Antidiabetic and Antioxidant Activities of the Twigs of Andrograhis paniculata on Streptozotocin-Induced Diabetic Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extraction
2.2. Chemicals and Reagents
2.3. Experimental Animals
2.4. Induction of Diabetes
2.5. Experimental Design
2.6. Oral Glucose Tolerance Test (OGTT)
2.7. Estimation of Livid Profile Parameters
2.8. Estimation of Liver Function Parameters
2.9. Estimation of Kidney Functions Parameters
2.10. Statistical Analysis
3. Results
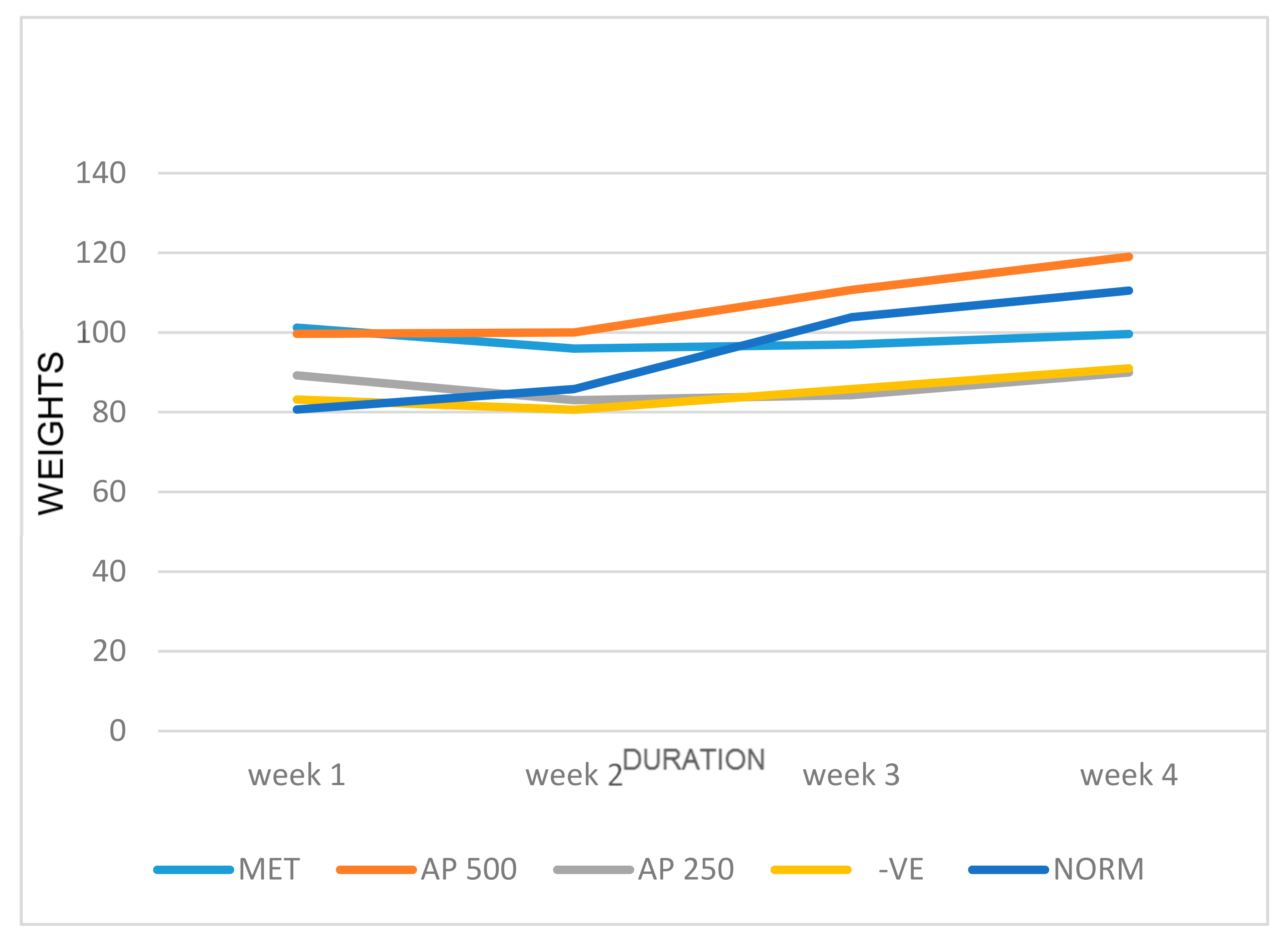
3.1. Effect of A. paniculata on Body Weight and Relative Organ Weights of STZ-Induced Diabetic Albino Rats
3.2. Effect of A. paniculata on Fasting Blood Glucose of STZ-Induced Diabetic Albino Rats
3.3. Effect of A. paniculata on Oral Glucose Tolerance Test in Non-Diabetic Albino Rats
3.4. Effect of A. paniculata on Oral Glucose Tolerance Test in Diabetic Albino Rats
3.5. Effect of A. paniculata and Metformin on Liver Function Test in Plasma
3.6. Effect of A. paniculata and Metformin on Lipid Profile in Plasma
3.7. Effect of A. paniculata and Metformin on Kidney Markers Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, G.C.; Sehgal, N.L.; Moloney, R.M.; Stafford, R.S. National Trends in Treatment of Type 2 Diabetes Mellitus. Arch. Int. Med. 2008, 168, 94–102. [Google Scholar] [CrossRef] [Green Version]
- WHO. Study Group on Diabetes Mellitus; Technical Report Series of a WHO Study Group; Second Report; A Meeting Held in Geneva, Switzerland, Published by World Health Organization. 1985. Available online: https://apps.who.int/iris/handle/10665/39592 (accessed on 17 June 2021).
