An Opinion on the Supplementation of Folic Acid 1 mg + Iron (Ferrous Sulfate) 90 mg in the Prevention and Treatment of Anemia
Abstract
1. Introduction
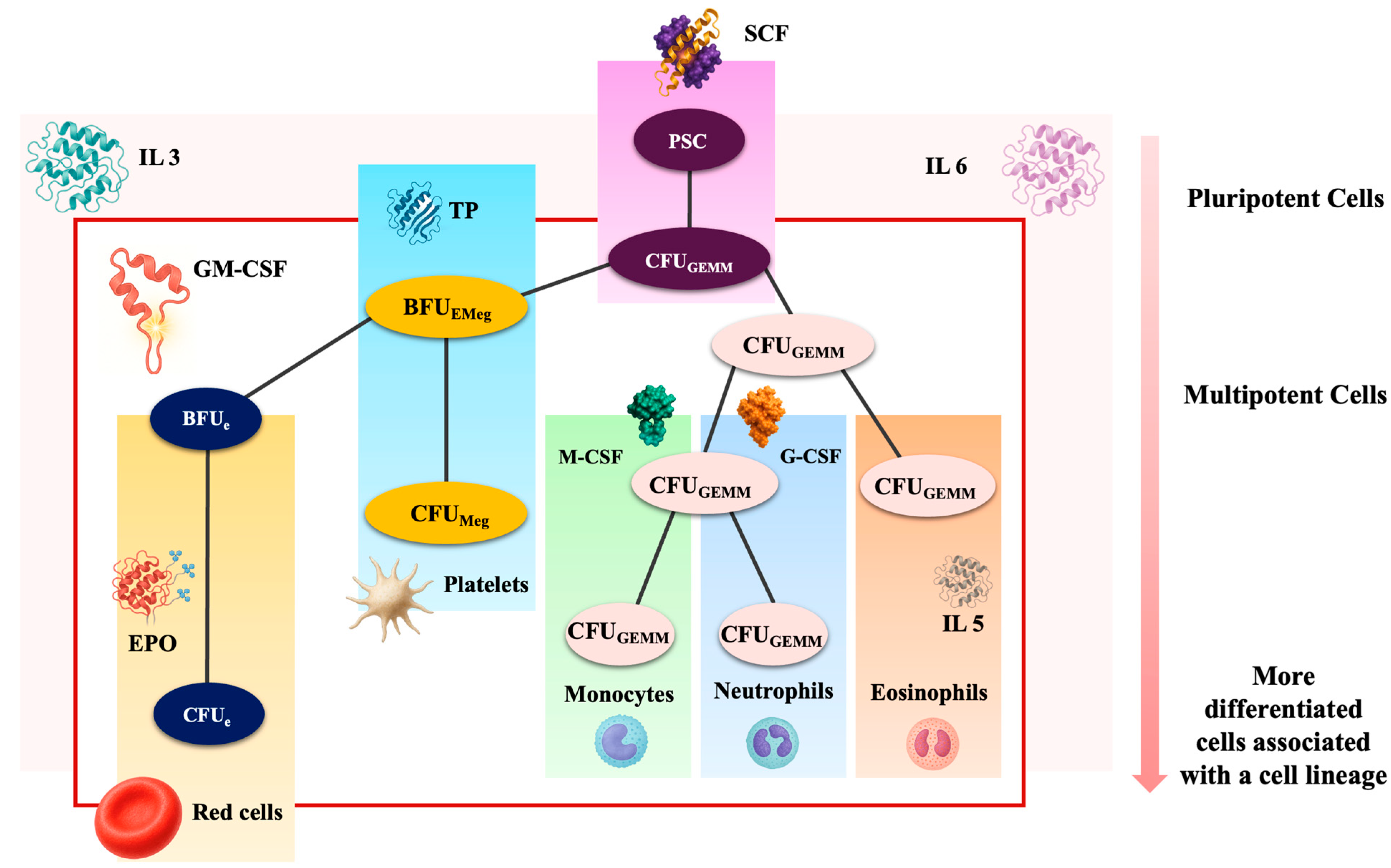
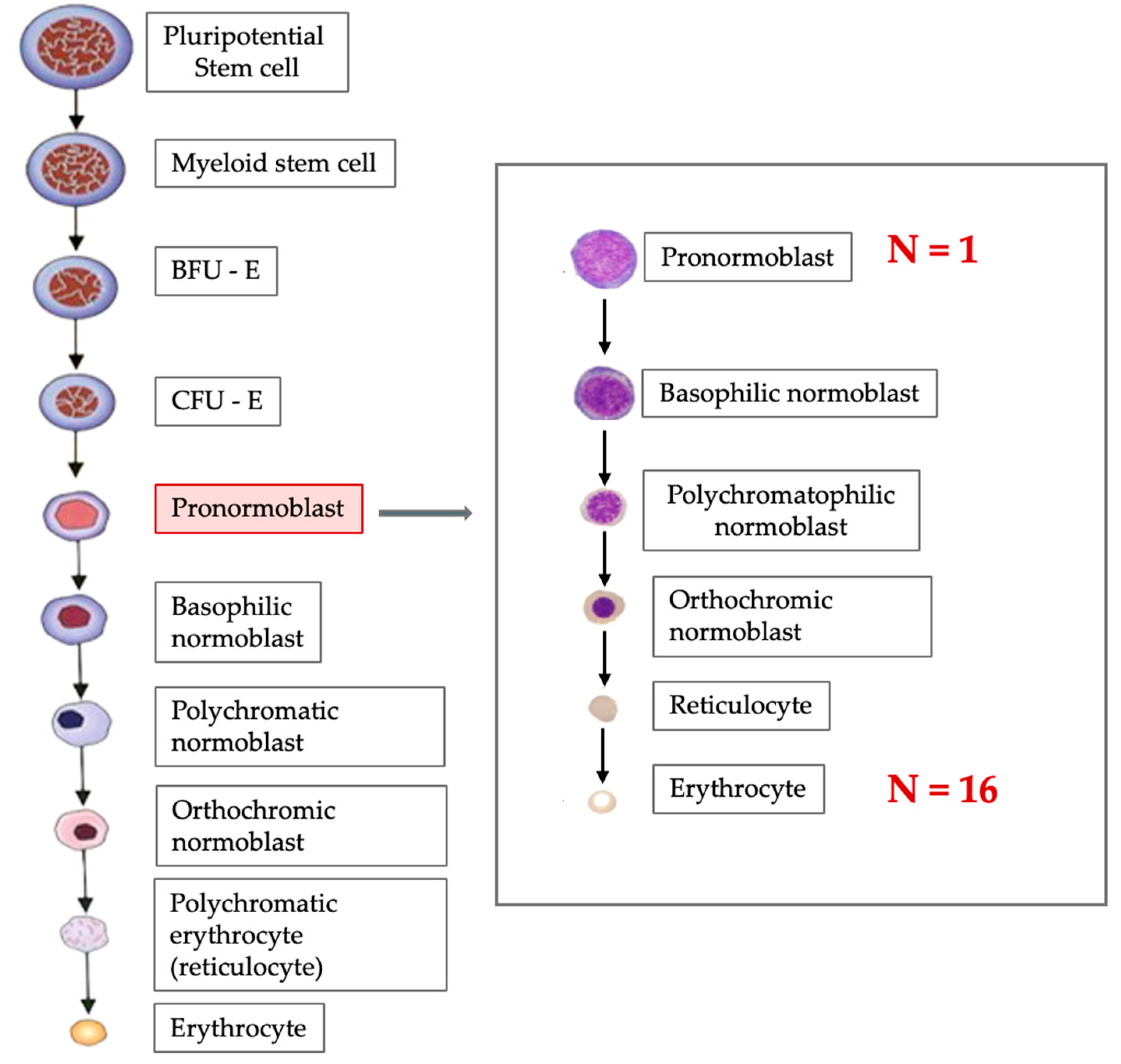
2. Erythropoiesis
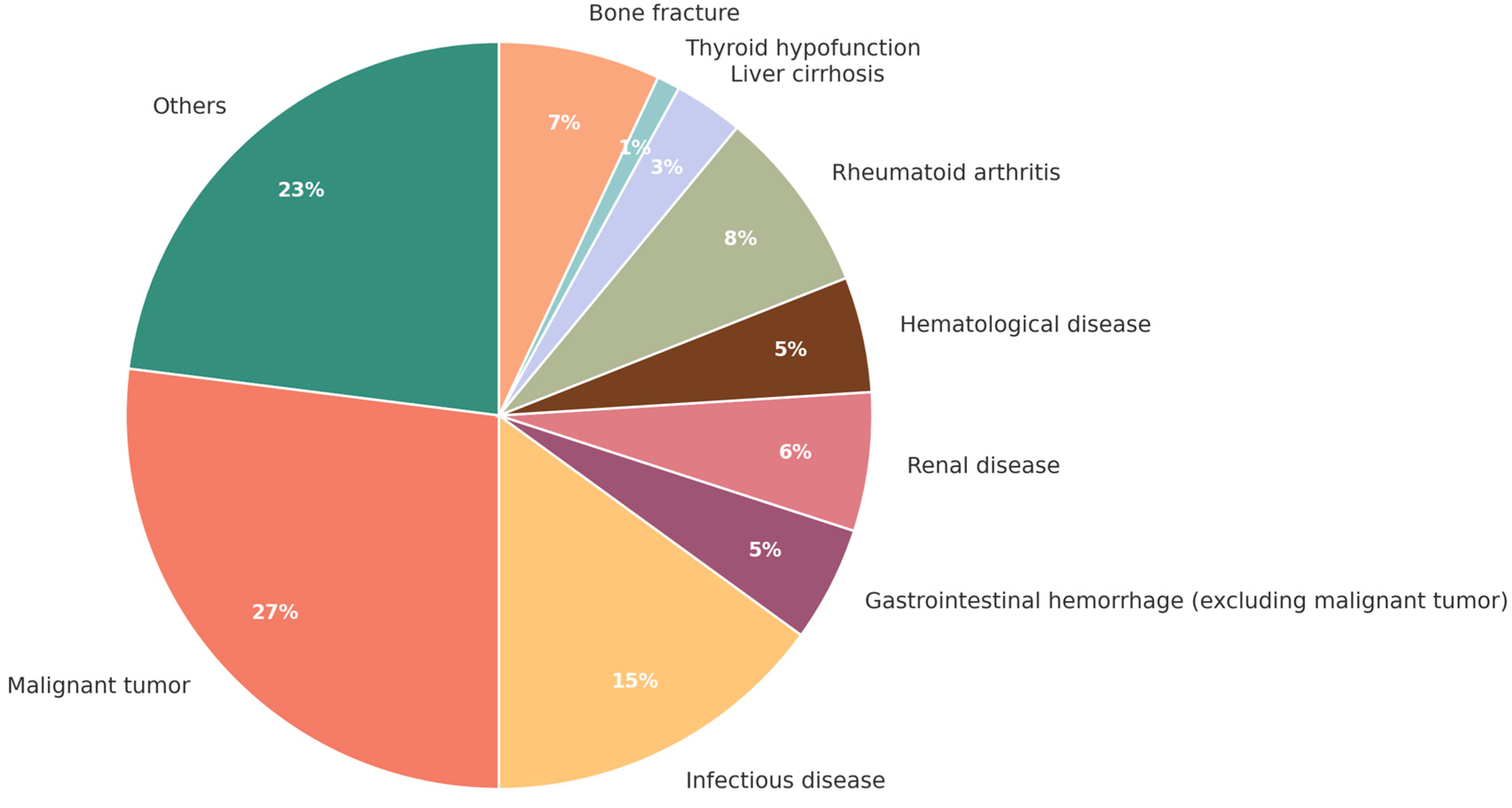
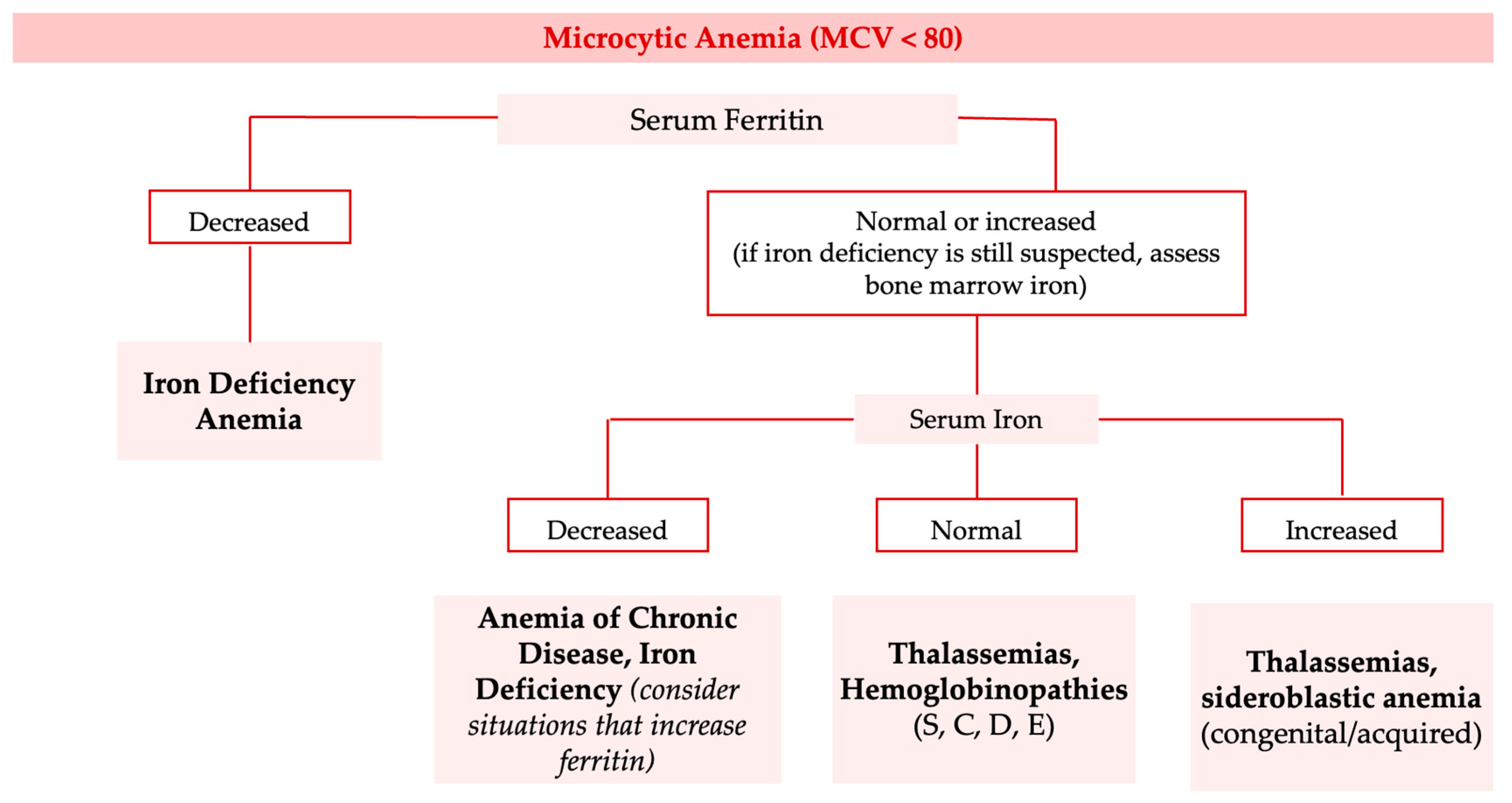
3. Anemias—Identification and Classification
4. Prevention of Anemia
5. Treatment of Anemia
- A fixed combination of folic acid 0.35 mg + ferrous sulfate 247.25 mg, approved for the prophylaxis and treatment of iron and folic acid deficiencies during pregnancy, postpartum, and prolonged lactation, or in cases where iron and folic acid were not regularly taken during lactation;
- A fixed combination of folic acid 0.35 mg + ferrous sulfate 325 mg, approved for the prophylaxis and treatment of anemia during pregnancy, for treating iron deficiency, and for preventing concurrent folic acid deficiency in adults. The safety profile of folic acid is well established, with no significant toxicity reported. Concerns raised in the past regarding potential harmful (e.g., carcinogenic) effects of high folic acid concentrations after supplementation have not been confirmed. A large meta-analysis involving 49,621 participants found no such risk [48]. On the contrary, prenatal folic acid supplementation has shown protective effects not only against neural tube defects but also against various pediatric cancers, reducing the incidence of leukemia, brain tumors, and neuroblastoma [49].
