Intraoral Sialadenoma Papilliferum: A Comprehensive Review of the Literature with Emphasis on Clinical and Histopathological Diagnostic Features
Abstract
1. Introduction
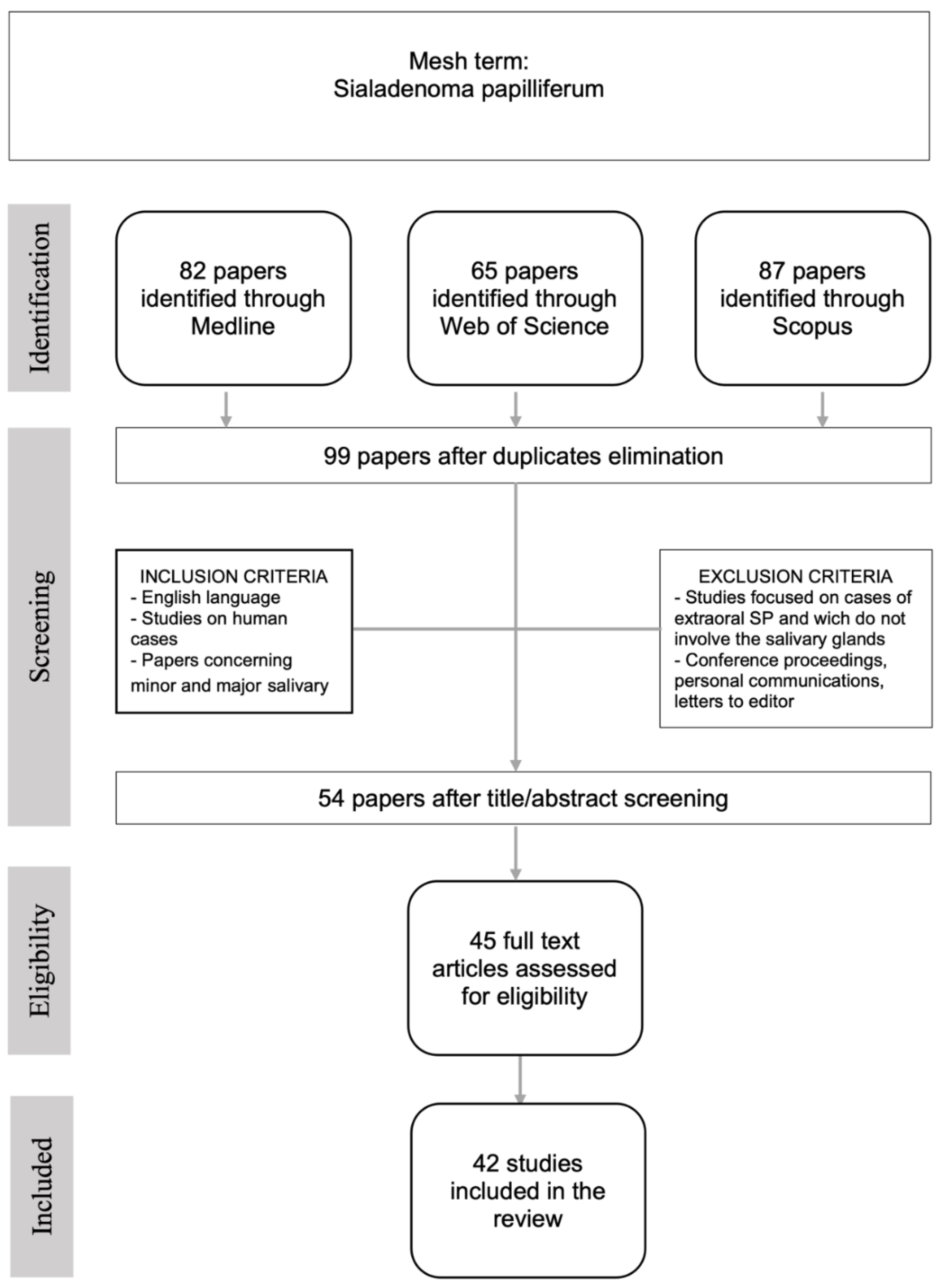
2. Materials and Methods
3. Results
3.1. Age and Sex
3.2. Clinical Features
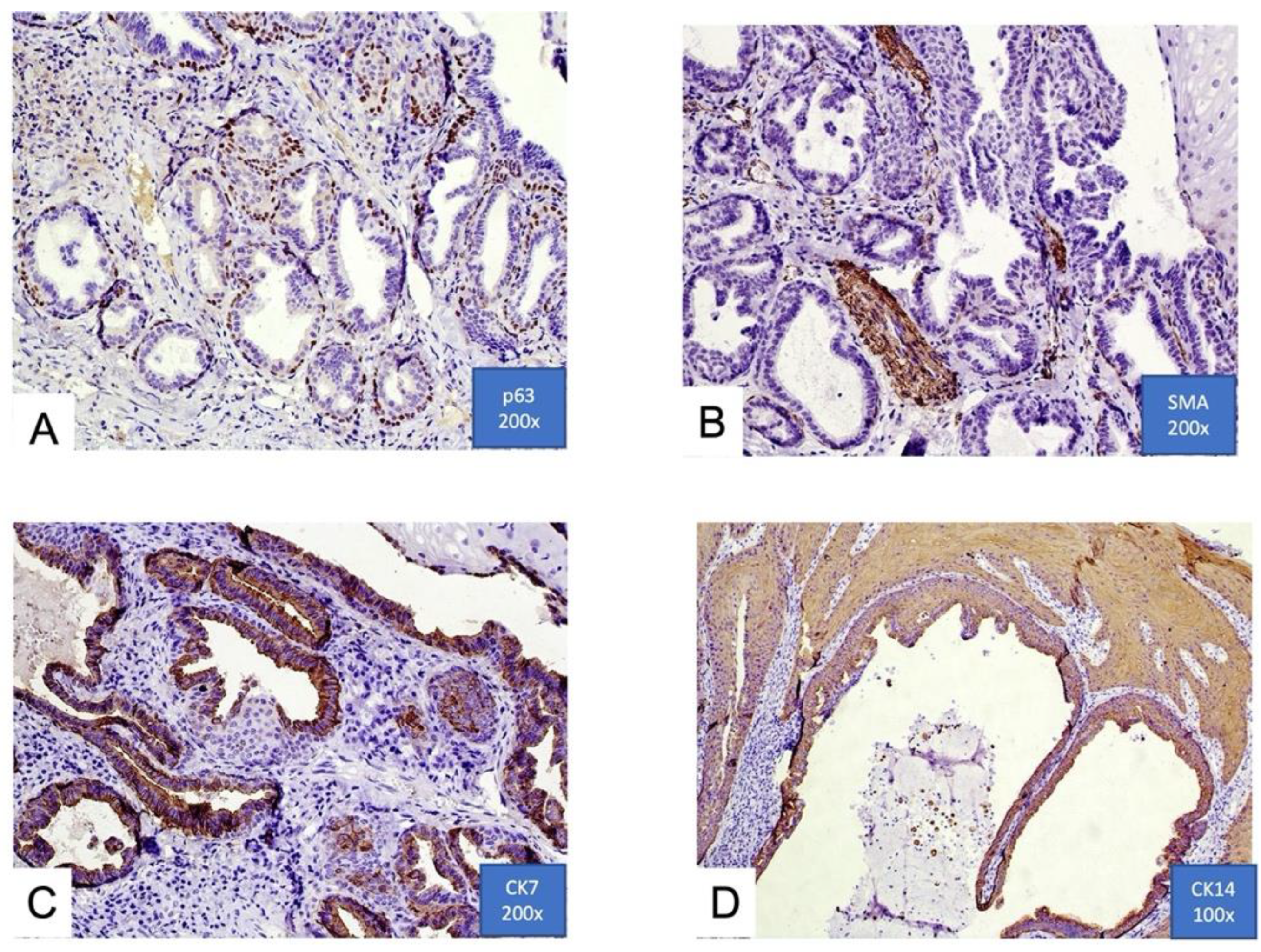
3.3. Histological Features
3.4. Treatment
3.5. Prognosis
3.6. Possible Malignant Transformation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abrams, A.M.; Finck, F.M. Sialadenoma papilliferum: A previously un-reported salivary gland tumor. Cancer 1969, 24, 1057–1063. [Google Scholar] [CrossRef]
- Seethala, R.R.; Stenman, G. Update from the 4th edition of the world health organization classification of head and neck tumours: Tumors of the salivary gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Waldron, C.A.; El-Mofty, S.K.; Gnepp, D.R. Tumors of the intraoral minor salivary glands: A demographic and histologic study of 426 cases. Oral Surg. Oral Med. Oral Pathol. 1988, 66, 323–333. [Google Scholar] [CrossRef]
- Seifert, G.; Brocheriou, C.; Cardesa, A.; Eveson, J.W. WHO international classification of tumors. Tentative histological classification of salivary gland tumours. Pathol. Res. Pract. 1990, 186, 555–581. [Google Scholar] [CrossRef]
- Crocker, D.J.; Christ, T.F.; Cavalaris, C.J. Sialadenoma Papilliferum: Report of Case. J. Oral Surg. 1972, 30, 520–521. [Google Scholar]
- Jensen, J.L.; Reingold, I.M. Sialadenoma Papillifemm of the Oral Cavity. Oral Surg. Oral Med. Oral Pathol. 1973, 35, 521–525. [Google Scholar] [CrossRef]
- Whittaker, J.S.; Mer, E.E. Papillary tumors of the minor salivary glands. J. Clin. Pathol. 1976, 29, 795–805. [Google Scholar] [CrossRef]
- Drummond, J.F.; Giansanti, J.S.; Sabes, W.R.; Smith, C.R. Sialadenoma papilliferum of the oral cavity. Oral Surg. Oral Med. Oral Pathol. 1978, 45, 72–75. [Google Scholar] [CrossRef]
- Freedman, P.D.; Lumerman, H. Sialoadenoma papilliferum. Oral Surg. 1978, 45, 88–94. [Google Scholar] [CrossRef]
- Solomon, M.P.; Rosen, Y.; Alfonso, A. Intraoral papillary squamous cell tumor of the soft palate with features of sialadenoma papilliferum? Malignant sialadenoma papilliferum. Cancer 1978, 42, 1859–1869. [Google Scholar]
- Mccoy, J.M.; Eckert, J.R.E.F. Sialoadenoma papilliferum. J. Oral Surg. 1980, 38, 691–693. [Google Scholar] [PubMed]
- Nasu, M.; Takagi, M.; Ishikawa, G. Sialoadenoma papilliferum. Report of a case. J. Oral Surg. 1981, 39, 367–369. [Google Scholar] [PubMed]
- Wertheimer, F.W.; Bank, K.; Ruskin, W.J. Sialadenoma papilliferum. Int. J. Oral Surg. 1983, 12, 190–193. [Google Scholar] [CrossRef]