- Crawford, J.M.; Cortran, R. Robbins Pathologic Basis of Disease; W.B. Saunders Co: Philadelphia, PA, USA, 2003; ISBN 0-8089-23021. [Google Scholar]
- Chinenye, S.; Young, E. State of Diabetes Care in Nigeria: A Review. Niger. Health J. 2011, 11, 101–106. [Google Scholar]
- Farnworth, N. Ethnopharmacology and Drug Development; John Wiley Books and Sons: Chichester, UK, 1994; ISBN 978-953-307-863-2. [Google Scholar]
- Sofowora, E.A. Medicinal Plants and Traditional Medicine in African, 2nd ed.; John Wiley Books and Sons: Ibadan, Nigeria, 1993; ISBN 8172361262. [Google Scholar]
- Chaudhary, G.; Goyal, S.; Poonia, P. Lawsonia inermis Linnaeus: A phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 91–98. [Google Scholar]
- Joy, P.; Thomas, J.; Mathew, S.; Skaria, B.P. Medicinal plants. Trop. Hortic. 1998, 2, 449–632. [Google Scholar]
- Kavishankar, G.; Lakshmidevi, N.; Murthy, S.M.; Prakash, H.; Niranjana, S. Diabetes and medicinal plants—A review. J. Pharm. Biomed. Sci. 2011, 2, 65–80. [Google Scholar]
- Akbar, S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Alt. Med. Rev. 2011, 16, 66–77. [Google Scholar]
- Kabir, M.H.; Hasan, N.; Rahman, M.M. A survey of medicinal plants used by the Deb barma clan of the Trivura tribe of Moulvibazar district, Bangladesh. J. Ethnobiol. Ethnomed. 2014, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Burkill, I.H.; Birtwistle, W.; Foxworthy, F.; Scrivenor, J.; Watson, J. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture and Co-Operatives: Kuala Lumpur, Malaysia, 1966.
