Advances in Structural Biology for Anesthetic Drug Mechanisms: Insights into General and Local Anesthesia
Abstract
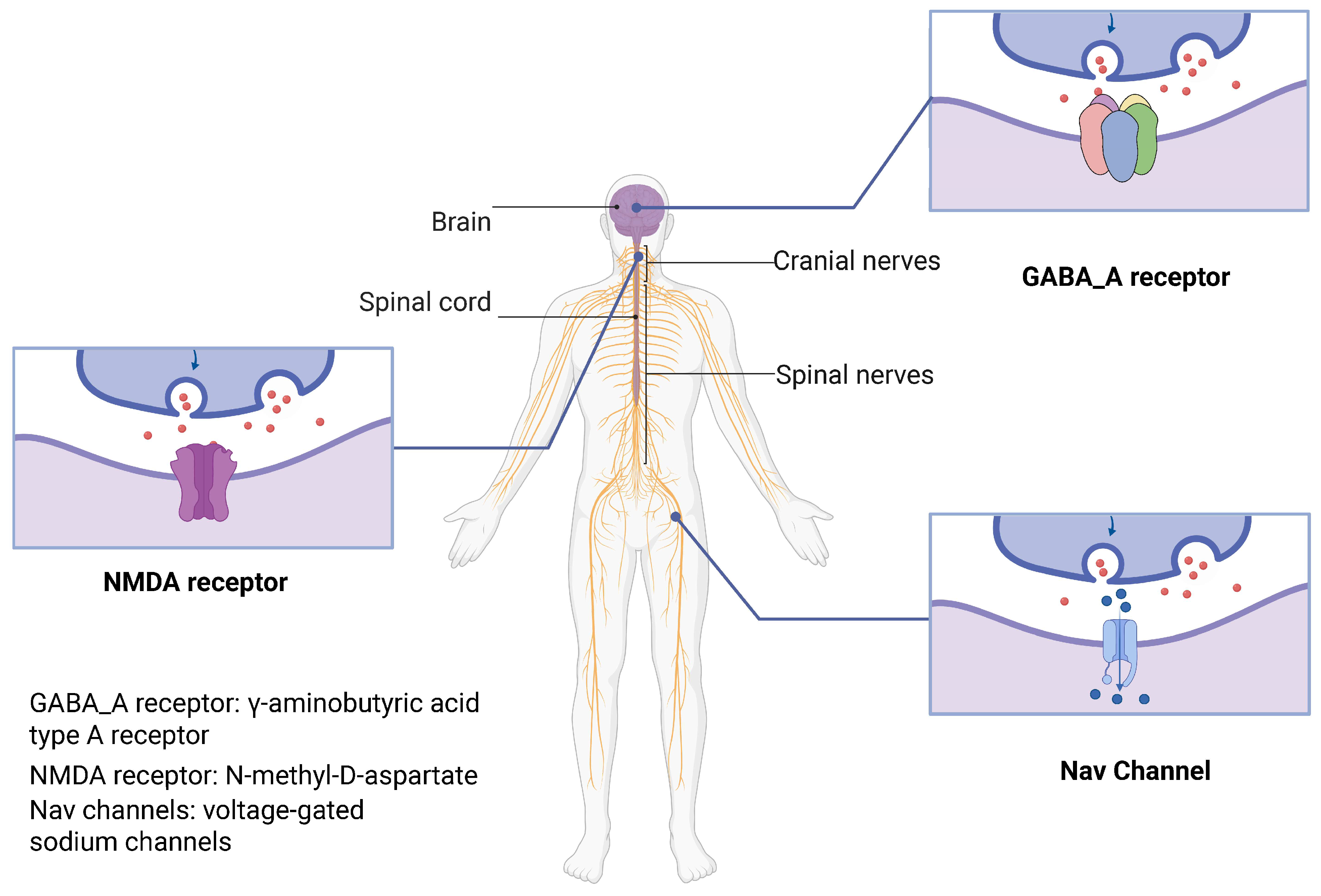
1. Introduction
2. Materials and Methods
3. Molecular Targets of General Anesthesia
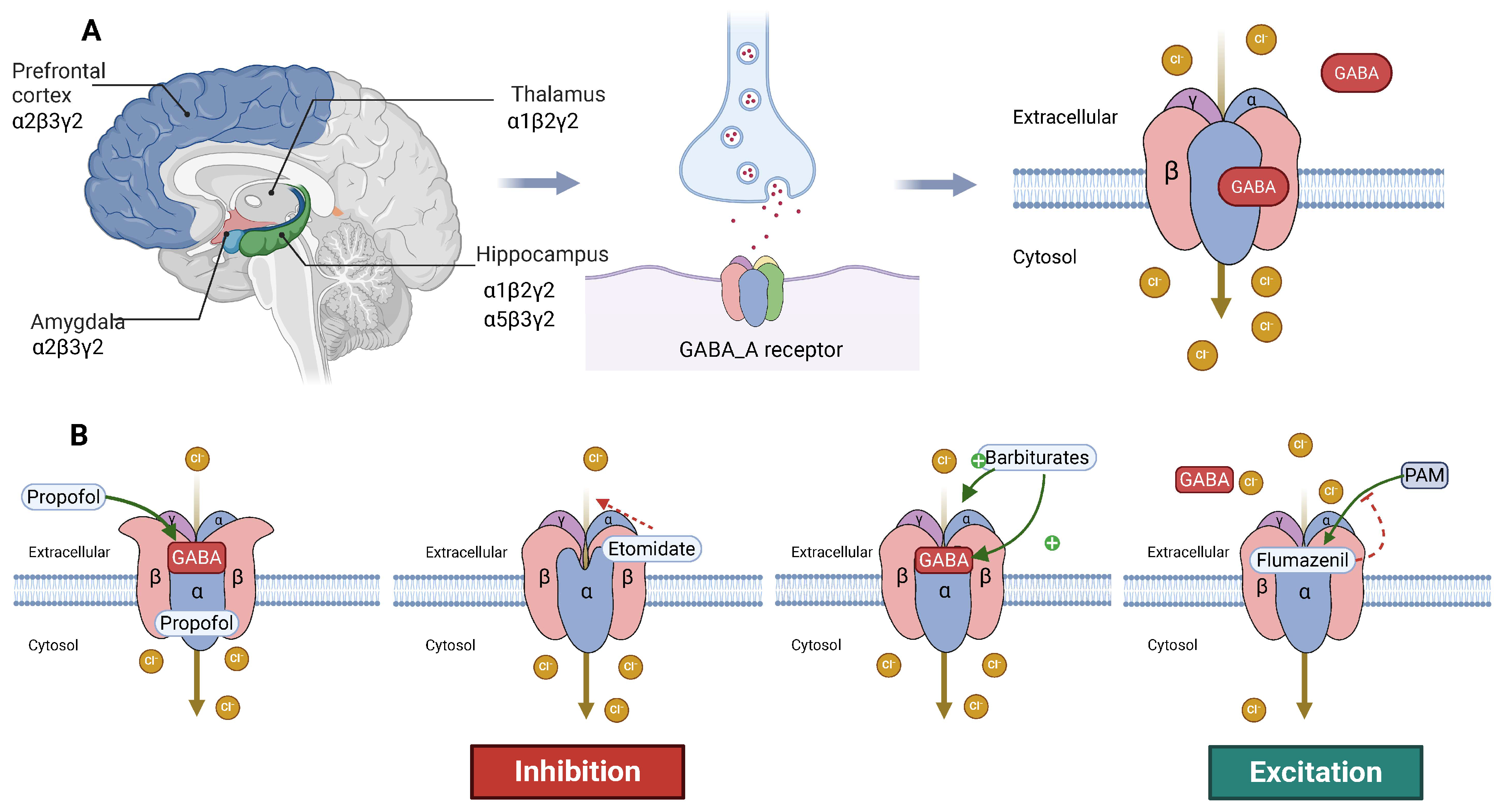
3.1. Structure and Function of the GABA_A Receptor
3.1.1. Basic Structure of the GABA_A Receptor
3.1.2. Mechanism of Action of Anesthetics on the GABA_A Receptor
3.1.3. Future Research Directions
3.2. Structure and Function of the NMDA Receptor
3.2.1. Basic Structure and Function of the NMDA Receptor
3.2.2. NMDA Receptor and General Anesthesia
3.2.3. Future Research Directions
4. Molecular Mechanisms of Local Anesthesia
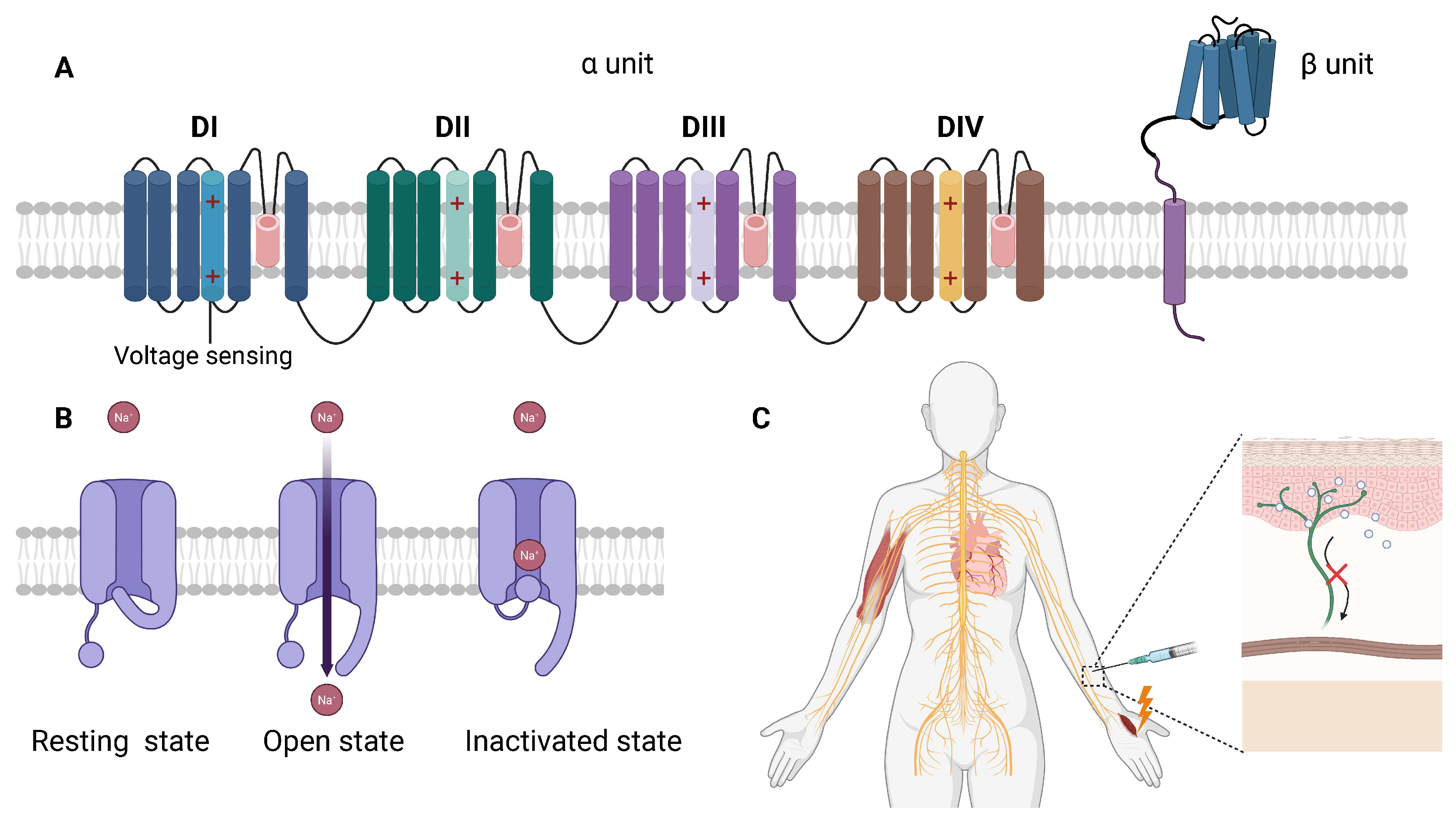
4.1. Nav Channels and the Mechanism of Local Anesthetics
4.1.1. Basic Structure and Function of Nav Channels
4.1.2. Mechanism of Action of Local Anesthetics
4.1.3. Future Research Directions
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Wang, Y.; Zhang, L.; Luo, M.; Wang, Y. Neural Network Mechanisms Underlying General Anesthesia: Cortical and Subcortical Nuclei. Neurosci. Bull. 2024, 40, 1995–2011. [Google Scholar] [CrossRef] [PubMed]
- Lirk, P.; Hollmann, M.W.; Strichartz, G. The Science of Local Anesthesia: Basic Research, Clinical Application, and Future Directions. Anesth. Analg. 2018, 126, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.; Aricescu, A.R. A structural perspective on GABAA receptor pharmacology. Curr. Opin. Struct. Biol. 2019, 54, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Sente, A. Gatekeepers of the brain: Identifying hidden mechanisms of type A GABA receptor signaling and assembly. Science 2024, 386, 738–739. [Google Scholar] [CrossRef]
- Wiedmann, F.; Rinne, S.; Donner, B.; Decher, N.; Katus, H.A.; Schmidt, C. Mechanosensitive TREK-1 two-pore-domain potassium (K2P) channels in the cardiovascular system. Prog. Biophys. Mol. Biol. 2021, 159, 126–135. [Google Scholar] [CrossRef]
- Philip, A.B.; Brohan, J.; Goudra, B. The Role of GABA Receptors in Anesthesia and Sedation: An Updated Review. CNS Drugs 2025, 39, 39–54. [Google Scholar] [CrossRef]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef]
- Garmon, E.H.; Huecker, M.R. Topical, Local, and Regional Anesthesia and Anesthetics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhao, M.; Zhou, M.; Lu, P.; Wang, Y.; Zeng, R.; Liu, L.; Zhu, S.; Kong, L.; Zhang, J. Local anesthetic delivery systems for the management of postoperative pain. Acta Biomater. 2024, 181, 1–18. [Google Scholar] [CrossRef]