- Puts, J.J.; Voorsmit, R.A.; Van Haelst, U.J. Sialocystadenoma papilliferum of the palate. J. Maxillofac. Surg. 1984, 12, 904. [Google Scholar] [CrossRef]
- Rennie, J.S.; MacDonald, D.G.; Critchlow, H.A. Sialadenoma papilliferum. A case report and review of the literature. Int. J. Oral Surg. 1984, 13, 452–454. [Google Scholar] [CrossRef]
- Shirasuna, K.; Watatani, K.; Mlyazakl, T. Ultrastructure of a sialadenoma papilliferum. Cancer 1984, 53, 468–474. [Google Scholar] [CrossRef]
- Bass, K.D.; Cosentino, B.J. Sialoadenoma papilliferum. J. Oral Maxillofac. Surg. 1985, 43, 302–304. [Google Scholar] [CrossRef]
- Regezi, J.A.; Lloyd, R.V.; Zarbo, R.J.; Mcclatchey, K.D. Minor salivary gland tumors. A histologic and immunohisto chemical study. Cancer 1985, 55, 108–115. [Google Scholar] [CrossRef]
- Fantasia, J.E.; Nocco, C.E.; Lally, E.T. UItrastructureof sialadenoma papilliferum. Arch. Pathol. Lab. Med. 1986, 110, 523–527. [Google Scholar]
- Mitre, B.K. Sialoadenoma papilliferum: Report of case and review of literature. J. Oral Maxillofac. Surg. 1986, 44, 469–474. [Google Scholar] [CrossRef]
- Papanicolaou, S.J.; Triantafyllou, A.G. Sialadenoma papilliferam of the oral cavity: A case report and review of the literature. J. Oral Med. 1987, 42, 57. [Google Scholar] [PubMed]
- Van der Wal, J.E.; Van der Waal, I. The rare sialadenoma papilliferum: Report of a case and review of the literature. Int. J. Oral Maxillofac. Surg. 1992, 21, 104–106. [Google Scholar] [CrossRef]
- Pimentel, M.T.; Lopez Amado, M.; Garcia, S.A. Recurrent sialadenoma papilliferum of the buccal mucosa. J. Laryngol. Otol. 1995, 109, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, E.; Favia, G.; Ricco, R. Sialadenoma papilliferum: An immunohistochemical study of five cases. J. Oral Pathol. Med. 1996, 25, 336–342. [Google Scholar] [CrossRef]
- Markopoulos, A.; Kayavis, I.; Papanayotou, P. Sialadenoma papilliferum of the oral cavity: Report of a case and literature review. J. Oral Maxillofac. Surg. 1997, 55, 1181–1184. [Google Scholar] [CrossRef]
- Asahina, I.; Masato, A. Sialadenoma papilliferum of the hard palate: A case report of a case and review of literature. J. Oral Maxillofac. Surg. 1997, 55, 1000–1003. [Google Scholar] [CrossRef]
- Argyres, M.I.; Golitz, L.E. Sialadenoma papilliferum of the palate: Case report and literature review. J. Cutan. Pathol. 1999, 26, 259–262. [Google Scholar] [CrossRef]
- Brannon, R.B.; Sciubba, J.J.; Giulani, M. Ductal papillomas of salivary gland origin: A report of 19 cases and a review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 68–77. [Google Scholar] [CrossRef]
