PDX1 Functions as a Tumor Suppressor in MCF7 Breast Cancer Cells: Implications for Chemotherapeutic Sensitivity
Abstract
1. Introduction
2. Materials and Methods
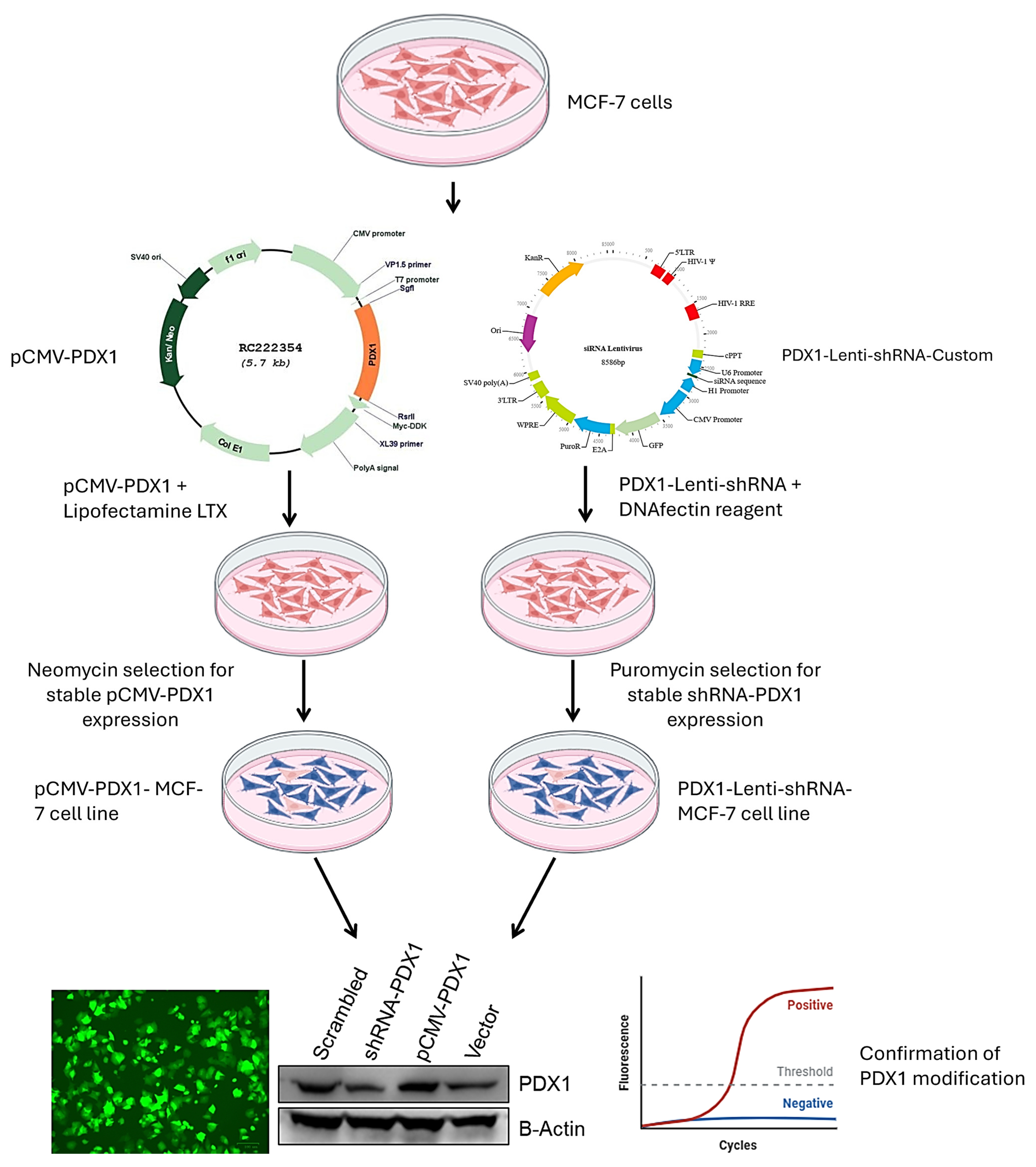
2.1. Cell Culture and Genetic Modulation
2.2. Gene and Protein Expression Analysis
2.3. Drug Treatment and Viability Assays
2.4. Statistical Analysis
3. Results
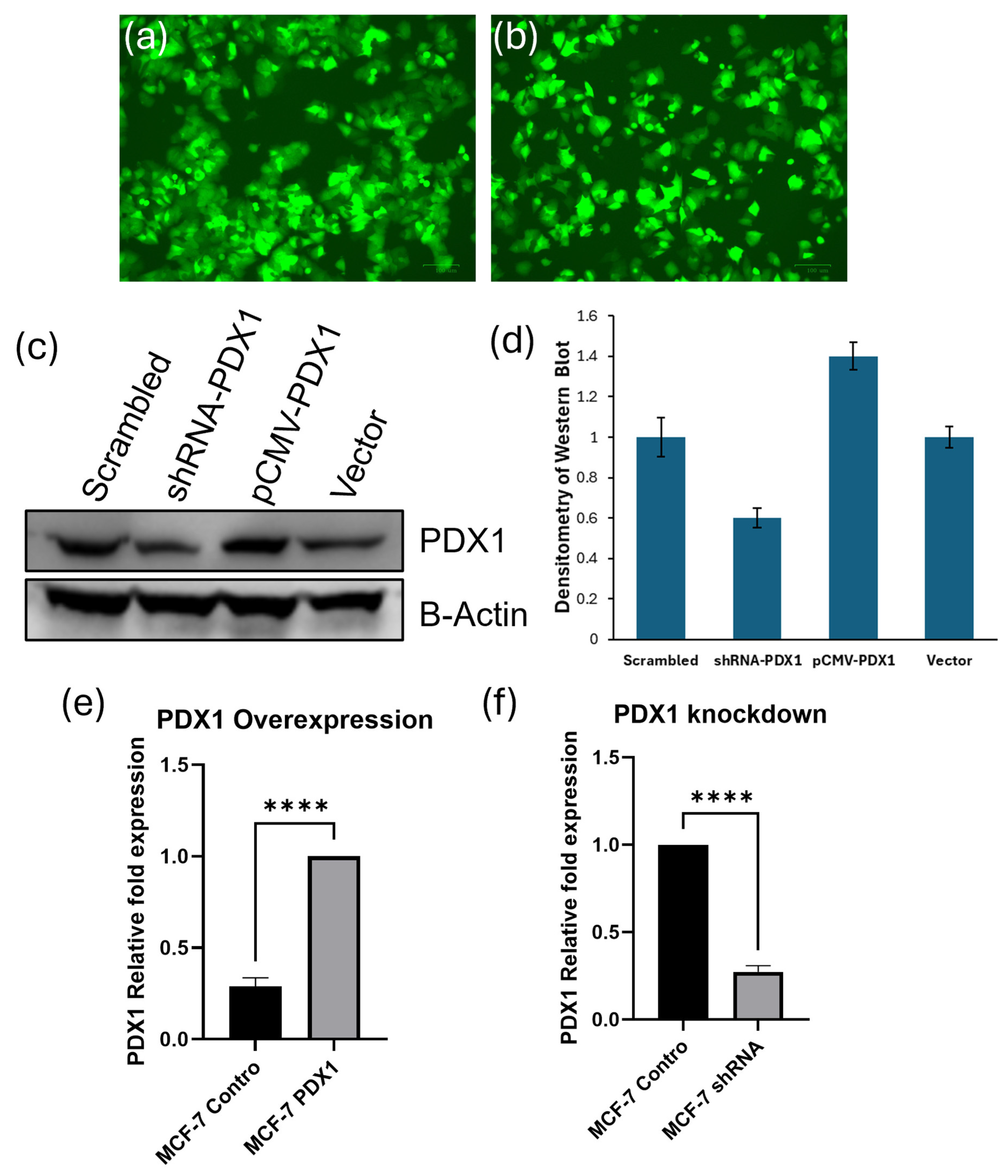
3.1. PDX1 Expression Is Effectively Modulated in MCF-7 Breast Cancer Cells
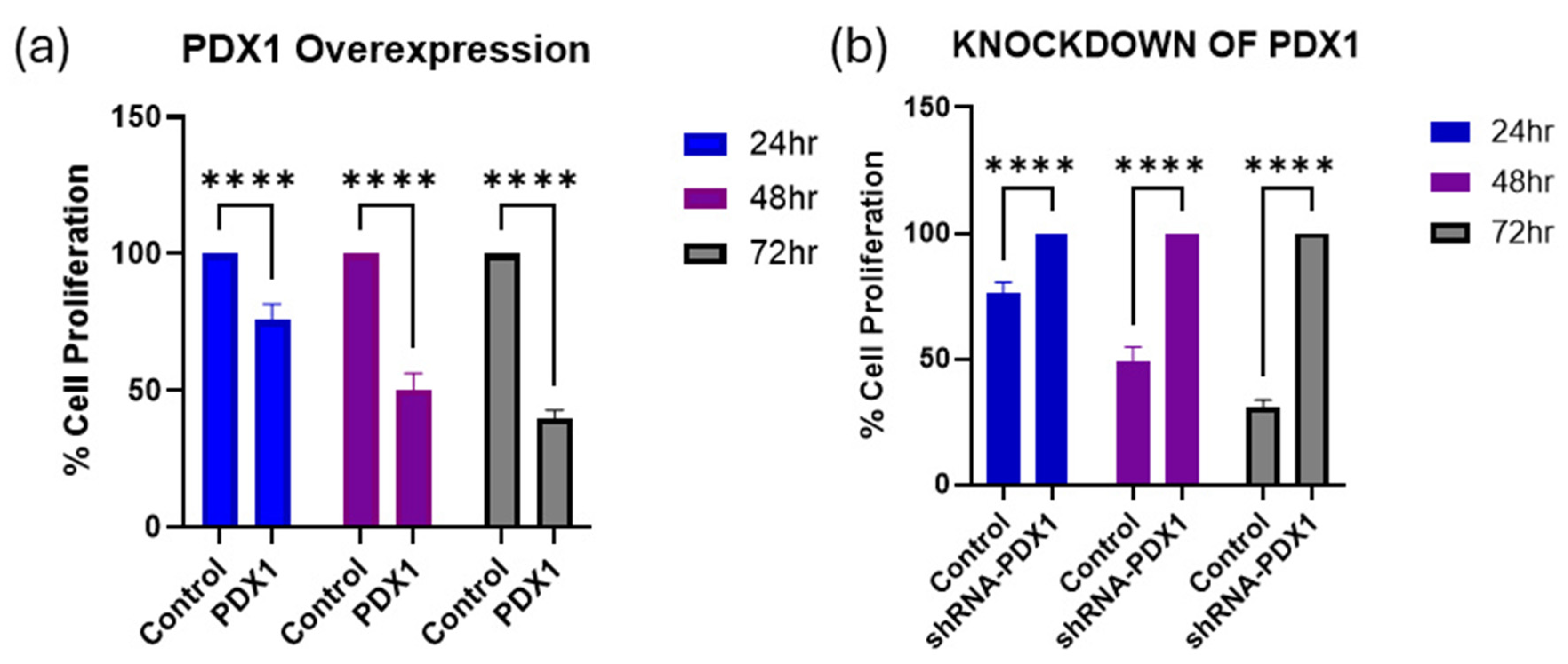
3.2. PDX1 Modulation Influences MCF-7 Cell Viability over Time
3.3. PDX1 Modulation Alters Chemosensitivity of MCF-7 Cells to Paclitaxel and Doxorubicin
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herman, J.F.; Mangala, L.S.; Mehta, K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 2006, 25, 3049–3058. [Google Scholar] [CrossRef]
- Lainetti, P.D.F.; Leis-Filho, A.F.; Laufer-Amorim, R.; Battazza, A.; Fonseca-Alves, C.E. Mechanisms of resistance to chemotherapy in breast cancer and possible targets in drug delivery systems. Pharmaceutics 2020, 12, 1193. [Google Scholar] [CrossRef]
- Chun, K.H.; Park, J.H.; Fan, S. Predicting and overcoming chemotherapeutic resistance in breast cancer. In Translational Research in Breast Cancer: Biomarker Diagnosis, Targeted Therapies and Approaches to Precision Medicine; Springer: Singapore, 2017; pp. 59–104. [Google Scholar]
