Ethyl 2-(3,5-Dioxo-2-p-tolyl-1,2,4-thiadiazolidin-4-yl) Acetate: A New Inhibitor of Insulin-Degrading Enzyme
Abstract
1. Introduction
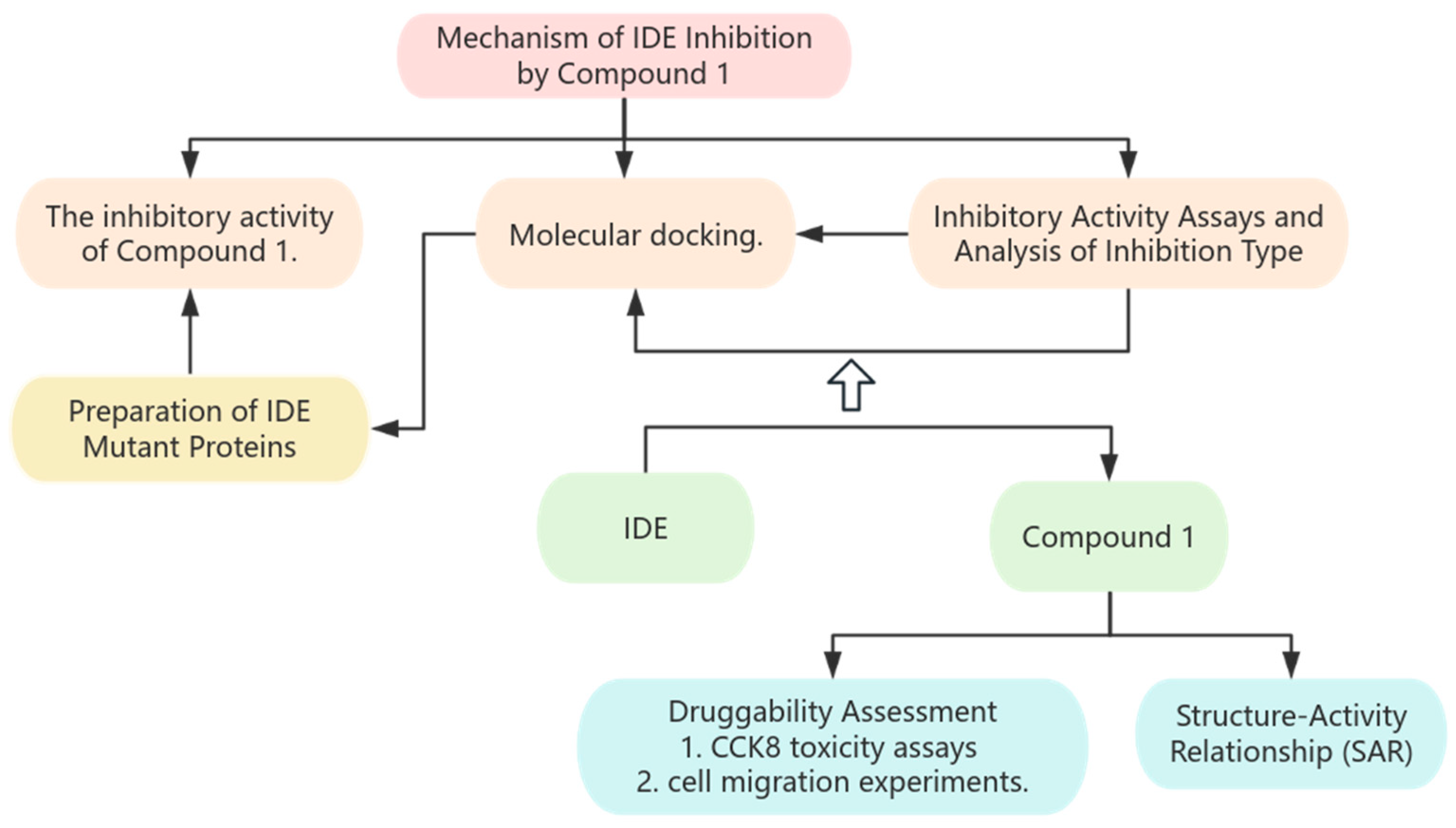
2. Materials and Methods
2.1. Synthesis of Thiazolidinedione Derivatives
2.2. Preparation of IDE
2.3. Enzymatic Assay
2.4. Molecular Docking
2.5. Cytotoxicity Assay and Cell Migration
2.5.1. Cytotoxicity Assay
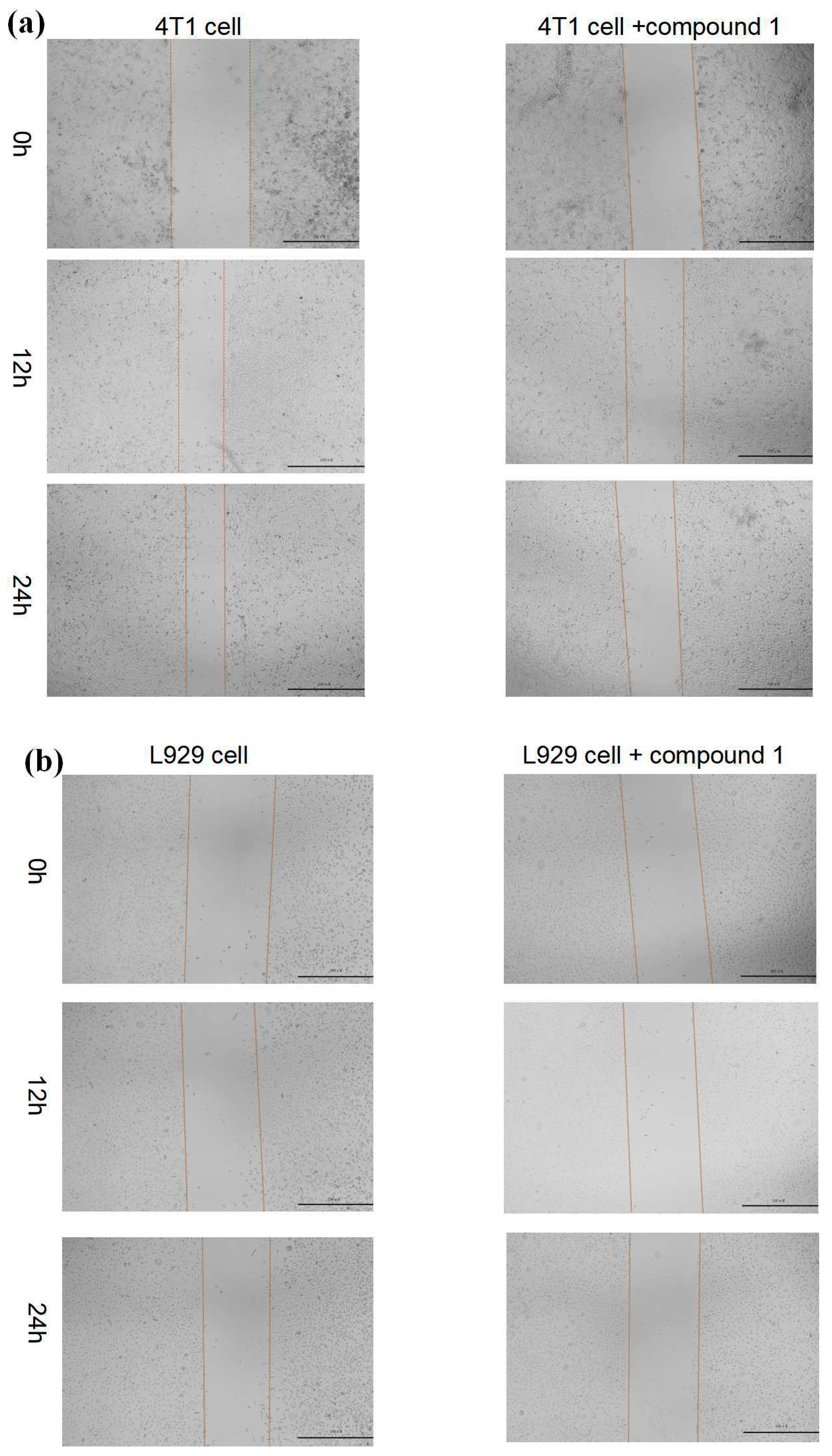
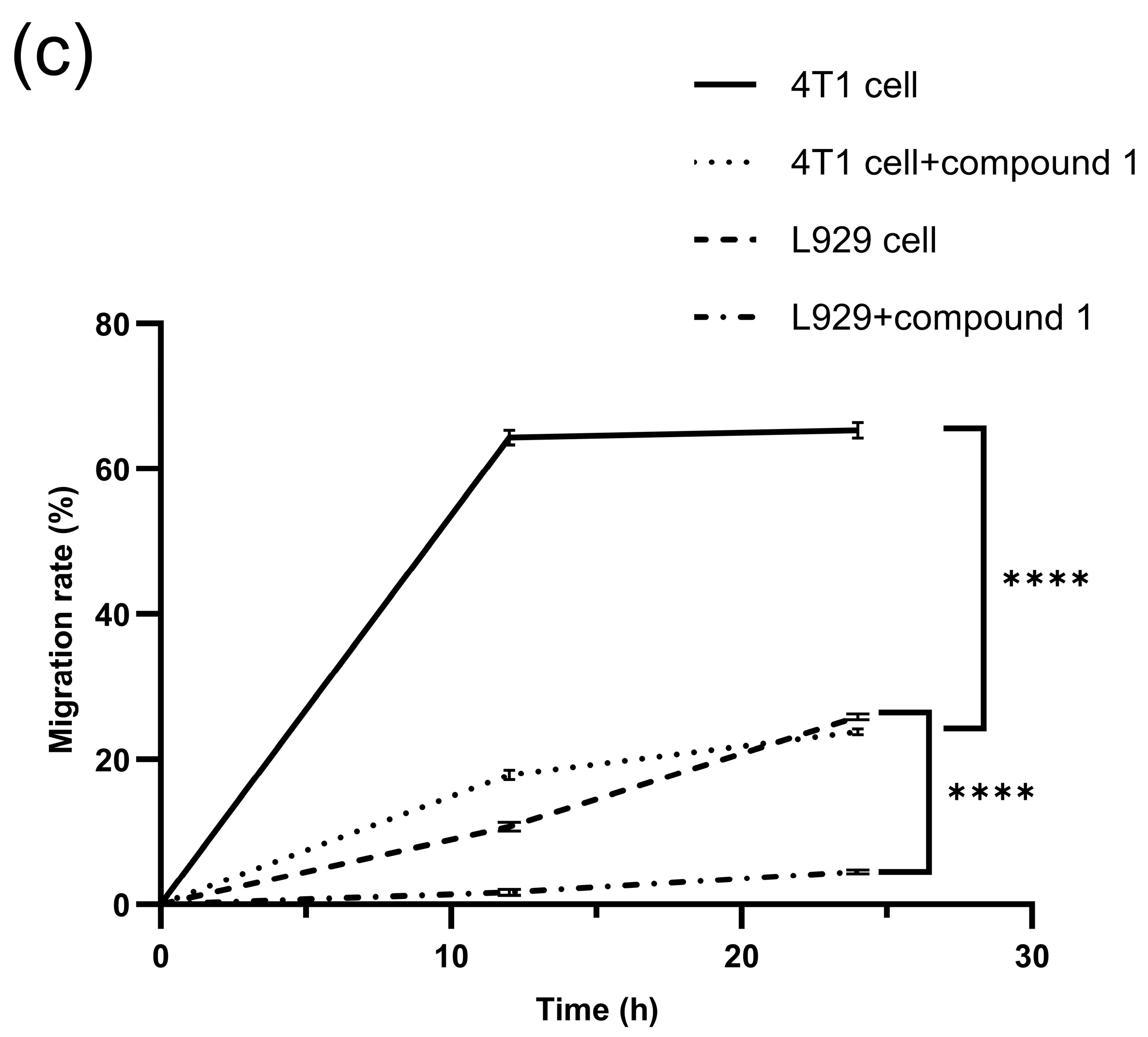
2.5.2. Cell Migration
2.6. Statistical Analyses
3. Results
3.1. Characterization of TDZD Compounds
3.1.1. Characterization of TDZD Compound 1
3.1.2. Characterization of TDZD Compound 2
3.1.3. Characterization of TDZD Compound 3
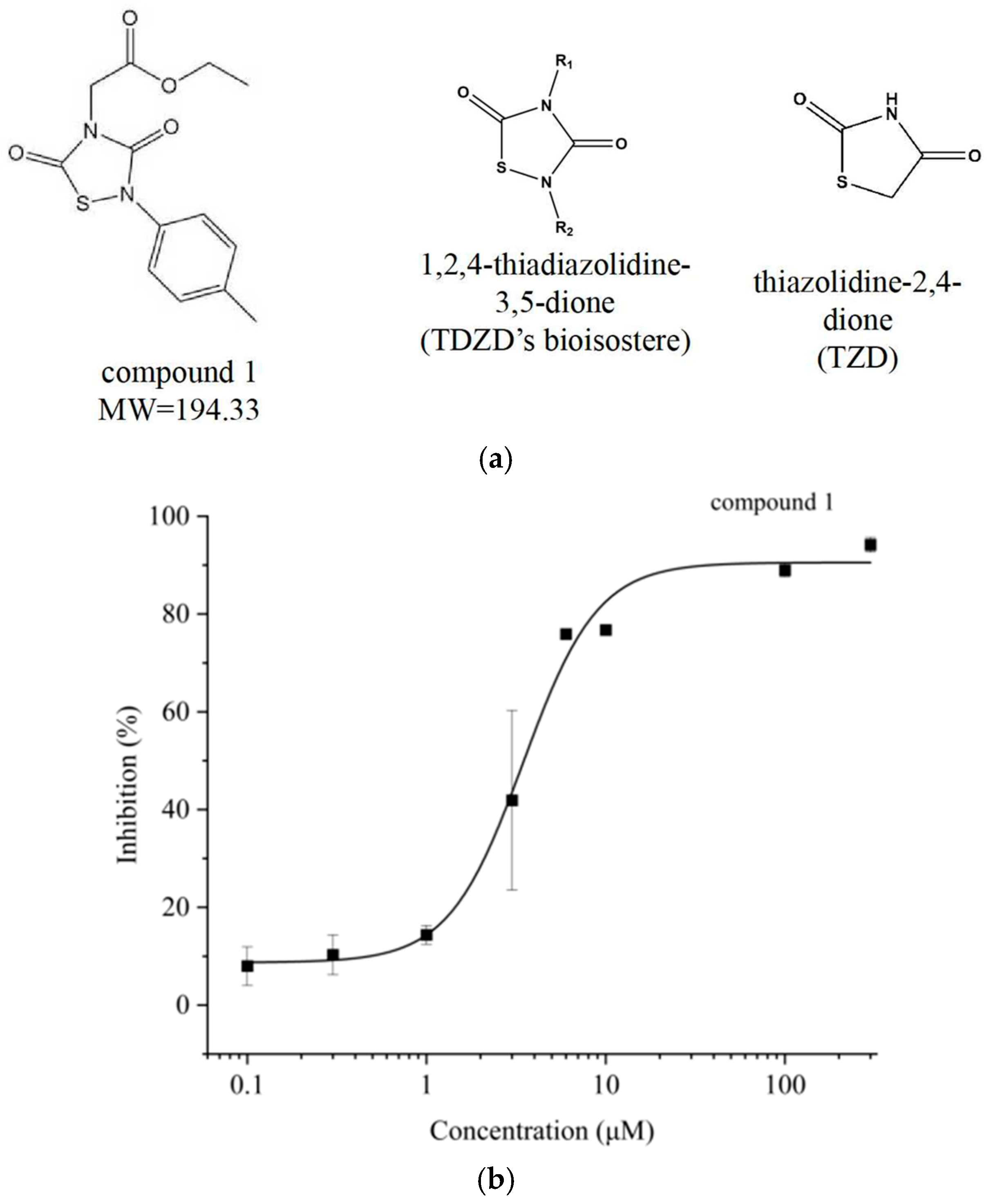
3.2. Discovery of TDZD Hit Compound
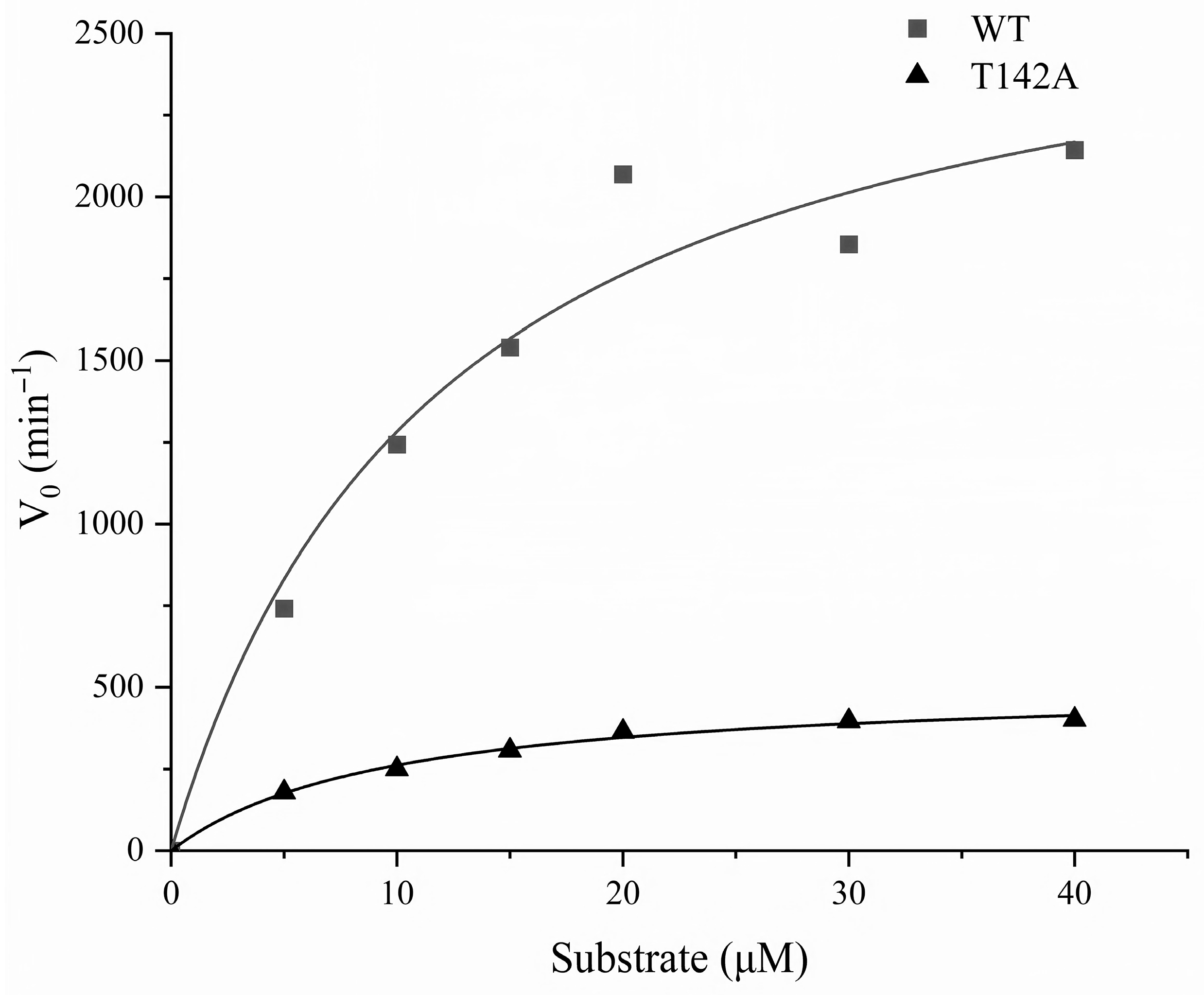
3.3. Compound 1 Inhibits IDE Competitively
3.4. Structure–Activity Relationship (SAR) Analysis of TDZDs
3.5. Docking of 1 in IDE
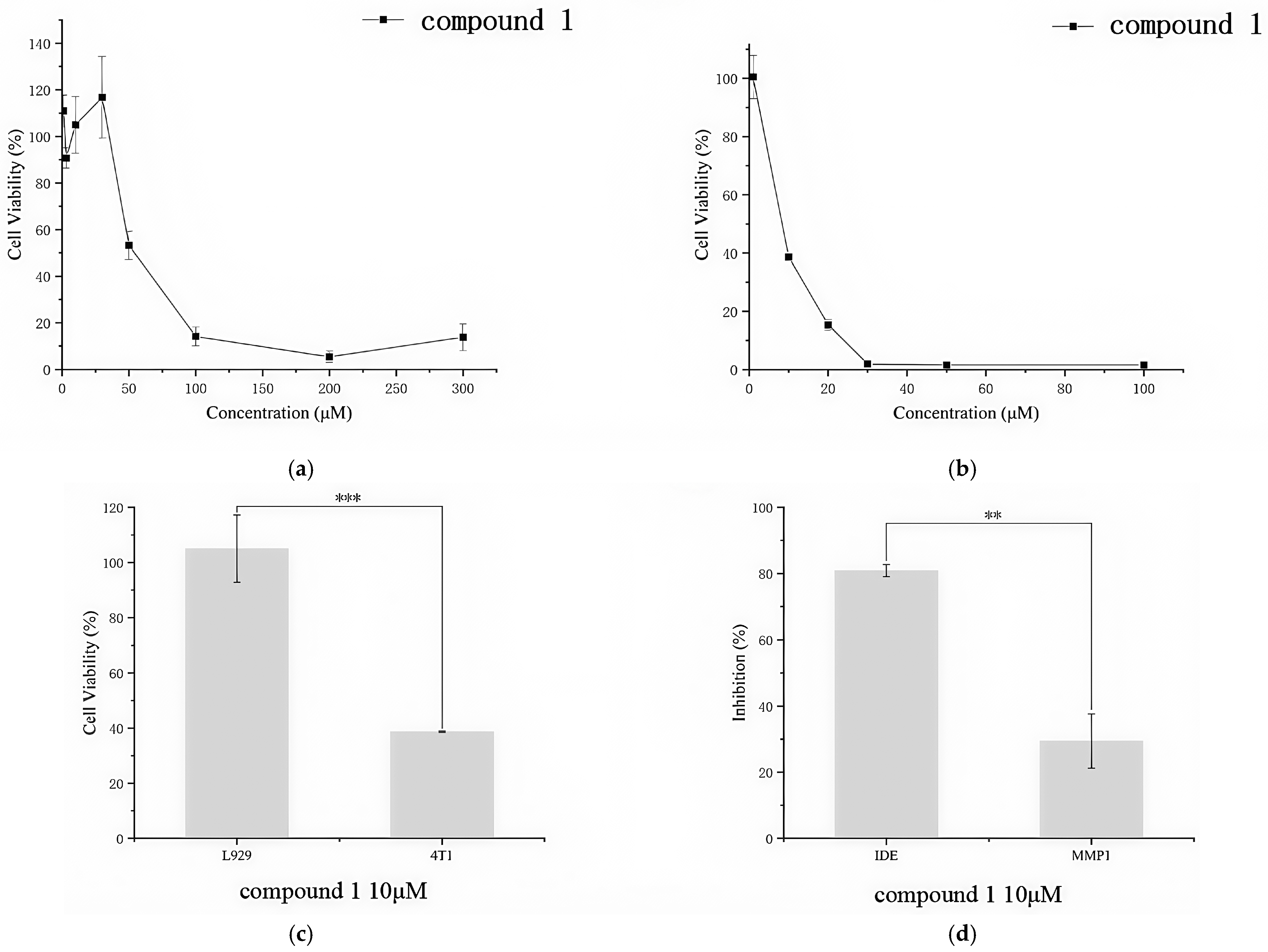
3.6. Cytotoxicity and Selectivity
3.7. Cell Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Leissring, M.A.; González-Casimiro, C.M.; Merino, B.; Suire, C.N.; Perdomo, G. Targeting Insulin-Degrading Enzyme in Insulin Clearance. Int. J. Mol. Sci. 2021, 22, 223. [Google Scholar] [CrossRef]
- Manolopoulou, M.; Guo, Q.; Malito, E.; Schilling, A.B.; Tang, W.J. Molecular basis of catalytic chamber-assisted unfolding and cleavage of human insulin by human insulin-degrading enzyme. J. Biol. Chem. 2009, 284, 14177–14188. [Google Scholar] [CrossRef]