- Ubaidat, M.A.; Robinson, R.A.; Belding, P.J.; Merryman, D.J. Sialadenoma papilliferum of the hard palate: Report of 2 cases and immunohistochemical evaluation. Arch. Pathol. Lab. Med. 2001, 125, 1595–1597. [Google Scholar] [CrossRef]
- Shimoda, M.; Kameyama, K.; Morinaga, S.; Tanaka, Y.; Hashiguchu, K.; Shimada, M.; Okara, Y. Malignant transformation of sialadenoma papilliferum of the palate: A case report. Virchows Arch. 2004, 445, 641–646. [Google Scholar] [CrossRef]
- Gomes, A.P.; Sobral, A.P.; Loducca, S.V.; de Araujo, V.C. Sialadenoma papilliferum: Immunohistochemical study. Int. J. Oral Maxillofac. Surg. 2004, 33, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Khurana, N.; Setia, N. Sialadenoma papilliferum in a young patient: A case report and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ponniah, I. A rare case of sialadenoma papilliferum with epithelial dysplasia and carcinoma in situ. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Vicente, O.P.; Marques, N.A.; Aytes, L.B.; Escoda, C.G. Minor salivary gland tumors: A clinicopathological study of 18 cases. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, 582–588. [Google Scholar]
- Liu, W.; Gnepp, D.R.; de Vries, E.; Bibawy, H.; Solomon, M.; Gloster, E.S. Mucoepidermoid carcinoma arising in a background of sialadenoma papilliferum: A case report. Head Neck Pathol. 2009, 3, 59–62. [Google Scholar] [CrossRef]
- Ide, F.; Kikuchi, K.; Kusama, K.; Kanazawa, H. Sialadenoma papilliferum with potentially malignant features. J. Clin. Pathol. 2010, 63, 362–364. [Google Scholar] [CrossRef]
- Kubota, Y.; Kubo, C.; Mori, Y. Sialadenoma Papilliferum of the Hard Palate: A Case Report. J. Oral Maxillofac. Surg. 2011, 70, 1609–1612. [Google Scholar] [CrossRef]
- Anuradha, A.; Ram Prasad, V.V.; Kashyap, B.; Srinivas, V. Sialadenoma papilliferum: Clinical misdiagnosis with a histological decree. Case Rep. Dent. 2012, 2012, 356271. [Google Scholar] [CrossRef]
- Reis de Sá Silva e Costa, F.E.; Vizcaíno Väzquez, J.R. Sialadenoma Papilliferum with inverted pattern in a young patient: A case report. Am. J. Case Rep. 2015, 16, 663–666. [Google Scholar] [CrossRef][Green Version]
- Santos, J.N.; Barros, A.C.; Gurgel, C.A.; Ramalho, L.M.P. Sialadenoma papilliferum of the tongue mimicking a malignant tumor. Braz. J. Otorhinolaryngol. 2013, 79, 404. [Google Scholar] [CrossRef]