- Kondratyeva, L.; Chernov, I.; Kopantzev, E.; Didych, D.; Kuzmich, A.; Alekseenko, I.; Kostrov, S.; Sverdlov, E. Pancreatic lineage specifier PDX1 increases adhesion and decreases motility of cancer cells. Cancers 2021, 13, 4390. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.C.; Chu, Y.; Tan, Y.Y.; Li, J.; Gao, S. Pancreatic and duodenal homeobox-1 in pancreatic ductal adenocarcinoma and diabetes mellitus. Chin. Med. J. 2020, 133, 344–350. [Google Scholar] [CrossRef]
- Roy, N.; Takeuchi, K.K.; Ruggeri, J.M.; Bailey, P.; Chang, D.; Li, J.; Leonhardt, L.; Puri, S.; Hoffman, M.T.; Gao, S.; et al. PDX1 dynamically regulates pancreatic ductal adenocarcinoma initiation and maintenance. Genes Dev. 2016, 30, 2669–2683. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Li, H.; Xiao, X.; Shen, L.; Fang, L. Downregulation of pancreatic-duodenal homeobox 1 expression in breast cancer patients: A mechanism of proliferation and apoptosis in cancer. Mol. Med. Rep. 2012, 6, 983–988. [Google Scholar] [CrossRef]
- Oz Puyan, F.; Can, N.; Ozyilmaz, F.; Usta, U.; Sut, N.; Tastekin, E.; Altaner, S. The relationship among PDX1, CDX2, and mucin profiles in gastric carcinomas; correlations with clinicopathologic parameters. J. Cancer Res. Clin. Oncol. 2011, 137, 1749–1762. [Google Scholar] [CrossRef]
