Alzheimer’s disease. J. Biol. Inorg. Chem. 2025, 30, 299–309. [Google Scholar] [CrossRef]
- Ramesh, M.; Balachandra, C.; Andhare, P.; Govindaraju, T. Rationally Designed Molecules Synergistically Modulate Multifaceted Aβ Toxicity, Microglial Activation, and Neuroinflammation. ACS Chem. Neurosci. 2022, 13, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Z.; Zhao, H.; Zhang, H.; Ren, J.; Qu, X. Polyoxometalates: Metallodrug agents for combating amyloid aggregation. Natl. Sci. Rev. 2024, 11, nwae226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bittner, N.; Funk, C.S.M.; Schmidt, A.; Bermpohl, F.; Brandl, E.J.; Algharably, E.E.A.; Kreutz, R.; Riemer, T.G. Psychiatric Adverse Events of Acetylcholinesterase Inhibitors in Alzheimer’s Disease and Parkinson’s Dementia: Systematic Review and Meta-Analysis. Drugs Aging 2023, 40, 953–964. [Google Scholar] [CrossRef]
- Kondinski, A.; Gumerova, N.I.; Rompel, A.; Falcaro, P.; Schreck, T. Data-Driven Polyoxometalate Chemistry. Chem. Eur. J. 2025, 31, e01528. [Google Scholar] [CrossRef] [PubMed]
- Hardman, T.C.; Aitchison, R.; Scaife, R.; Edwards, J.; Slater, G. The future of clinical trials and drug development: 2050. Drugs Context. 2023, 12, 2023-2-2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hua, J.A.; Wei, X.M.; Ma, X.; Jiao, J.Z.; Chai, B.H.; Wu, C.B.; Zhang, C.L.; Niu, Y.L. A {Cd4Cl2O14} cluster functionalized sandwich-type tungstoarsenate as a conformation modulator for misfolding Aβ peptides. Cryst. Eng. Comm. 2022, 24, 1171–1176. [Google Scholar] [CrossRef]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef]
- Gao, N.; Liu, Z.; Zhang, H.; Liu, C.; Yu, D.; Ren, J.; Qu, X. Site-Directed Chemical Modification of Amyloid by Polyoxometalates for Inhibition of Protein Misfolding and Aggregation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115336. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ning, X.; Lv, J.; Wei, Y.; Zhang, H.; Li, M. Enantioselective modulation of amyloid burden and memory deficits by chiralpolyoxometalates for Alzheimer’s disease treatment. Inorg. Chem. Front. 2023, 10, 5347–5356. [Google Scholar] [CrossRef]
- Diaz, A.; Muñoz-Arenas, G.; Venegas, B.; Vázquez-Roque, R.; Flores, G.; Guevara, J.; Gonzalez-Vergara, E.; Treviño, S. Metforminium Decavanadate (MetfDeca) Treatment Ameliorates Hippocampal Neurodegeneration and Recognition Memory in a Metabolic Syndrome Model. Neurochem. Res. 2021, 46, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Copple, I.M. The Keap1-Nrf2 Cell Defense Pathway—A Promising Therapeutic Target? Adv. Pharmacol. 2012, 63, 43–79. [Google Scholar]
- la Torre, A.; Lo Vecchio, F.; Angelillis, V.S.; Gravina, C.; D’Onofrio, G.; Greco, A. Reinforcing Nrf2 Signaling: Help in the Alzheimer’s Disease Context. Int. J. Mol. Sci. 2025, 26, 1130. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Zhao, Z.; Zhang, L.; Su, H.; Zhao, Y.; Sun, Y.; Li, C.; Wang, Y.; Hua, J. An interesting nano-linear nickel-modified Strandberg-type phosphomolybdate acting as a conformation modulator anti-misfolding peptide. J. Mol. Struct. 2025, 1329, 141498. [Google Scholar] [CrossRef]
- Fang, L.; Peng, R.; Xia, L.; Zhuang, G.-L. Active Learning-Assisted Exploration of [PO40Mo12]3− for Alzheimer’s Therapy Insights. Adv. Sci. 2025, 12, e08702. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-Nanoparticle-Based Multifunctional Amyloid-β Inhibitor against Alzheimer’s Disease. Chem. A Eur. J. 2015, 21, 829–835. [Google Scholar] [CrossRef]
- Gao, N.; Dong, K.; Zhao, A.; Sun, H.; Wang, Y.; Ren, J.; Qu, X. Polyoxometalate-based nanozyme: Design of a multifunctional enzyme for multi-faceted treatment of Alzheimer’s disease. Nano Res. 2016, 9, 1079–1090. [Google Scholar] [CrossRef]