- Affholter, J.A.; Fried, V.A.; Roth, R.A. Human insulin-degrading enzyme shares structural and functional homologies with E. coli protease III. Science 1988, 242, 1415–1418. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Hamel, F.G.; Bennett, R.; Ryan, M.P.; Roth, R.A. Human red blood cell insulin-degrading enzyme and rat skeletal muscle insulin protease share antigenic sites and generate identical products from insulin. J. Biol. Chem. 1990, 265, 2984–2987. [Google Scholar] [CrossRef] [PubMed]
- Tundo, G.; Ciaccio, C.; Sbardella, D.; Boraso, M.; Viviani, B.; Coletta, M.; Marini, S. Somatostatin modulates insulin-degrading-enzyme metabolism: Implications for the regulation of microglia activity in AD. PLoS ONE 2012, 7, e34376. [Google Scholar] [CrossRef]
- Bennett, R.G.; Hamel, F.G.; Duckworth, W.C. Insulin inhibits the ubiquitin-dependent degrading activity of the 26S proteasome. Endocrinology 2000, 141, 2508–2517. [Google Scholar] [CrossRef]
- Ciaccio, C.; Tundo, G.R.; Grasso, G.; Spoto, G.; Marasco, D.; Ruvo, M.; Gioia, M.; Rizzarelli, E.; Coletta, M. Somatostatin: A novel substrate and a modulator of insulin-degrading enzyme activity. J. Mol. Biol. 2009, 385, 1556–1567. [Google Scholar] [CrossRef]
- Fernández-Gamba, A.; Leal, M.C.; Morelli, L.; Castaño, E.M. Insulin-degrading enzyme: Structure-function relationship and its possible roles in health and disease. Curr. Pharm. Des. 2009, 15, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.V.; Gehm, B.D.; Rosner, M.R. An evolutionarily conserved enzyme degrades transforming growth factor-alpha as well as insulin. J. Cell Biol. 1989, 109, 1301–1307. [Google Scholar] [CrossRef]