- Zeng, S.; Qing, Q.; Xu, W.; Yu, S.; Zheng, M.; Tan, H.; Peng, J.; Huang, J. Personalized anesthesia and precision medicine: A comprehensive review of genetic factors, artificial intelligence, and patient-specific factors. Front. Med. 2024, 11, 1365524. [Google Scholar] [CrossRef]
- Liu, X.; Xue, Z.; Luo, M.; Ke, B.; Lv, J. Anesthetic drug discovery with computer-aided drug design and machine learning. Anesthesiol. Perioper. Sci. 2024, 2, 7. [Google Scholar] [CrossRef]
- Franks, N.P. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008, 9, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Li, G.D. GABAA receptors as molecular targets of general anesthetics: Identification of binding sites provides clues to allosteric modulation. Can. J. Anaesth. 2011, 58, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.C.; Liu, S.J. Role of HCN channels in the nervous system: Membrane excitability and various modulations. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2014, 30, 506–510. [Google Scholar] [PubMed]
- Kim, J.J.; Hibbs, R.E. Direct Structural Insights into GABAA Receptor Pharmacology. Trends Biochem. Sci. 2021, 46, 502–517. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lester, H.A.; Lummis, S.C. The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 2010, 43, 449–499. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Engin, E.; Liu, J.; Rudolph, U. α2-containing GABAA receptors: A target for the development of novel treatment strategies for CNS disorders. Pharmacol. Ther. 2012, 136, 142–152. [Google Scholar] [CrossRef]
- Mohamad, F.H.; Has, A.T.C. The alpha5-Containing GABAA Receptors-a Brief Summary. J. Mol. Neurosci. 2019, 67, 343–351. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, A.; Nan, X.; Yang, L.; Zhang, D.; Zhang, Z.; Liu, H. The Application and Pharmaceutical Development of Etomidate: Challenges and Strategies. Mol. Pharm. 2024, 21, 5989–6006. [Google Scholar] [CrossRef]
- Hung, C.C.; Chen, P.L.; Huang, W.M.; Tai, J.J.; Hsieh, T.J.; Ding, S.T.; Hsieh, Y.W.; Liou, H.H. Gene-wide tagging study of the effects of common genetic polymorphisms in the α subunits of the GABAA receptor on epilepsy treatment response. Pharmacogenomics 2013, 14, 1849–1856. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, H.; Clark, S.; Gouaux, E. Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology. Nature 2023, 622, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Lindemeyer, A.K.; Wallner, M.; Li, X.; Huynh, K.W.; Zhou, Z.H. Cryo-electron microscopy reveals informative details of GABAA receptor structural pharmacology: Implications for drug discovery. Ann. Transl. Med. 2019, 7, S144. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.; Rajkumar, N.; Singh, S.; Dubey, R.; Yadav, G. A Comparative Study of Propofol and Isoflurane Anaesthesia using Butorphanol in Neurosurgery. Indian J. Anaesth. 2009, 53, 324–329. [Google Scholar] [PubMed]