- Ajaya, R.; Kumar, K.; Sridevi, N.; Vijaya, K.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharm. 2004, 92, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.J.; Yadava, N.S.; Tandon, J.S. Antisecretory (antidiarrhoeal) activity of Indian medicinal plants against Escherichia colientero toxin-induced secretion in rabbit and guinea pig ileal loop models. Int. J. Pharmacogn. 1993, 31, 198–204. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, A.; Tyagi, S. Quantitative HPLC analysis of andrographolide in Andrographis paniculata at two different stages of life cycle of plant. Acta Chim. Pharm. Indica 2012, 2, 1–7. [Google Scholar]
- Nanduri, S.; Nyavanandi, V.K.; Sanjeeva, S.; Thunuguntla, R. Synthesis and structure-activity relationshivs of andrographolide analogues as novel cytotoxic agents. Bioorg. Med. Chem. Lett. 2004, 14, 4711–4717. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.C.; Hung, C.R.; Chen, W.C.; Cheng, J.T. Antihyperglycemic effect of andrographolide in STZ-induced diabetic rats. Planta Med. 2003, 69, 1075–1079. [Google Scholar] [PubMed]
- Yu, B.C.; Chang, C.K.; Su, C.F.; Cheng, J.T. Mediation of β-endorphin in andrographolide-induced plasma glucose lowering action in type I diabetes-like animals. Naunyn-Schmiedeberg Arch. Pharmacol. 2008, 377, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chen, C.F.; Chiou, W.F. Andrographolide prevents oxygen radical production by human neutrophils: Possible mechanism(s) involved in its anti-inflammatory effect. Br. J. Pharmacol. 2002, 135, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Wiart, C.; Kumar, K.; Yusof, M.Y.; Hamimah, H.; Fauzi, Z.M.; Sulaiman, M. Antiviral properties of ent-labdene diterpenes of Andrographis paniculata Nees, inhibitors of herpes simplex virus type 1. Phytother. Res. 2005, 19, 1069–1070. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Zhari, I.; Mariam, A. Analysis of urinary andrographolides and antioxidant status after oral administration of Andrographis paniculata leaf extract in rats. Food Chem. Toxicol. 2008, 46, 3616–3620. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Tan, B.H. Mechanisms of cardiovascular activity of Andrographis paniculata in the anaesthetized rat. J. Ethnopharmacol. 1997, 56, 97–101. [Google Scholar] [CrossRef]
- Trivedi, N.P.; Rawal, U.M. Hepatoprotective and antioxidant property of Andrographis paniculata (Nees) in BHC induced liver damage in mice. Ind. J. Exp. Biol. 2001, 39, 41–46. [Google Scholar]
- Rana, A.C.; Avadhoot, Y. Hepatoprotective effects of Andrograhphis paniculata against carbon tetrachloride-induced liver damage. Arch. Pharm. Res. 1991, 14, 93–95. [Google Scholar] [CrossRef]
- Akbarsha, M.A.; Murugaian, P. Aspects of the male reproductive toxicity/male antifertility property of andrographolide in albino rats: Effect on the testis and the cauda epididymidal spermatozoa. Phytother. Res. 2000, 14, 432–435. [Google Scholar] [CrossRef]
- Oyebode, O.; Kandala, N.B.; Chilton, P.J.; Lilford, R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Oladele, S.; Ayo, J.; Adaudi, A. Medicinal and physiological properties of flavonoids, coumarin derivatives and anthraqoinones of plant origin. West Afr. J. Pharmacol. Drug Res. 2010, 11. [Google Scholar] [CrossRef]