- Liang, W.G.; Ren, M.; Zhao, F.; Tang, W.J. Structures of human CCL18, CCL3, and CCL4 reveal molecular determinants for quaternary structures and sensitivity to insulin-degrading enzyme. J. Mol. Biol. 2015, 427 Pt B, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Yfanti, C.; Mengele, K.; Gkazepis, A.; Weirich, G.; Giersig, C.; Kuo, W.L.; Tang, W.J.; Rosner, M.; Schmitt, M. Expression of metalloprotease insulin-degrading enzyme insulysin in normal and malignant human tissues. Int. J. Mol. Med. 2008, 22, 421–431. [Google Scholar] [PubMed]
- Radulescu, R.T.; Hufnagel, C.; Luppa, P.; Hellebrand, H.; Kuo, W.L.; Rosner, M.R.; Harbeck, N.; Giersig, C.; Meindl, A.; Schmitt, M.; et al. Immunohistochemical demonstration of the zinc metalloprotease insulin-degrading enzyme in normal and malignant human breast: Correlation with tissue insulin levels. Int. J. Oncol. 2007, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Weirich, G.; Mengele, K.; Yfanti, C.; Gkazepis, A.; Hellmann, D.; Welk, A.; Giersig, C.; Kuo, W.L.; Rosner, M.R.; Tang, W.J.; et al. Immunohistochemical evidence of ubiquitous distribution of the metalloendoprotease insulin-degrading enzyme (IDE; insulysin) in human non-malignant tissues and tumor cell lines. Biol. Chem. 2008, 389, 1441–1445. [Google Scholar] [CrossRef]