- Masiulis, S.; Desai, R.; Uchański, T.; Serna Martin, I.; Laverty, D.; Karia, D.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Kotecha, A.; et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef]
- Laverty, C.; Oliver, C.; Moss, J.; Nelson, L.; Richards, C. Persistence and predictors of self-injurious behaviour in autism: A ten-year prospective cohort study. Mol. Autism 2020, 11, 8. [Google Scholar] [CrossRef]
- Uchanski, T.; Masiulis, S.; Fischer, B.; Kalichuk, V.; Lopez-Sanchez, U.; Zarkadas, E.; Weckener, M.; Sente, A.; Ward, P.; Wohlkonig, A.; et al. Megabodies expand the nanobody toolkit for protein structure determination by single-particle cryo-EM. Nat. Methods 2021, 18, 60–68. [Google Scholar] [CrossRef]
- Maitra, R.; Reynolds, J.N. Subunit dependent modulation of GABAA receptor function by neuroactive steroids. Brain Res. 1999, 819, 75–82. [Google Scholar] [CrossRef]
- Bonin, R.P.; Orser, B.A. GABAA receptor subtypes underlying general anesthesia. Pharmacol. Biochem. Behav. 2008, 90, 105–112. [Google Scholar] [CrossRef]
- Li, Z.; Jin, X.; Wu, T.; Zhao, X.; Wang, W.; Lei, J.; Pan, X.; Yan, N. Structure of human Nav1.5 reveals the fast inactivation-related segments as a mutational hotspot for the long QT syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2100069118. [Google Scholar] [CrossRef]
- Pavel, M.A.; Petersen, E.N.; Wang, H.; Lerner, R.A.; Hansen, S.B. Studies on the mechanism of general anesthesia. Proc. Natl. Acad. Sci. USA 2020, 117, 13757–13766. [Google Scholar] [CrossRef]
- Forman, S.A. Clinical and molecular pharmacology of etomidate. Anesthesiology 2011, 114, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sridhar, A.; Teng, J.; Howard, R.J.; Lindahl, E.; Hibbs, R.E. Structural and dynamic mechanisms of GABAA receptor modulators with opposing activities. Nat. Commun. 2022, 13, 4582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, P. GABAA Receptor Subunit (γ2, δ, β1-3) Variants in Genetic Epilepsy: A Comprehensive Summary of 206 Clinical Cases. J. Child. Neurol. 2024, 39, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A. Extrasynaptic δ-subunit containing GABAA receptors. J. Integr. Neurosci. 2021, 20, 173–184. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Wu, H.; Lei, L.; Xu, S.; Shen, X.; Guo, X.; Shen, R.; Xia, X.; Liu, Y.; et al. Postoperative cognitive dysfunction: Current developments in mechanism and prevention. Med. Sci. Monit. 2014, 20, 1908–1912. [Google Scholar] [CrossRef]