- Dowling, R.; Godwin, J.P.; Stambolic, V. Understanding the benefit of metformin use in cancer treatment. Biomedcent. Med. 2011, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davern, T.J.; Scharschmidt, B.F. Biochemical liver tests. In Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, diagnosis, management. Saunders 2002, 7, 1227–1228. [Google Scholar]
- Gatsing, D.; Aliyu, R.; Kuiate, J.R.; Garba, I.H.; Jaryum, K.H.; Tedongma, N.; Tchouanguep, M.F.; Adoga, G.I. Toxicological evaluation of the aqueous extract of Allium sativum bulbs on laboratory mice and rats. Cameroon J. Exp. Biol. 2005, 1, 39–45. [Google Scholar] [CrossRef]
- Nduka, N. Clinical Biochemistry for Students of Pathology; Longman Nig. PLC: Abuja, Nigeria, 1999; Volume 1, pp. 157–168. [Google Scholar]
- Muntner, P.; Coresh, J.; Smith, J.C.; Eckfeldt, J.; Klag, M.J. Plasma livids and the risk of developing renal dysfunction: The atherosclerosis risk in communities study. Kidney Int. 2000, 58, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Aliyu, R.; Adebayo, A.H.; Gatsing, D.; Garba, I.H. The Effects of Ethanolic Leaf Extract of Commivhora africana (Burseraceae) on Rat Liver and Kidney Functions. J. Pharmacol. Toxicol. 2006, 2, 373–379. [Google Scholar] [CrossRef]
- Srisawat, N.; Itoste, E.E.A.; Kellum, J.A. Modern classification of acute kidney injury. Blood Purif. 2010, 29, 300–307. [Google Scholar] [CrossRef] [PubMed]
| GROUPS | DOSAGE (mg/kg) |
|---|---|
| Sham control | Distilled water (oral) |
| Metformin-100 mg | 60 mg/kg STZ (iv) + Metformin (oral) 100 mg/kg |
| Diabetic group | 60 mg/kg STZ (iv) |
| A. paniculata 250 mg | 60 mg/kg STZ (iv) + A. paniculata (oral) (250 mg/kg) |
| A. paniculata 500 mg | 60 mg/kg STZ (iv) + A. paniculata (oral) (500 mg/kg) |
| Group | Liver (g) | Kidney (g) | Spleen (g) |
|---|---|---|---|
| Sham control | 3.53 ± 0.09 # | 0.72 ± 0.04 #n | 0.54 ± 0.05 # |
| Metformin-100 mg | 4.51 ± 0.3 n* | 0.93 ± 0.03 * | 0.30 ± 0.06 * |
| Diabetic group | 3.61 ± 0.17 # | 0.90 ± 0.05 * | 0.38 ± 0.04 |
| A. paniculata 250 mg | 4.11 ± 0.64 | 0.91 ± 0.11 * | 0.70 ± 0.14 #n |
| A. paniculata 500 mg | 4.4 ± 0.26 * | 0.90 ± 0.09 | 0.73 ± 0.13 #n |
| Groups | % Reduction |
|---|---|
| Sham control | 7.96 |
| Metformin-100 mg | 81.73 |
| Diabetic group | 15.8 |
| A. paniculata 250 mg | 81.21 |
| A. paniculata 500 mg | 85.8 |
| Group | Zero | 30 Minutes | 60 Minutes | 120 Minutes |
|---|---|---|---|---|
| Sham control | 82.83 ± 6.61 | 151.83 ± 28.20 | 157.17 ± 12.05 # | 116.93 ± 7.52 # |
| Metformin-100 mg | 75.33 ± 2.96 | 105.67 ± 25.62 | 95.33 ± 12.33 * | 64.00 ± 8.14 * |
| A. paniculata-500 mg | 75.33 ± 4.26 | 162.33 ± 32.13 | 162.00 ± 10.50 # | 137.67 ± 21.17 # |
| Groups | Zero Time | 30 Minutes | 60 Minutes | 120 Minutes | 150 Minutes |
|---|---|---|---|---|---|
| Sham control | 82.8 ± 6.61 n | 151.8 ± 28.2 n | 157.2 ± 12.1 n | 116.2 ± 7.5 n | 89.3 ± 6.4 n |
| Metformin 100 mg | 188.2 ± 64.4 n | 159.2 ± 45.4 n | 94.6 ± 26.8 n | 74.6 ± 12.9 n | 70.8 ± 11.6 n |
| Diabetic group | 421.0 ± 42.4 #* | 495.4 ± 64.1 #* | 466.4 ± 76.2 #* | 423.4 ± 85.6 #* | 362.4 ± 78.9 #* |
| A. paniculata-250 mg | 236.8 ± 108.7 | 544.0 ± 19.1 #* | 490.0 ± 41.2 #* | 479.3 ± 46.5 #* | 358.5 ± 40.8 #* |
| A. paniculata-500 mg | 397.0 ± 125.3 * | 484.7 ± 75.3 #* | 454.7 ± 80.6 #* | 446.3 ± 98.7 #* | 377.3 ± 110.6 #* |
| Groups | Total Bilirubin (μmol/L) | Direct Bilirubin (μmol/L) |
|---|---|---|
| Sham control | 32.89 ± 1.7 | 22.02 ± 6.0 #n |