- Fowler, C.B.; Damm, D.D. Sialadenoma Papilliferum: Analysis of Seven New Cases and Review of the Literature. Head Neck Pathol. 2018, 12, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Ogawa, T.; Chikazu, D. Sialadenoma papilliferum in the buccal mucosa detected on (18)F-fluorodeoxyglucose-positron emission tomography. Br. J. Oral Maxillofac. Surg. 2017, 55, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Sunil, S.; Babu, S.S.; Panicker, S.; Pratap, N. Sialadenoma papilliferum: A rare case report and review of literature. J. Cancer Res. Ther. 2017, 13, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Atarbashi-Moghadam, S.; Lotfi, A.; Moshref, M.; Mokhtari, S. Sialadenoma papilliferum of the hard palate: A rare case report. Indian J. Pathol. Microbiol. 2019, 62, 163–164. [Google Scholar] [PubMed]
- Kronenberg, J.; Horowitz, A.; Leventon, G. Sialoadenoma papilliferum of the parotid gland. J. Laryngol. Otol. 1989, 103, 1089–1090. [Google Scholar] [CrossRef]
- Loehn, B.; Sutton, C.; Jastram-Belcher, J.; Harton, A.; Anderson, D.; Walvekar, R.R. Sialadenoma papilliferum of the parotid gland: Case report and review of literature. Head Neck 2013, 35, E74–E76. [Google Scholar] [CrossRef]
- Grushka, M.; Podoshin, L.; Boss, J.H.; Fradis, M. Sialadenoma papilliferum of the parotid gland. Laryngoscope 1984, 94, 231–233. [Google Scholar] [CrossRef]
- Takasugi, N.; Yoshida, H.; Tani, M.; Ikeda, H.; Kitayoshi, M.; Iseki, T.; Akiyama, H.; Kotaki, S.; Ikeda, C.; Tominaga, K. Sialadenoma papilliferum: Special staining and immunohistochemical staining. J. Oral Maxillofac. Surg. 2021, 33, 358–361. [Google Scholar] [CrossRef]
- Melo, G.M.; Cervantes, O.; Abrahao, M.; Covolan, L.; Ferreira, E.S.; Baptista, H.A. A brief history of salivary gland surgery. Rev. Colégio Bras. Cir. 2017, 44, 403–412. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Bishop, J.A.; Yu Fong Chang, J. Sialadenoma Papilliferum. Surg. Pathol. Clin. 2021, 14, 43–51. [Google Scholar] [CrossRef]
- Vaidya, A.D.; Pantvaidya, G.H.; Metgudmath, R.; Kane, S.V.; D’Cruz, A.K. Minor salivary gland tumors of the oral cavity: A case series with review of literature. J. Cancer Res. Ther. 2012, 8 (Suppl. S1), S111–S115. [Google Scholar] [PubMed]


| Title | Authors | Year | n° Cases Reported | Age, Sex | Oral Subsite | Size of Lesions (cm) |
|---|---|---|---|---|---|---|
| Sialoadenoma papilliferum. A Previously Unreported Salivary Gland Tumor [1] | Abrams, A.M. and Finck, F.M. | 1969 | 1 | 57, M | Hard and soft right palate junction | 1.5 |
| Sialadenoma papilliferum: report of case [5] | Crocker, D.J., et al. | 1972 | 1 | 71, M | Left buccal mucosa | 0.6 |
| SialadenomaPapillifemm of the Oral Cavity [6] | Jensen, J.L. and Reingold, I.M. | 1973 | 1 | 48, M | Hard palate | 0.8 |