- Heurteaux, C.; Guy, N.; Laigle, C.; Blondeau, N.; Duprat, F.; Mazzuca, M.; Lang-Lazdunski, L.; Widmann, C.; Zanzouri, M.; Romey, G.; et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004, 23, 2684–2695. [Google Scholar] [CrossRef]
- Nuno-Perez, A.; Mondoloni, S.; Tchenio, A.; Lecca, S.; Mameli, M. Biophysical and synaptic properties of NMDA receptors in the lateral habenula. Neuropharmacology 2021, 196, 108718. [Google Scholar] [CrossRef]
- Luscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef]
- Bender, P.A.; Chakraborty, S.; Durham, R.J.; Berka, V.; Carrillo, E.; Jayaraman, V. Bi-directional allosteric pathway in NMDA receptor activation and modulation. Nat. Commun. 2024, 15, 8841. [Google Scholar] [CrossRef]
- Ferreira, J.S.; Schmidt, J.; Rio, P.; Aguas, R.; Rooyakkers, A.; Li, K.W.; Smit, A.B.; Craig, A.M.; Carvalho, A.L. GluN2B-Containing NMDA Receptors Regulate AMPA Receptor Traffic through Anchoring of the Synaptic Proteasome. J. Neurosci. 2015, 35, 8462–8479. [Google Scholar] [CrossRef]
- Pochwat, B.; Nowak, G.; Szewczyk, B. An update on NMDA antagonists in depression. Expert Rev. Neurother. 2019, 19, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.P.; Nicole, O.; Groc, L. NMDA receptor functions in health and disease: Old actor, new dimensions. Neuron 2023, 111, 2312–2328. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Murata, K.; Wolf, M. Cryo-electron microscopy for structural analysis of dynamic biological macromolecules. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 324–334. [Google Scholar] [CrossRef]
- Zhu, S.; Stein, R.A.; Yoshioka, C.; Lee, C.H.; Goehring, A.; McHaourab, H.S.; Gouaux, E. Mechanism of NMDA Receptor Inhibition and Activation. Cell 2016, 165, 704–714. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, F.; Zhang, T.; Lv, S.; Zhou, L.; Du, D.; Lin, H.; Guo, F.; Luo, C.; Zhu, S. Structural basis of ketamine action on human NMDA receptors. Nature 2021, 596, 301–305. [Google Scholar] [CrossRef]
- Hashimoto, K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem. Pharmacol. 2020, 177, 113935. [Google Scholar] [CrossRef]
- Chou, T.H.; Tajima, N.; Romero-Hernandez, A.; Furukawa, H. Structural Basis of Functional Transitions in Mammalian NMDA Receptors. Cell 2020, 182, 357–371.e13. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Liu, J.; Yang, Y.; Wang, R.; Zhang, D.; Zhu, T. Development of NMDA receptors contributes to the enhancement of electroencephalogram oscillations under volatile anesthetics in rats. Front. Neural Circuits 2022, 16, 1065374. [Google Scholar] [CrossRef]
- Olsen, R.W. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology 2018, 136, 10–22. [Google Scholar] [CrossRef]