- Sbardella, D.; Fasciglione, G.F.; Gioia, M.; Ciaccio, C.; Tundo, G.R.; Marini, S.; Coletta, M. Human matrix metalloproteinases: An ubiquitarian class of enzymes involved in several pathological processes. Mol. Asp. Med. 2012, 33, 119–208. [Google Scholar] [CrossRef]
- Grasso, G.; Pietropaolo, A.; Spoto, G.; Pappalardo, G.; Tundo, G.R.; Ciaccio, C.; Coletta, M.; Rizzarelli, E. Copper(I) and copper(II) inhibit Aβ peptides proteolysis by insulin-degrading enzyme differently: Implications for metallostasis alteration in Alzheimer’s disease. Chemistry 2011, 17, 2752–2762. [Google Scholar] [CrossRef]
- Gennari, A.; Sormani, M.; Puntoni, M.; Martini, V.; Amaro, A.; Bruzzi, P.; Pfeffer, U. Identification of a Prognostic Signature Based on the Expression of Genes Related to the Insulin Pathway in Early Breast Cancer. Breast Care 2021, 16, 299–306. [Google Scholar] [CrossRef]
- Tundo, G.R.; Sbardella, D.; De Pascali, S.A.; Ciaccio, C.; Coletta, M.; Fanizzi, F.P.; Marini, S. Novel Platinum(II) compounds modulate insulin-degrading enzyme activity and induce cell death in neuroblastoma cells. JBIC J. Biol. Inorg. Chem. 2015, 20, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lesire, L.; Leroux, F.; Deprez-Poulain, R.; Deprez, B. Insulin-Degrading Enzyme, an Under-Estimated Potential Target to Treat Cancer? Cells 2022, 11, 1228. [Google Scholar] [CrossRef]
- Tundo, G.R.; Sbardella, D.; Ciaccio, C.; Bianculli, A.; Orlandi, A.; Desimio, M.G.; Arcuri, G.; Coletta, M.; Marini, S. Insulin-degrading enzyme (IDE): A novel heat shock-like protein. J. Biol. Chem. 2013, 288, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Pietzsch, S.; Stapel, B.; Ricke-Hoch, M.; Lee, C.W.; Bankstahl, J.P.; Scherr, M.; Heineke, J.; Scharf, G.; Haghikia, A.; et al. Insulin supplementation attenuates cancer-induced cardiomyopathy and slows tumor disease progression. JCI Insight 2017, 2, e93098. [Google Scholar] [CrossRef] [PubMed]
- Garczorz, W.; Kosowska, A.; Francuz, T. Antidiabetic Drugs in Breast Cancer Patients. Cancers 2024, 16, 299. [Google Scholar] [CrossRef]
- Radulescu, R.T. Intracellular insulin in human tumors: Examples and implications. Diabetol. Metab. Syndr. 2011, 3, 5. [Google Scholar] [CrossRef]
- Radulescu, R.T. One for all and all for one: RB defends the cell while IDE, PTEN and IGFBP-7 antagonize insulin and IGFs to protect RB. Med. Hypotheses 2007, 69, 1018–1020. [Google Scholar] [CrossRef]
- Radulescu, R.T.; Doklea, E.D.; Kehe, K.; Mückter, H. Nuclear colocalization and complex formation of insulin with retinoblastoma protein in HepG2 human hepatoma cells. J. Endocrinol. 2000, 166, R1–R4. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, R.T.; Bellitti, M.R.; Ruvo, M.; Cassani, G.; Fassina, G. Binding of the LXCXE insulin motif to a hexapeptide derived from retinoblastoma protein. Biochem. Biophys. Res. Commun. 1995, 206, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Eichelbaum, E.J.; Wang, H.; Vesely, D.L. Atrial natriuretic peptide and long acting natriuretic peptide inhibit ERK 1/2 in prostate cancer cells. Anticancer Res. 2006, 26, 4143–4148. [Google Scholar] [PubMed]
- Vesely, D.L. Cardiac hormones for the treatment of cancer. Endocr. Relat. Cancer 2013, 20, R113–R125. [Google Scholar] [CrossRef]