6. Limitations and Future Perspectives
7. Suggestions and Recommendations Based on Clinical Studies/Experiences
8. Recommendation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Garcia-Casal, M.N.; Dary, O.; Jefferds, M.E.; Pasricha, S. Diagnosing anemia: Challenges at the public health level. Ann. N. Y. Acad. Sci. 2023, 1524, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice—Does Hemoglobin Change with Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef]
- Kundrapu, S.; Noguez, J. Laboratory Assessment of Anemia. Adv. Clin. Chem. 2018, 83, 197–225. [Google Scholar] [CrossRef]
- De Franceschi, L.; Iolascon, A.; Taher, A.; Cappellini, M.D. Clinical management of iron deficiency anemia in adults: Systemic review on advances in diagnosis and treatment. Eur. J. Intern. Med. 2017, 42, 16–23. [Google Scholar] [CrossRef]
- Hillman, R.S.; Finch, C.A. Erythropoiesis. N. Engl. J. Med. 1971, 285, 99–101. [Google Scholar] [CrossRef]
- Chung, E.Y.; Palmer, S.C.; Saglimbene, V.M.; Craig, J.C.; Tonelli, M.; Strippoli, G.F. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: A network meta-analysis. Cochrane Database Syst. Rev. 2023, 2023, CD010590. [Google Scholar] [CrossRef]
- Zivot, A.; Lipton, J.M.; Narla, A.; Blanc, L. Erythropoiesis: Insights into pathophysiology and treatments in 2017. Mol. Med. 2018, 24, 11. [Google Scholar] [CrossRef]
- Cha, H.J. Erythropoiesis: Insights from a genomic perspective. Exp. Mol. Med. 2024, 56, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Andrès, E.; Serraj, K.; Federici, L.; Vogel, T.; Kaltenbach, G. Anemia in elderly patients: New insight into an old disorder. Geriatr. Gerontol. Int. 2012, 13, 519–527. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; Zon, L.I. Hematopoiesis. Development 2013, 140, 2463–2467. [Google Scholar] [CrossRef] [PubMed]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An Evolving Paradigm for Stem Cell Biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef]
- Orkin, S.H. Hematopoiesis: How does it happen? Curr. Opin. Cell Biol. 1995, 7, 870–877. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M.; McLaren, G.D. Iron absorption and metabolism. Curr. Opin. Gastroenterol. 2009, 25, 129–135. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.E.; Umbreit, J.N. Iron absorption and transport: An update. Am. J. Hematol. 2000, 64, 287–298. [Google Scholar] [CrossRef]