- Brenna, C.T.A.; Goldstein, B.I.; Zarate, C.A., Jr.; Orser, B.A. Repurposing General Anesthetic Drugs to Treat Depression: A New Frontier for Anesthesiologists in Neuropsychiatric Care. Anesthesiology 2024, 141, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Zarate, C.A., Jr.; Thompson, S.M. Molecular Pharmacology and Neurobiology of Rapid-Acting Antidepressants. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Suzuki, K.; Kavalali, E.T.; Monteggia, L.M. Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol. Med. 2023, 29, 364–375. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef]
- Zafirova, Z.; Sheehan, C.; Hosseinian, L. Update on nitrous oxide and its use in anesthesia practice. Best Pract. Res. Clin. Anaesthesiol. 2018, 32, 113–123. [Google Scholar] [CrossRef]
- Stroebel, D.; Paoletti, P. Architecture and function of NMDA receptors: An evolutionary perspective. J. Physiol. 2021, 599, 2615–2638. [Google Scholar] [CrossRef]
- Miladinovic, T.; Nashed, M.G.; Singh, G. Overview of Glutamatergic Dysregulation in Central Pathologies. Biomolecules 2015, 5, 3112–3141. [Google Scholar] [CrossRef]
- Abbott, J.A.; Wen, H.; Liu, B.; Gupta, S.S.; Iacobucci, G.J.; Zheng, W.; Popescu, G.K. Allosteric inhibition of NMDA receptors by low dose ketamine. Mol. Psychiatry 2024, 30, 1009–1018. [Google Scholar] [CrossRef]
- Chou, T.H.; Epstein, M.; Michalski, K.; Fine, E.; Biggin, P.C.; Furukawa, H. Structural insights into binding of therapeutic channel blockers in NMDA receptors. Nat. Struct. Mol. Biol. 2022, 29, 507–518. [Google Scholar] [CrossRef]
- Takahashi, H.; Xia, P.; Cui, J.; Talantova, M.; Bodhinathan, K.; Li, W.; Saleem, S.; Holland, E.A.; Tong, G.; Pina-Crespo, J.; et al. Pharmacologically targeted NMDA receptor antagonism by NitroMemantine for cerebrovascular disease. Sci. Rep. 2015, 5, 14781. [Google Scholar] [CrossRef]
- Apkarian, V.A.; Hashmi, J.A.; Baliki, M.N. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 2011, 152, S49–S64. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Alami, A.; Alexander, G.C.; Mattison, D.R. Safety and effectiveness of NMDA receptor antagonists for depression: A multidisciplinary review. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022, 42, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Lijffijt, M.; Ramakrishnan, N.; Vo-Le, B.; Vo-Le, B.; Iqbal, S.; Iqbal, T.; O’Brien, B.; Smith, M.A.; Swann, A.C.; et al. Does mismatch negativity have utility for NMDA receptor drug development in depression? Braz. J. Psychiatry 2022, 44, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Piacherski, V.; Marachkou, A. Pharmacological and clinical implications of local anaesthetic mixtures: A narrative review. Anaesthesia 2022, 77, 939. [Google Scholar] [CrossRef]