- Nguyen, J.P.; Frost, C.D.; Lane, M.L.; Skelton, W.P.I.V.; Skelton, M.; Vesely, D.L. Novel dual inhibitors of vascular endothelial growth factor and VEGFR2 receptor. Eur. J. Clin. Investig. 2012, 42, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, X.; Li, P.; Yang, Y.; Zhang, W.; Zhao, S.; Zeng, Y.; Zhou, X.; Zeng, L.H.; Yang, G. Modified natriuretic peptides and their potential roles in cancer treatment. Biomed. J. 2022, 45, 118–131. [Google Scholar] [CrossRef]
- Bennett, R.G.; Hamel, F.G.; Duckworth, W.C. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes 2003, 52, 2315–2320. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G. Insulin degradation: Progress and potential. Endocr. Rev. 1998, 19, 608–624. [Google Scholar]
- Leissring, M.A.; Malito, E.; Hedouin, S.; Reinstatler, L.; Sahara, T.; Abdul-Hay, S.O.; Selkoe, D.J.; Gregory, D.; Dennis, J.; Ross, L.; et al. Designed inhibitors of insulin-degrading enzyme regulate the catabolism and activity of insulin. PLoS ONE 2010, 5, e10504. [Google Scholar] [CrossRef]
- Charton, J.; Gauriot, M.; Guo, Q.; Hennuyer, N.; Marechal, X.; Dumont, J.; Hamdane, M.; Pottiez, V.; Landry, V.; Sperandio, O.; et al. Imidazole-derived 2-[N-carbamoylmethyl-alkylamino]acetic acids, substrate-dependent modulators of insulin-degrading enzyme in amyloid-beta hydrolysis. Eur. J. Med. Chem. 2014, 79, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Charton, J.; Gauriot, M.; Totobenazara, J.; Hennuyer, N.; Dumont, J.; Bosc, D.; Marechal, X.; Elbakali, J.; Herledan, A.; Wen, X.; et