- Ma, J.; Chen, M.; Wang, J.; Xia, H.H.; Zhu, S.; Liang, Y.; Gu, Q.; Qiao, L.; Dai, Y.; Zou, B.; et al. Pancreatic duodenal homeobox-1 (PDX1) functions as a tumor suppressor in gastric cancer. Carcinog. 2008, 29, 1327–1333. [Google Scholar] [CrossRef]
- Li, Z.; Qian, J.; Li, J.; Zhu, C. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2019, 18, 435–442. [Google Scholar] [CrossRef]
- Li, P.; Zhong, D.; Gong, P.Y. Synergistic effect of paclitaxel and verapamil to overcome multi-drug resistance in breast cancer cells. Biochem. Biophys. Res. Comm 2019, 516, 183–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wei, Y.; Li, M.; Yu, S.; Ye, M.; Zhang, H.; Chen, S.; Liu, W.; Zhang, J. MiR-129-3p promotes docetaxel resistance of breast cancer cells via CP110 inhibition. Sci. Rep. 2015, 5, 15424. [Google Scholar] [CrossRef]
- Huang, B.; Qu, Z.; Ong, C.W.; Tsang, Y.H.; Xiao, G.; Shapiro, D.; Salto-Tellez, M.; Ito, K.; Ito, Y.; Chen, L.F. RUNX3 acts as a tumor suppressor in breast cancer by targeting estrogen receptor α. Oncogene 2012, 31, 527–534. [Google Scholar] [CrossRef]