- Meretsky, C.R.; Plitt, V.E.; Friday, B.L.; Schiuma, A.T.; Ajebli, M. A Comparative Analysis of the Efficacy of Local Anesthetics and Systemic Anesthetics in the Red-Headed Versus Non-Red-Headed Patient Population: A Comprehensive Review. Cureus 2024, 16, e61797. [Google Scholar] [CrossRef]
- Jain, E.; Bubanale, S.C. Comparative study to assess the effect of ropivacaine and a mixture of lidocaine and bupivacaine on intraocular pressure after peribulbar anesthesia for cataract surgery. Indian J. Ophthalmol. 2022, 70, 3844–3848. [Google Scholar] [CrossRef]
- Stewart, Z.E. Safety of local anesthetics in cervical nerve root injections: A narrative review. Skelet. Radiol. 2023, 52, 1893–1900. [Google Scholar] [CrossRef]
- Payandeh, J.; Gamal El-Din, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 2012, 486, 135–139. [Google Scholar] [CrossRef]
- Beyder, A.; Strege, P.R.; Bernard, C.; Farrugia, G. Membrane permeable local anesthetics modulate NaV1.5 mechanosensitivity. Channels 2012, 6, 308–316. [Google Scholar] [CrossRef][Green Version]
- Cortada, E.; Brugada, R.; Verges, M. Role of protein domains in trafficking and localization of the voltage-gated sodium channel β2 subunit. J. Biol. Chem. 2024, 300, 107833. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Q.; Huang, G.; Jin, X.; Li, J.; Pan, X.; Yan, N. Dissection of the structure-function relationship of NaV channels. Proc. Natl. Acad. Sci. USA 2024, 121, e2322899121. [Google Scholar] [CrossRef] [PubMed]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The NaV1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef]
- Simkin, D.; Bendahhou, S. Skeletal muscle Na+ channel disorders. Front. Pharmacol. 2011, 2, 63. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Liu, P.; Xue, X.; Zhang, C.; Peng, L.; Shen, W.; Yang, S.; Wang, F. The Role of Photobiomodulation to Modulate Ion Channels in the Nervous System: A Systematic Review. Cell. Mol. Neurobiol. 2024, 44, 79. [Google Scholar] [CrossRef]
- Wang, T.; Huang, W.; Xu, K.; Sun, Y.; Zhang, Q.C.; Yan, C.; Li, Z.; Yan, N. CryoSeek II: Cryo-EM analysis of glycofibrils from freshwater reveals well-structured glycans coating linear tetrapeptide repeats. Proc. Natl. Acad. Sci. USA 2025, 122, e2423943122. [Google Scholar] [CrossRef]
- Shen, H.; Liu, D.; Wu, K.; Lei, J.; Yan, N. Structures of human NaV1.7 channel in complex with auxiliary subunits and animal toxins. Science 2019, 363, 1303–1308. [Google Scholar] [CrossRef]