| Metformin-100 mg | 29.32 ± 2.4 n | 76.31 ± 8.0 *n |
| Diabetic group | 36.07 ± 1.1 # | 46.84 ± 0.4 *# |
| A. paniculata 250 mg | 42.46 ± 2.3 *#n | 111.96 ± 5.9 *#n |
| A. paniculata 500 mg | 24.94 ± 2.1 *n | 22.47 ± 1.0 #n |
| Group | ALP (U/l) | ALT (U/l) |
|---|---|---|
| Sham control | 187.99 ± 13.27 n | 68.52 ± 3.8 # |
| Metformin-100 mg | 233.13 ± 26.17 n | 86.68 ± 6.1 * |
| Diabetic group | 398.26 ± 77.56 *# | 77.24 ± 5.6 |
| A. paniculata 250 mg | 356.96 ± 0.000 * | 79.12 ± 3.0 |
| A. paniculata 500 mg | 392.8 ± 0.00 *# | 52.03 ± 13.2 #n |
| Group | Total Protein (g/L) | Albumin (g/L) |
|---|---|---|
| Sham control | 68.26 ± 3.5 | 43.7 ± 4.1 |
| Metformin-100 mg | 64.51 ± 4.4 | 36.1 ± 8.6 |
| Diabetic group | 63.26 ± 3.0 | 45.5 ± 8.1 |
| A. paniculata 250 mg | 68.60 ± 2.8 | 32.7 ± 0.21 |
| A. paniculata 500 mg | 71.66 ± 5.1 | 22.9 ± 3.5 *n |
| Group | HDL-Cholesterol (Mmol/L) | LDL (Mmol/L) |
|---|---|---|
| Sham control | 1.51 ± 0.2 # | 4.07 ± 1.1 n |
| Metformin-100 mg | 2.87 ± 0.14 * | 2.59 ± 0.11 *n |
| Diabetic group | 1.67 ± 0.22 # | 6.61 ± 0.50 *# |
| A. paniculata 250 mg | 1.69 ± 0.34 | 6.78 ± 1.1 # |
| A. paniculata 500 mg | 0.07 ± 1.41 *#n | 5.66 ± 1.1 # |
| Group | Triglyceride (Mmol/L) | Cholesterol (Mmol/L) |
|---|---|---|
| Sham control | 0.48 ± 0.13 | 4.5 ± 1.8 n |
| Metformin-100 mg | 1.19 ± 0.47 | 5.04 ± 0.99 n |
| Diabetic group | 1.14 ± 0.29 | 8.80 ± 0.38 *# |
| A. paniculata 250 mg | 0.64 ± 0.19 | 8.77 ± 0.89 * |
| A. paniculata 500 mg | 0.44 ± 0.07 | 5.94 ± 0.92 |
| Group | Potassium (mEg/L) | Inorganic Phosphorus (Mmol/L) |
|---|---|---|
| Sham control | 10.67 ± 0.14 #n | 5.22 ± 1.3 |
| Metformin-100 mg | 13.99 ± 1.8 *n | 4.80 ± 0.74 |
| Diabetic group | 6.55 ± 0.61 *# | 3.64 ± 0.56 |
| A. paniculata 250 mg | 7.53 ± 0.50 *# | 3.78 ± 1.50 |
| A. paniculata 500 mg | 7.33 ± 0.99 *# | 3.87 ± 0.89 |
| Group | Creatinine (μmol/L) |
|---|---|
| Sham control | 73.5 ± 2.2 |
| Metformin-100 mg | 90.19 ± 8.8 |
| Diabetic group | 81.69 ± 13.2 |
| A. paniculata 250 mg | 91.5 ± 0.00 *#n |
| A. paniculata 500 mg | 75.02 ± 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunlana, O.O.; Adetuyi, B.O.; Esalomi, E.F.; Rotimi, M.I.; Popoola, J.O.; Ogunlana, O.E.; Adetuyi, O.A. Antidiabetic and Antioxidant Activities of the Twigs of Andrograhis paniculata on Streptozotocin-Induced Diabetic Male Rats. BioChem 2021, 1, 238-249. https://doi.org/10.3390/biochem1030017
Ogunlana OO, Adetuyi BO, Esalomi EF, Rotimi MI, Popoola JO, Ogunlana OE, Adetuyi OA. Antidiabetic and Antioxidant Activities of the Twigs of Andrograhis paniculata on Streptozotocin-Induced Diabetic Male Rats. BioChem. 2021; 1(3):238-249. https://doi.org/10.3390/biochem1030017
Chicago/Turabian StyleOgunlana, Olubanke O., Babatunde O. Adetuyi, Elohor F. Esalomi, Miracle I. Rotimi, Jacob O. Popoola, Oluseyi E. Ogunlana, and Oluwatosin A. Adetuyi. 2021. "Antidiabetic and Antioxidant Activities of the Twigs of Andrograhis paniculata on Streptozotocin-Induced Diabetic Male Rats" BioChem 1, no. 3: 238-249. https://doi.org/10.3390/biochem1030017
APA StyleOgunlana, O. O., Adetuyi, B. O., Esalomi, E. F., Rotimi, M. I., Popoola, J. O., Ogunlana, O. E., & Adetuyi, O. A. (2021). Antidiabetic and Antioxidant Activities of the Twigs of Andrograhis paniculata on Streptozotocin-Induced Diabetic Male Rats. BioChem, 1(3), 238-249. https://doi.org/10.3390/biochem1030017