- Lam, S.; Lodder, K.; Teunisse, A.F.A.S.; Rabelink, M.J.W.E.; Schutte, M.; Jochemsen, A.G. Role of Mdm4 in drug sensitivity of breast cancer cells. Oncogene 2010, 29, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Antonyak, M.A.; Miller, A.M.; Jansen, J.M.; Boehm, J.E.; Balkman, C.E.; Wakshlag, J.J.; Page, R.L.; Cerione, R.A. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J. Biol. Chem. 2004, 279, 41461–41467. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, S.; Zhu, S.; Yi, M.; Luo, S.; Wu, K. MiRNA-mediated EMT and CSCs in cancer chemoresistance. Exp. Hematol. Oncol. 2021, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ly, R.C.; Frazier, C.V.; Yu, J.; Qin, S.; Fan, X.Y.; Goetz, M.P.; Boughey, J.C.; Weinshilboum, R.; Wang, L. The novel function of tumor protein D54 in regulating pyruvate dehydrogenase and metformin cytotoxicity in breast cancer. Cancer Metab. 2019, 7, 1. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of breast cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Adekiya, T.A.; Moore, M.; Thomas, M.; Lake, G.; Hudson, T.; Adesina, S.K. Preparation, optimization, and in-vitro evaluation of brusatol-and docetaxel-loaded nanoparticles for the treatment of prostate cancer. Pharmaceutics 2024, 16, 114. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest Graph™ IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 21 January 2025).