| Papillary tumors of the minor salivary glands [7] | Whittaker, J.P. and Mer, E.E. | 1976 | 2 | 50, M | Hard and soft palate junction | 0.6 |
| 65, M | Hard palate | N/A | ||||
| Sialoadenoma papilliferum of the oral cavity [8] | Drummond, J.F., et al. | 1978 | 1 | 71, M | Left mandibular retromolar area | 0.5 |
| Sialoadenoma papilliferum [9] | Freedman, P.D. and Lumerman, H. | 1978 | 2 | 68, M | Hard palate lateral to the midline | 0.3 |
| 68, M | Left hard palate | 0.5 | ||||
| Intraoral papillary squamous cell tumor of the soft palate with features of sialadenoma papilliferum? Malignant sialadenoma papilliferum [10] | Solomon, M.P., et al. | 1978 | 1 | 62, M | Soft palate | 4.0 |
| Sialoadenoma papilliferum [11] | Mccoy, J.M. and Eckert, J.R.E.F. | 1980 | 1 | 77, F | Right buccal mucosa | 0.7 |
| Sialoadenoma papilliferum. Report of a case [12] | Nasu, M., et al. | 1981 | 1 | 61, M | Hard palate | 0.6 |
| Sialoadenoma papilliferum [13] | Wertheimer, F.W., et al. | 1983 | 2 | 32, F | Hard palate | 0.4 |
| 43, M | Soft palate | 0.5 | ||||
| Sialocystadenoma papilliferum of the palate [14] | Puts, J.J., et al. | 1984 | 1 | 71, M | Hard palate | 1.6 |
| Sialadenoma papilliferum. A case report and review of the literature [15] | Rennie, J.S., et al. | 1984 | 1 | 78, F | Hard and soft palate junction | 1.0 |
| Ultrastructure of a sialadenoma papilliferum [16] | Kanemitsu, S., et al. | 1984 | 1 | 58, M | Hard palate | 0.7 |
| Sialoadenoma papilliferum [17] | Bass, K.D. and Cosentino, B.J. | 1985 | 1 | 76, F | Left faucial pillar | 1.0 |
| Minor salivary gland tumors. A histologic and immunohisto chemical study [18] | Regezi, J.A., et al. | 1985 | 2 | 63, M | Hard palate | N/A |
| 79, F | Hard palate | N/A | ||||
| UItrastructure of sialadenoma papilliferum [19] | Fantasia, J.E., et al. | 1986 | 5 | 87, F | Hard palate | N/A |
| 77, M | Buccal mucosa | N/A | ||||
| 48, F | Hard palate | N/A | ||||
| 45, M | Hard palate | N/A | ||||
| 60, F | Mucosa upper lip | N/A | ||||
| Sialoadenoma papilliferum: report of case and review of literature [20] | Mitre, B.K. | 1986 | 1 | 42, F | Hard and soft palate junction | 0.4 |
| Sialadenoma papilliferum of the oral cavity: a case report and review of the literature [21] | Papanicolaou, S. and Triantafyllou, A.G. | 1987 | 1 | 46, M | Hard palate | 0.5 |
| The rare sialoadenoma papilliferum—report of a case and review of the literature [22] | Van der Wal, J.E. and van der Waal, I. | 1991 | 1 | 46, M | Hard and soft palate junction | 0.5 |