- von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B.; Stoffel, N.U. Effect of dietary factors and time of day on iron absorption from oral iron supplements in iron deficient women. Am. J. Hematol. 2023, 98, 1356–1363. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.G. Folic Acid. Crit. Rev. Clin. Lab. Sci. 2001, 38, 183–223. [Google Scholar] [CrossRef]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef]
- Jelkmann, W. Physiology and Pharmacology of Erythropoietin. Transfus. Med. Hemother. 2013, 40, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Marques, F.; Nunes, A.R.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of anaemia and iron deficiency in Portugal: The EMPIRE study. Intern. Med. J. 2016, 46, 470–478. [Google Scholar] [CrossRef]
- Robalo Nunes, A.; Fonseca, C.; Marques, F.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of anemia and iron deficiency in older Portuguese adults: An EMPIRE substudy. Geriatr. Gerontol. Int. 2017, 17, 1814–1822. [Google Scholar] [CrossRef]
- Katsumi, A.; Abe, A.; Tamura, S.; Matsushita, T. Anemia in older adults as a geriatric syndrome: A review. Geriatr. Gerontol. Int. 2021, 21, 549–554. [Google Scholar] [CrossRef]
- Stauder, R.; Thein, S.L. Anemia in the elderly: Clinical implications and new therapeutic concepts. Haematologica 2014, 99, 1127–1130. [Google Scholar] [CrossRef]
- Masatsugu, O.H.T.A. Management of Anemia in the Elderly. JMAJ 2009, 52, 219–223. [Google Scholar] [CrossRef]
- Vohra, R.; Hussain, A.; Dudyala, A.K.; Pahareeya, J.; Khan, W.; Mumtaz, W. Multi-class classification algorithms for the diagnosis of anemia in an outpatient clinical setting. PLoS ONE 2022, 17, e0269685. [Google Scholar] [CrossRef]
- Clinton, O.; Micheal, K.; Namyalo, A.K.; Mary, M.; Mike, M.; Muwanguzi, E.; Okongo, B.; Wagubi, R. Anaemia, Morphological Classification and Its Associated Risk Factors Among Lactating Mothers at Mbarara City Council Health Centre IV, Southwestern Uganda. J. Blood Med. 2022, 13, 473–481. [Google Scholar] [CrossRef]
- Iolascon, A.; Andolfo, I.; Russo, R.; Sanchez, M.; Busti, F.; Swinkels, D.; Martinez, P.A.; Bou-Fakhredin, R.; Muckenthaler, M.U.; Unal, S.; et al. Recommendations for diagnosis, treatment, and prevention of iron deficiency and iron deficiency anemia. HemaSphere 2024, 8, e108. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Intermittent Iron and Folic Acid Supplementation in Menstruating Women; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Roche, M.L.; Samson, K.L.; Green, T.J.; Karakochuk, C.D.; Martinez, H. Perspective: Weekly Iron and Folic Acid Supplementation (WIFAS): A Critical Review and Rationale for Inclusion in the Essential Medicines List to Accelerate Anemia and Neural Tube Defects Reduction. Adv. Nutr. Int. Rev. J. 2021, 12, 334–342. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Cuthbert, A.; Weeks, J.; Venkatramanan, S.; Larvie, D.Y.; De-Regil, L.M.; Garcia-Casal, M.N.; Cochrane Central Editorial Service. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2024, 2024, CD004736. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations on Antenatal Care; WHO: Geneva, Switzerland, 2016. [Google Scholar]
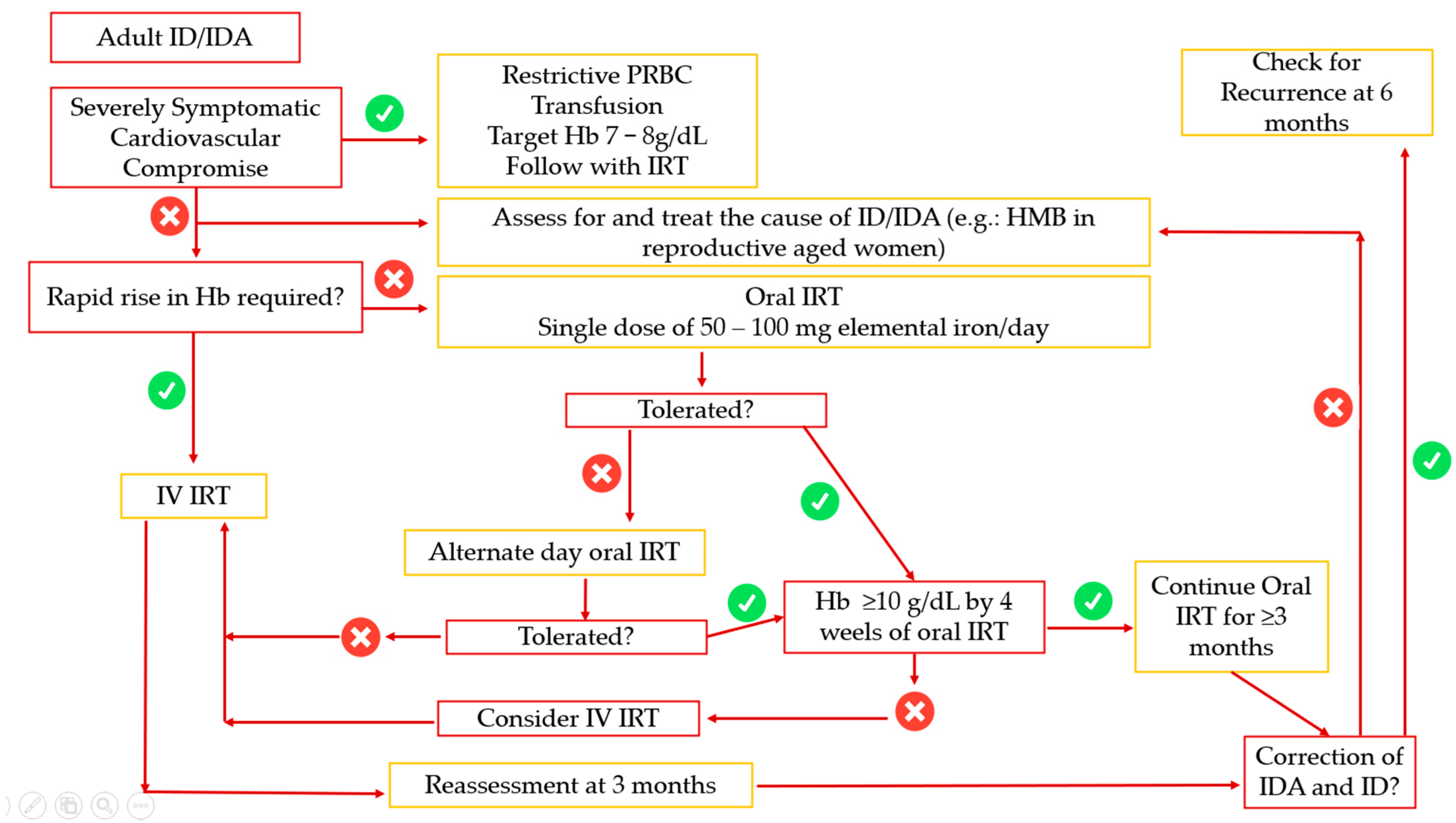
- Stoffel, N.U.; von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B. Oral iron supplementation in iron-deficient women: How much and how often? Mol. Asp. Med. 2020, 75, 100865. [Google Scholar] [CrossRef]
- Simic, S.; Karczewski, M.; Klapdor, S.; Nowak, A.; Schubert, M.; Moretti, D.; Swinkels, D.W.; Beuschlein, F.; Saleh, L.; Suter, P.; et al. Krayenbuehl PA. Effectiveness of low-dose iron treatment in non-anaemic iron-deficient women: A prospective open-label single-arm trial. Swiss Med. Wkly. 2023, 153, 40079. [Google Scholar] [CrossRef] [PubMed]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef]
- Al-Naseem, A.; Sallam, A.; Choudhury, S.; Thachil, J. Iron deficiency without anaemia: A diagnosis that matters. Clin. Med. 2021, 21, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Sánchez, A.; Aponte-Castillo, J.A.; Parra-Peña, R.I.; Ramos-Peñafiel, C.O. Dietary recommendations in deficiency anaemia. Rev. Med. Hosp. Gen. Mex. 2015, 78, 144–150. [Google Scholar]
- Lozoff, B. Iron Deficiency and Child Development. Food Nutr. Bull. 2007, 28 (Suppl. 4), S560–S571. [Google Scholar] [CrossRef]
- Pasupathy, E.; Kandasamy, R.; Thomas, K.; Basheer, A. Alternate day vs daily oral iron: A randomized trial. Sci. Rep. 2023, 13, 1818. [Google Scholar]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef]
- Pantopoulos, K. Oral iron supplementation: New formulations, old questions. Haematologica 2024, 109, 2790–2801. [Google Scholar] [CrossRef]
- DeLoughery, T.G.; Jackson, C.S.; Ko, C.W.; Rockey, D.C. AGA Update on Iron Deficiency Anemia. Clin. Gastroenterol. Hepatol. 2024, 22, 1575–1583. [Google Scholar] [CrossRef]