- Ma, M.; Gao, N.; Sun, Y.; Du, X.; Ren, J.; Qu, X. Redox-Activated Near-Infrared-Responsive Polyoxometalates Used for Photothermal Treatment of Alzheimer’s Disease. Adv. Healthc. Mater. 2018, 7, e1800320. [Google Scholar] [CrossRef]
- Gao, L.; Wang, J.; Bi, Y. Nanotechnology for Neurodegenerative Diseases: Recent Progress in Brain-Targeted Delivery, Stimuli-Responsive Platforms, and Organelle-Specific Therapeutics. Int. J. Nanomed. 2025, 20, 11015–11044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.; He, H.; Du, B.; He, Y. Nanoscale drug formulations for the treatment of Alzheimer’s disease progression. RSC Adv. 2025, 15, 4031–4078. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, J.; Gong, Y.; Zhou, H.; Chen, X.; Zhu, X.; Zhao, Y.; Wen, Y.; Qin, X.; Liu, J. Peptide-modified Mo polyoxometalate nanoparticles suppress Zn2+-induced Aβ aggregation. ChemNanoMat 2019, 5, 897–910. [Google Scholar] [CrossRef]
- Díaz, A.; Vázquez-Roque, R.; Carreto-Meneses, K.; Moroni-González, D.; Moreno-Rodríguez, J.A.; Treviño, S. Polyoxidovanadates as a pharmacological option against brain aging. Polyoxidovanadates as a pharmacological option against brain aging. J. Chem. Neuroanat. 2023, 129, 102256. [Google Scholar] [CrossRef]
- Huang, Y.; Chang, Y.; Liu, L.; Wang, J. Nanomaterials for Modulating the Aggregation of β-Amyloid Peptides. Molecules 2021, 26, 4301. [Google Scholar] [CrossRef]
- Lo, C.H.; Cheong, L.Y.T.; Zeng, J. Nanoplatforms Targeting Intrinsically Disordered Protein Aggregation for Translational Neuroscience Applications. Nanomaterials 2025, 15, 704. [Google Scholar] [CrossRef] [PubMed]
- Baracaldo-Santamaría, D.; Avendaño-Lopez, S.S.; Ariza-Salamanca, D.F.; Rodriguez-Giraldo, M.; Calderon-Ospina, C.A.; González-Reyes, R.E.; Nava-Mesa, M.O. Role of Calcium Modulation in the Pathophysiology and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 9067. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Kumari, S.; Dhapola, R.; Sharma, P.; Paidlewar, M.; Vellingiri, B.; Medhi, B.; HariKrishnaReddy, D. Calcium dysregulation in Alzheimer’s disease: Unraveling the molecular nexus of neuronal dysfunction and therapeutic opportunities. Biochem Pharmacol. 2025, 242 Pt 2, 117211. [Google Scholar] [CrossRef]
- Alzheimer’s Association Calcium Hypothesis Workgroup. Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimer’s Dement. 2017, 13, 178–182.e17. [Google Scholar] [CrossRef]
- Popugaeva, E.; Bezprozvanny, I.; Chernyuk, D. Reversal of calcium dysregulation as potential approach for treating Alzheimer’s disease. Curr. Alzheimer Res. 2020, 17, 344. [Google Scholar] [CrossRef]
- Chen, J.; Yang, W.-Z.; Chen, H.; Ding, X.; Chen, H.; Zhan, C.-H.; Jin, Z. Targeting protein aggregation: The promising application of polyoxometalates in neurodegenerative diseases. Inorg. Chem. Front. 2024, 11, 7238–7255. [Google Scholar] [CrossRef]
- Maret, W. The quintessence of metallomics: A harbinger of a different life science based on the periodic table of the bioelements. Metallomics 2022, 14, mfac051. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Ma, B. Feature Papers in BioChem. BioChem 2025, 5, 17. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aureliano, M.; Mateus, J.; Rijo, D.M. Polyoxometalates’ Progress for the Treatment of Alzheimer’s Disease. BioChem 2025, 5, 41. https://doi.org/10.3390/biochem5040041
Aureliano M, Mateus J, Rijo DM. Polyoxometalates’ Progress for the Treatment of Alzheimer’s Disease. BioChem. 2025; 5(4):41. https://doi.org/10.3390/biochem5040041
Chicago/Turabian StyleAureliano, Manuel, João Mateus, and David Manjua Rijo. 2025. "Polyoxometalates’ Progress for the Treatment of Alzheimer’s Disease" BioChem 5, no. 4: 41. https://doi.org/10.3390/biochem5040041
APA StyleAureliano, M., Mateus, J., & Rijo, D. M. (2025). Polyoxometalates’ Progress for the Treatment of Alzheimer’s Disease. BioChem, 5(4), 41. https://doi.org/10.3390/biochem5040041