| Recurrent sialadenoma papilliferum of the buccal mucosa [23] | Pimentel, M.T.Y., et al. | 1995 | 1 | 65, F | Buccal mucosa | 2.0 |
| Sialadenoma papilliferum: an immunohistochemical study of five cases [24] | Maiorano, E., et al. | 1996 | 5 | 56, M | Hard palate | 0.5 |
| 37, F | Hard palate | 1.0 | ||||
| 60, M | Cheek | 0.8 | ||||
| 46, M | Hard palate | 1.4 | ||||
| 50, M | Hard palate | 1.8 | ||||
| Sialadenoma papilliferum of the oral cavity: report of a case and literature review [25] | Markopoulos, A., et al. | 1997 | 1 | 50, M | Hard palate | 0.5 |
| Sialadenoma papilliferum of the hard palate: a case report of a case and review of literature [26] | Asahina, I. and Masato, A. | 1997 | 1 | 50, M | Hard palate | 0.4 |
| Sialadenoma papilliferum of the palate: case report and literature review [27] | Argyres, M.I. and Golitz, L.E. | 1998 | 1 | 50, M | Hard palate | 0.5 |
| Ductal papillomas of salivary gland origin: A report of 19 cases and a review of the literature [28] | Brannon, R.B., et al. | 2001 | 3 | 69, F | Hard palate | N/A |
| 53, F | Soft palate | N/A | ||||
| 31, F | Hard palate | N/A | ||||
| Sialadenoma papilliferum of the hard palate—Report of 2 cases and immunohistochemical evaluation [29] | Ubaidat, M.A., et al. | 2001 | 2 | 72, M | Hard palate | 0.4 |
| 58, M | Hard palate | 0.5 | ||||
| Malignant transformation of sialadenoma papilliferum of the palate: a case report [30] | Shimoda, M., et al. | 2004 | 1 | 79, F | Hard and soft palate junction | 4.0 |
| Sialadenoma papilliferum: Immunohistochemical study [31] | Gomes, A.P.N., et al. | 2004 | 2 | 53, M | Hard palate | 1.0 |
| 52, F | Soft palate | 0.5 | ||||
| Sialadenoma papilliferum in a young patient: a case report and review of the literature [32] | Mahajan, D., et al. | 2007 | 1 | 18, M | Upper lip | 0.8 |
| A rare case of sialadenoma papilliferum with epithelial dysplasia and carcinoma in situ [33] | Ponniah, I. | 2007 | 1 | 30, M | Floor of the mouth | 1.5 |
| Minor salivary gland tumors: A clinicopathological study of 18 cases [34] | Vicente, O.P., et al. | 2008 | 1 | 46, F | Hard palate | N/A |
| Mucoepidermoid carcinoma arising in a background of sialadenoma papilliferum: A case report [35] | Liu, W., et al. | 2009 | 1 | 82, F | Left base of the tongue | N/A |
| Sialadenoma papilliferum with potentially malignant features [36] | Ide, F., et al. | 2010 | 1 | 67, M | Right retromolar alveolar ridge | 3.0 |
| Sialadenoma papilliferum of the hard palate: A case report [37] | Kubota, Y., et al. | 2012 | 1 | 62, M | Hard palate | 1.0 |
| Sialadenoma papilliferum: clinical misdiagnosis with a histological decree [38] | Anuradha, A., et al. | 2012 | 1 | 65, M | Floor of the mouth | 1.0 |