- Liu, H.; Zhu, X.; Wei, Y.; Song, C.; Wang, Y. Recent advances in targeted gene silencing and cancer therapy by nanoparticle-based delivery systems. Biomed. Pharmacother. 2023, 157, 114065. [Google Scholar] [CrossRef]
- Youssef, E.; Fletcher, B.; Palmer, D. Enhancing precision in cancer treatment: The role of gene therapy and immune modulation in oncology. Front. Med. 2025, 11, 1527600. [Google Scholar] [CrossRef]
- Naghizadeh, S.; Mohammadi, A.; Duijf, P.H.; Baradaran, B.; Safarzadeh, E.; Cho, W.C.S.; Mansoori, B. The role of miR-34 in cancer drug resistance. J. Cell. Physiol. 2020, 235, 6424–6440. [Google Scholar] [CrossRef]
- Li, K.; Tian, S.; Sun, K.; Su, Q.; Mei, Y.; Niu, W. ROS-responsive polyprodrug micelles carrying suicide genes in combination with chemotherapy and gene therapy for prostate cancer treatment. RSC Adv. 2024, 14, 5577–5587. [Google Scholar] [CrossRef]

| Gene Abbreviation | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Taqman (5′-3′) |
|---|---|---|---|
| B-actin | GAACTGCCTGACTACCTCATG | CGAAGTCCAGGGCAACATAG | TGCGTGACA/ZEN/TCAAAGAGAAGCTGTGC |
| PDX1 | TGAAGTCTACCAAAGCTCACG | TCCTTCTCCAGCTCTAGCA | CCTGCCCACTGGCCTTTCCA |
| Treatments | Control | shRNA-PDX1 | Control | pCMV-PDX1 |
|---|---|---|---|---|
| Paclitaxel | 1.313 nM | 2.652 nM | 1.472 nM | 0.966 nM |
| Doxorubicin | 1.327 µM | 1.361 µM | 1.663 µM | 1.488 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekiya, T.A. PDX1 Functions as a Tumor Suppressor in MCF7 Breast Cancer Cells: Implications for Chemotherapeutic Sensitivity. BioChem 2025, 5, 20. https://doi.org/10.3390/biochem5030020
Adekiya TA. PDX1 Functions as a Tumor Suppressor in MCF7 Breast Cancer Cells: Implications for Chemotherapeutic Sensitivity. BioChem. 2025; 5(3):20. https://doi.org/10.3390/biochem5030020
Chicago/Turabian StyleAdekiya, Tayo Alex. 2025. "PDX1 Functions as a Tumor Suppressor in MCF7 Breast Cancer Cells: Implications for Chemotherapeutic Sensitivity" BioChem 5, no. 3: 20. https://doi.org/10.3390/biochem5030020
APA StyleAdekiya, T. A. (2025). PDX1 Functions as a Tumor Suppressor in MCF7 Breast Cancer Cells: Implications for Chemotherapeutic Sensitivity. BioChem, 5(3), 20. https://doi.org/10.3390/biochem5030020