- Shen, A.; Chen, D.; Kaur, M.; Bartels, P.; Xu, B.; Shi, Q.; Martinez, J.M.; Man, K.M.; Nieves-Cintron, M.; Hell, J.W.; et al. β-blockers augment L-type Ca2+ channel activity by targeting spatially restricted β2AR signaling in neurons. eLife 2019, 8, e49464. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Huang, Z.; Li, Y.; Yang, B.; Gong, J.; Jiang, D. Structural basis for NaV1.7 inhibition by pore blockers. Nat. Struct. Mol. Biol. 2022, 29, 1208–1216. [Google Scholar] [CrossRef]
- Kwong, K.; Carr, M.J. Voltage-gated sodium channels. Curr. Opin. Pharmacol. 2015, 22, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Getachew, M.; Tesfaye, H.; Yihunie, W.; Ayenew, T.; Alemu, S.; Dagnew, E.M.; Biyazin, Y.; Abebe, D.; Degefu, N.; Abebaw, A. Sustained release local anesthetics for pain management: Relevance and formulation approaches. Front. Pain Res. 2024, 5, 1383461. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br. J. Anaesth. 2002, 89, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Strichartz, G.R. State-Dependent Inhibition of Sodium Channels by Local Anesthetics: A 40-Year Evolution. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2012, 6, 120–127. [Google Scholar] [CrossRef]
- Kılınç, G. Management of local anesthetic toxicity and importance of lipid infusion. J. Surg. Med. 2019, 3, 406–410. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Mizogami, M. Interaction of local anesthetics with biomembranes consisting of phospholipids and cholesterol: Mechanistic and clinical implications for anesthetic and cardiotoxic effects. Anesthesiol. Res. Pract. 2013, 2013, 297141. [Google Scholar] [CrossRef]
- Aulestia-Viera, P.V.; Braga, M.M.; Borsatti, M.A. The effect of adjusting the pH of local anaesthetics in dentistry: A systematic review and meta-analysis. Int. Endod. J. 2018, 51, 862–876. [Google Scholar] [CrossRef]
- Kim, J.S.; Meeker, S.; Ru, F.; Tran, M.; Zabka, T.S.; Hackos, D.; Undem, B.J. Role of NaV1.7 in postganglionic sympathetic nerve function in human and guinea-pig arteries. J. Physiol. 2024, 602, 3505–3518. [Google Scholar] [CrossRef]
- Klint, J.K.; Smith, J.J.; Vetter, I.; Rupasinghe, D.B.; Er, S.Y.; Senff, S.; Herzig, V.; Mobli, M.; Lewis, R.J.; Bosmans, F.; et al. Seven novel modulators of the analgesic target NaV 1.7 uncovered using a high-throughput venom-based discovery approach. Br. J. Pharmacol. 2015, 172, 2445–2458. [Google Scholar] [CrossRef]
- Neal, J.M.; Bernards, C.M.; Butterworth, J.F.t.; Di Gregorio, G.; Drasner, K.; Hejtmanek, M.R.; Mulroy, M.F.; Rosenquist, R.W.; Weinberg, G.L. ASRA practice advisory on local anesthetic systemic toxicity. Reg. Anesth. Pain Med. 2010, 35, 152–161. [Google Scholar] [CrossRef]