al. Structure-activity relationships of imidazole-derived 2-[N-carbamoylmethyl-alkylamino]acetic acids, dual binders of human insulin-degrading enzyme. Eur. J. Med. Chem. 2015, 90, 547–567. [Google Scholar] [CrossRef]
- Deprez-Poulain, R.; Hennuyer, N.; Bosc, D.; Liang, W.G.; Enée, E.; Marechal, X.; Charton, J.; Totobenazara, J.; Berte, G.; Jahklal, J.; et al. Catalytic site inhibition of insulin-degrading enzyme by a small molecule induces glucose intolerance in mice. Nat. Commun. 2015, 6, 8250. [Google Scholar] [CrossRef]
- Maianti, J.P.; McFedries, A.; Foda, Z.H.; Kleiner, R.E.; Du, X.Q.; Leissring, M.A.; Tang, W.-J.; Charron, M.J.; Seeliger, M.A.; Saghatelian, A.; et al. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature 2014, 511, 94–98. [Google Scholar] [CrossRef]
- Kajal, K.; Singh, G.; Pradhan, T.; Bhurta, D.; Monga, V. The medicinal perspective of 2, 4-thiazolidinediones based ligands as antimicrobial, antitumor and antidiabetic agents: A review. Arch. Pharm. 2022, 355, e2100517. [Google Scholar] [CrossRef]
- Colca, J.R. The TZD insulin sensitizer clue provides a new route into diabetes drug discovery. Expert. Opin. Drug Discov. 2015, 10, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diab Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.S.; Prasad, D.N.; Singh, R.K. An Insight into the Synthesis and SAR of 2, 4-Thiazolidinediones (2, 4-TZD) as Multifunctional Scaffold: A Review. Mini Rev. Med. Chem. 2020, 20, 308–330. [Google Scholar] [CrossRef]