- Clevenger, B.; Gurusamy, K.; Klein, A.A.; Murphy, G.J.; Anker, S.D.; Richards, T. Systematic review and meta-analysis of iron therapy in anaemic adults without chronic kidney disease: Updated and abridged Cochrane review. Eur. J. Heart Fail. 2016, 18, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Vollset, S.E.; Clarke, R.; Lewington, S.; Ebbing, M.; Halsey, J.; Lonn, E.; Armitage, J.; Manson, J.E.; Hankey, G.J.; Spence, J.D.; et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: Meta-analyses of data on 50,000 individuals. Lancet 2013, 381, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J.; Ponka, P. The Roles of Folate, Vitamin B12, and Iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef] [PubMed]
- E Butterworth, C.; Tamura, T. Folic acid safety and toxicity: A brief review. Am. J. Clin. Nutr. 1989, 50, 353–358. [Google Scholar] [CrossRef]
- Parodi, E.; Giraudo, M.T.; Davitto, M.; Ansaldi, G.; Mondino, A.; Garbarini, L.; Franzil, A.; Mazzone, R.; Russo, G.; Ramenghi, U. Reticulocyte Parameters. J. Pediatr. Hematol. 2012, 34, e249–e252. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Junaid, M.A. Folic acid in pregnancy and disease. J. Biomed. Sci. 2014, 21, 77. [Google Scholar] [CrossRef]
- Bar-Oz, B.; Koren, G.; Nguyen, P.; Kapur, B.M. Folate fortification—Are we there yet? Reprod. Toxicol. 2008, 25, 408–412. [Google Scholar] [CrossRef]
| Anemia in Elderly Patients |
|---|
| Alteration of cognitive function |
| Iatrogenic injury |
| Descompasation of underlying disorders |
| Hospitalization |
| Increased mortality |
| Increased debility |
| Reduction in mobility and bone density; Increased risk of falls and fractures |
| Reduction of quality of life |
| Cause | Percentage of All Anemia Cases |
|---|---|
| Deficiency of iron | 16.6 |
| Deficiency of folate | 6.4 |
| Deficiency of vitamin B12 | 5.9 |
| Deficiency of folate and vitamin B12 | 2.0 |
| Deficiency of iron, folate and vitamin B12 | 3.4 |
| Renal insufficiency | 8.2 |
| Inflammation (chronic disease) | 19.7 |
| Renal insufficiency and inflammation | 4.3 |
| Non-Regenerative | Regenerative |
|---|---|
| Aplastic anaemia | Haemolysis |
| Pure red cell aplasia | Immune |
| Myelodysplasic syndrome | Non-immune
|
| Deficiency states | |
| Marrow infiltration/fibrosis | |
| Inflammatory anaemia | |