- Syed, O.; Nemr, C.; Knezevic, N.N. Exparel and Beyond: Novel Local Anesthetics, Where We are and What is Coming up. Curr. Anesthesiol. Rep. 2024, 14, 490–496. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Zhang, Q.; Deng, Y.; Li, X.; Peng, L.; Zuo, X.; Piao, M.; Kuang, X.; Sheng, S.; et al. Recent advances in polymer-based drug delivery systems for local anesthetics. Acta Biomater. 2019, 96, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, H.C., Jr.; Riegelhaupt, P.M.; Kelz, M.B.; Solt, K.; Eckenhoff, R.G.; Orser, B.A.; Goldstein, P.A. Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharmacol. Sci. 2019, 40, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, J.V.; Pajouhesh, H.; Beckley, J.T.; Delwig, A.; Du Bois, J.; Hunter, J.C. Challenges and Opportunities for Therapeutics Targeting the Voltage-Gated Sodium Channel Isoform NaV1.7. J. Med. Chem. 2019, 62, 8695–8710. [Google Scholar] [CrossRef]
- Butterworth, J.F.t. Models and mechanisms of local anesthetic cardiac toxicity: A review. Reg. Anesth. Pain Med. 2010, 35, 167–176. [Google Scholar] [CrossRef]
- Plakhotnik, J.; Zhang, L.; Estrada, M.; Coles, J.G.; Lonnqvist, P.A.; Maynes, J.T. Local Anesthetic Cardiac Toxicity Is Mediated by Cardiomyocyte Calcium Dynamics. Anesthesiology 2022, 137, 687–703. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.; Li, J.; Yan, D.; Hu, Y.; Wu, P.; Fang, F.; McQuillan, P.M.; Hang, W.; Leng, J.; et al. General anesthetic agents induce neurotoxicity through astrocytes. Neural Regen. Res. 2024, 19, 1299–1307. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, Z.; Zhou, H.; Yan, R.; Li, Y.; Zhang, X.; Bao, L.; Yang, Y.; Zhang, J.; Song, S. Advances in Structural Biology for Anesthetic Drug Mechanisms: Insights into General and Local Anesthesia. BioChem 2025, 5, 18. https://doi.org/10.3390/biochem5020018
Liu H, Liu Z, Zhou H, Yan R, Li Y, Zhang X, Bao L, Yang Y, Zhang J, Song S. Advances in Structural Biology for Anesthetic Drug Mechanisms: Insights into General and Local Anesthesia. BioChem. 2025; 5(2):18. https://doi.org/10.3390/biochem5020018
Chicago/Turabian StyleLiu, Hanxiang, Zheng Liu, Huixian Zhou, Rongkai Yan, Yuzhen Li, Xiaofeng Zhang, Lingyu Bao, Yixin Yang, Jinming Zhang, and Siyuan Song. 2025. "Advances in Structural Biology for Anesthetic Drug Mechanisms: Insights into General and Local Anesthesia" BioChem 5, no. 2: 18. https://doi.org/10.3390/biochem5020018
APA StyleLiu, H., Liu, Z., Zhou, H., Yan, R., Li, Y., Zhang, X., Bao, L., Yang, Y., Zhang, J., & Song, S. (2025). Advances in Structural Biology for Anesthetic Drug Mechanisms: Insights into General and Local Anesthesia. BioChem, 5(2), 18. https://doi.org/10.3390/biochem5020018