| Comp. | R1 | R2 | IC50 | p-Value |
|---|---|---|---|---|
| 1 | 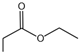 |  | 3.60 | 2.09329 ± 0.41497 |
| 2 | 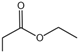 | 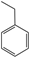 | 3.23 | 1.24981 ± 0.52639 |
| 3 | 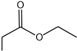 |  | 7.59 | 6.55547 ± 0.25285 |
| IDE | Vmax (min−1) | Km (μM) | IC50 (μM) |
|---|---|---|---|
| WT | 2814.32 | 11.93 | 3.5 |
| T142A | 501.05 | 9.01 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xiao, S.; Miao, H.; Lu, C.; Zhao, Q.; Shao, Z.; Chen, T. Ethyl 2-(3,5-Dioxo-2-p-tolyl-1,2,4-thiadiazolidin-4-yl) Acetate: A New Inhibitor of Insulin-Degrading Enzyme. BioChem 2025, 5, 27. https://doi.org/10.3390/biochem5030027
Zhang Y, Xiao S, Miao H, Lu C, Zhao Q, Shao Z, Chen T. Ethyl 2-(3,5-Dioxo-2-p-tolyl-1,2,4-thiadiazolidin-4-yl) Acetate: A New Inhibitor of Insulin-Degrading Enzyme. BioChem. 2025; 5(3):27. https://doi.org/10.3390/biochem5030027
Chicago/Turabian StyleZhang, Yonghong, Shu Xiao, Hongsheng Miao, Changrui Lu, Qi Zhao, Zhiyu Shao, and Ting Chen. 2025. "Ethyl 2-(3,5-Dioxo-2-p-tolyl-1,2,4-thiadiazolidin-4-yl) Acetate: A New Inhibitor of Insulin-Degrading Enzyme" BioChem 5, no. 3: 27. https://doi.org/10.3390/biochem5030027
APA StyleZhang, Y., Xiao, S., Miao, H., Lu, C., Zhao, Q., Shao, Z., & Chen, T. (2025). Ethyl 2-(3,5-Dioxo-2-p-tolyl-1,2,4-thiadiazolidin-4-yl) Acetate: A New Inhibitor of Insulin-Degrading Enzyme. BioChem, 5(3), 27. https://doi.org/10.3390/biochem5030027