| Erytropoietin underproduction | Haemorrhage |
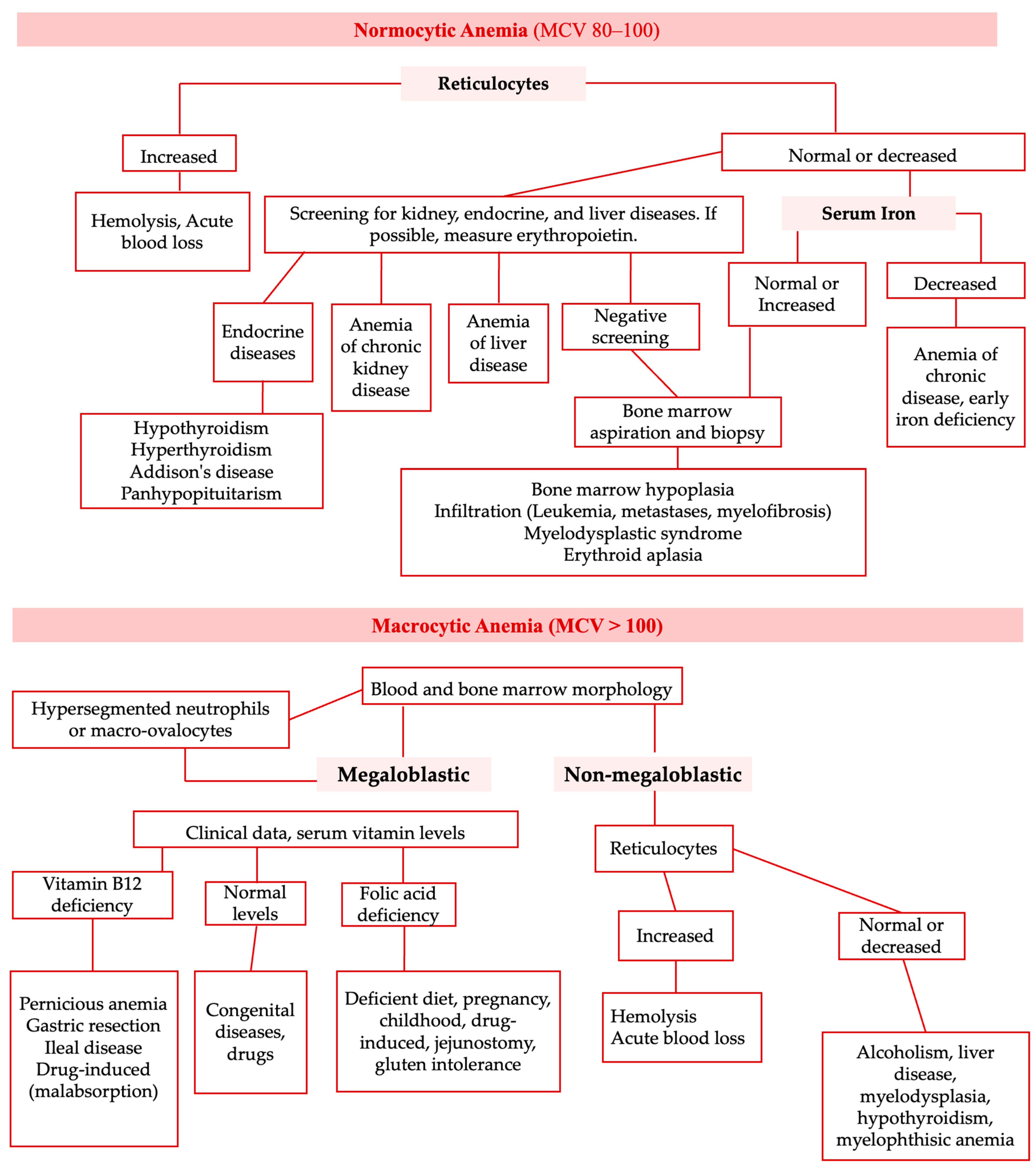
| Normocytic (MCV 80-100 fL) | Microcytic (MCV < 80 fL) | Macrocytic (MCV >100 fL) |
|---|---|---|
| - Hemorrhagic anemia - Early iron deficiency anemia - Anemia of chronic disease - Anemia associated with bone marrow suppression - Anemia associated with chronic renal insufficiency - Anemia associated with endocrine dysfunction - Autoimmune hemolytic anemia - Anemia associated with hypothyroidism or hypopituitarism - Hereditary spherocytosis - Hemolytic anemia associated with paroxysmal nocturnal hemoglobinuria | - Iron deficiency anemia - Thalassemias - Anemia of chronic disease - Sideroblastic anemia - Anemia associated with copper deficiency - Anemia associated with lead poisoning | - Folic acid deficiency anemia - Anemia associated with vitamin B12 deficiency - Drug-induced hemolytic anemia - Anemia associated with reticulocytosis - Anemia associated with liver disease - Anemia associated with etanol abuse - Anemia associated with acute myelodysplastic syndrome |
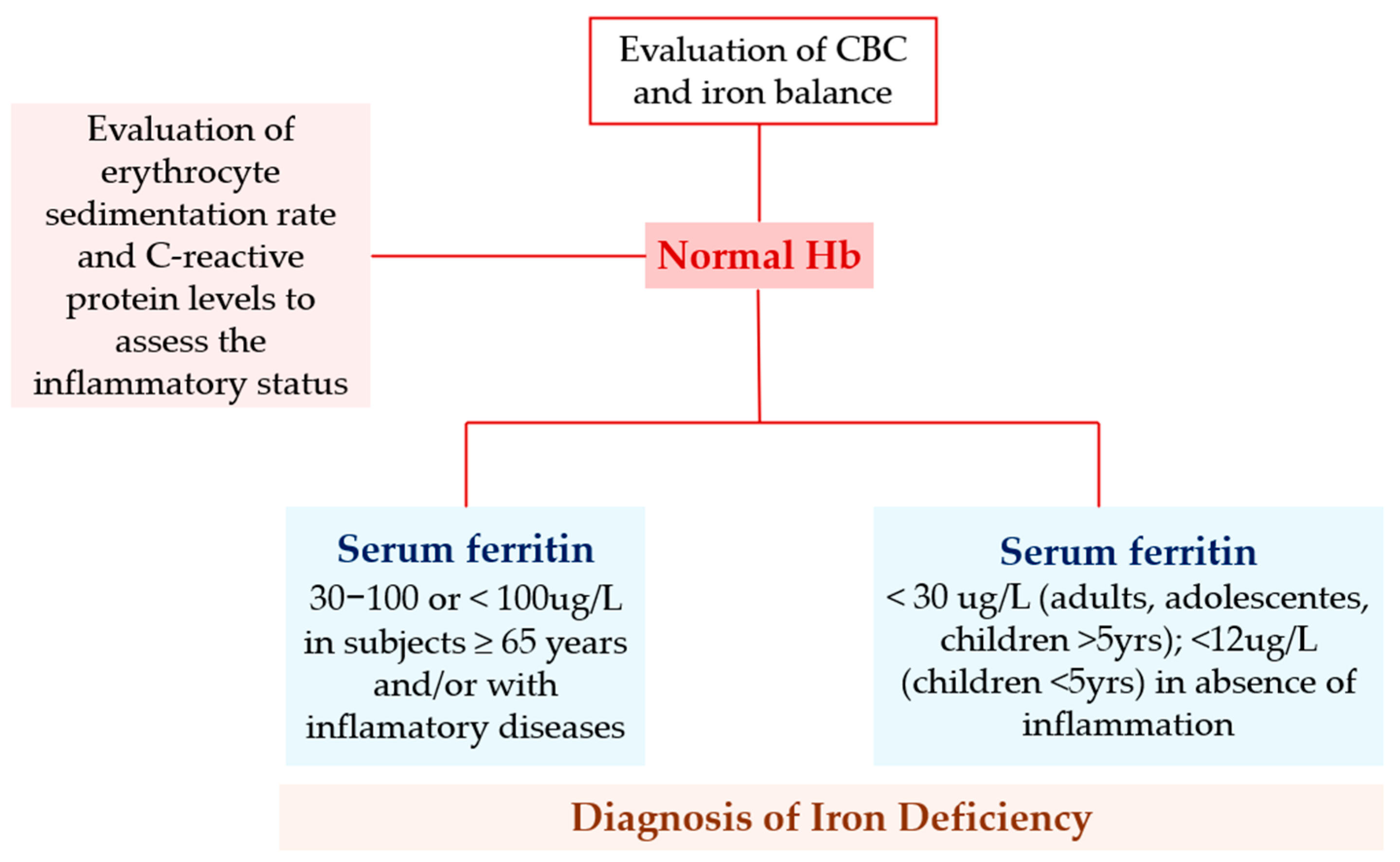
| Evaluation | ||
|---|---|---|
| History | Physical Examination | Laboratory Investigations |
|
|
|
| Reticulocyte Count | |
|---|---|
| Low | High |
Hypoproliferative anemia:
| Increased loss of red blood cells:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, J.; Silva, J.B.; José, C.V.; Ribeiro, H. An Opinion on the Supplementation of Folic Acid 1 mg + Iron (Ferrous Sulfate) 90 mg in the Prevention and Treatment of Anemia. BioChem 2025, 5, 30. https://doi.org/10.3390/biochem5030030
Gomes J, Silva JB, José CV, Ribeiro H. An Opinion on the Supplementation of Folic Acid 1 mg + Iron (Ferrous Sulfate) 90 mg in the Prevention and Treatment of Anemia. BioChem. 2025; 5(3):30. https://doi.org/10.3390/biochem5030030
Chicago/Turabian StyleGomes, João, Joana Brandão Silva, César Vinícius José, and Hugo Ribeiro. 2025. "An Opinion on the Supplementation of Folic Acid 1 mg + Iron (Ferrous Sulfate) 90 mg in the Prevention and Treatment of Anemia" BioChem 5, no. 3: 30. https://doi.org/10.3390/biochem5030030
APA StyleGomes, J., Silva, J. B., José, C. V., & Ribeiro, H. (2025). An Opinion on the Supplementation of Folic Acid 1 mg + Iron (Ferrous Sulfate) 90 mg in the Prevention and Treatment of Anemia. BioChem, 5(3), 30. https://doi.org/10.3390/biochem5030030