| Sialadenoma Papilliferum with inverted pattern in a young patient: a case report [39] | Reis de Sá Silva e Costa, F.E., et al. | 2015 | 1 | 20, M | Upper lip buccal mucosa | 1.3 |
| Sialadenoma papilliferum of the tongue mimicking a malignant tumor [40] | Santos, J.N., et al. | 2013 | 1 | 32, F | Posteriore lateral border of the tongue | 1.0 |
| Sialadenoma Papilliferum: Analysis of Seven New Cases and Review of the Literature [41] | Fowler, C.B. and Damm, D.D. | 2017 | 7 | 55, F | Hard palate | 0.3 |
| 50, M | Hard palate | 0.8 | ||||
| 62, M | Hard palate | N/A | ||||
| 63, M | Hard palate | N/A | ||||
| 57, M | Palate | 0.4 | ||||
| 48, F | Hard palate | 0.5 | ||||
| 76, F | Hard palate | 1.3 | ||||
| Sialadenoma papilliferum in the buccal mucosa detected on (18)F-fluorodeoxyglucose-positron emission tomography [42] | Miyamoto, S., et al. | 2017 | 1 | 53, M | Left buccal mucosa | 0.8 |
| Sialadenoma papilliferum: A rare case report and review of literature [43] | Sunil, S., et al. | 2017 | 1 | 58, F | Hard palate | 1.0 |
| Sialadenoma papilliferum of the hard palate: A rare case report [44] | Atarbashi-Moghadam, S., et al. | 2019 | 1 | 50, F | Hard palate | 1.0 |
| Sialadenoma papilliferum: Special staining and immunohistochemical staining [45] | Takasugi, N., et al. | 2021 | 1 | 83, F | Hard palate | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonelli, R.; Paes de Almeida, O.; Bologna-Molina, R.; Meleti, M. Intraoral Sialadenoma Papilliferum: A Comprehensive Review of the Literature with Emphasis on Clinical and Histopathological Diagnostic Features. Oral 2022, 2, 242-250. https://doi.org/10.3390/oral2030023
Antonelli R, Paes de Almeida O, Bologna-Molina R, Meleti M. Intraoral Sialadenoma Papilliferum: A Comprehensive Review of the Literature with Emphasis on Clinical and Histopathological Diagnostic Features. Oral. 2022; 2(3):242-250. https://doi.org/10.3390/oral2030023
Chicago/Turabian StyleAntonelli, Rita, Oslei Paes de Almeida, Ronell Bologna-Molina, and Marco Meleti. 2022. "Intraoral Sialadenoma Papilliferum: A Comprehensive Review of the Literature with Emphasis on Clinical and Histopathological Diagnostic Features" Oral 2, no. 3: 242-250. https://doi.org/10.3390/oral2030023
APA StyleAntonelli, R., Paes de Almeida, O., Bologna-Molina, R., & Meleti, M. (2022). Intraoral Sialadenoma Papilliferum: A Comprehensive Review of the Literature with Emphasis on Clinical and Histopathological Diagnostic Features. Oral, 2(3), 242-250. https://doi.org/10.3390/oral2030023








