Imatinib in Targeted Therapy: Advances in Biomedical Applications and Drug Delivery Systems
Abstract
1. Introduction
2. Methods
3. Chemical Properties and Pharmacological Profile of IMT
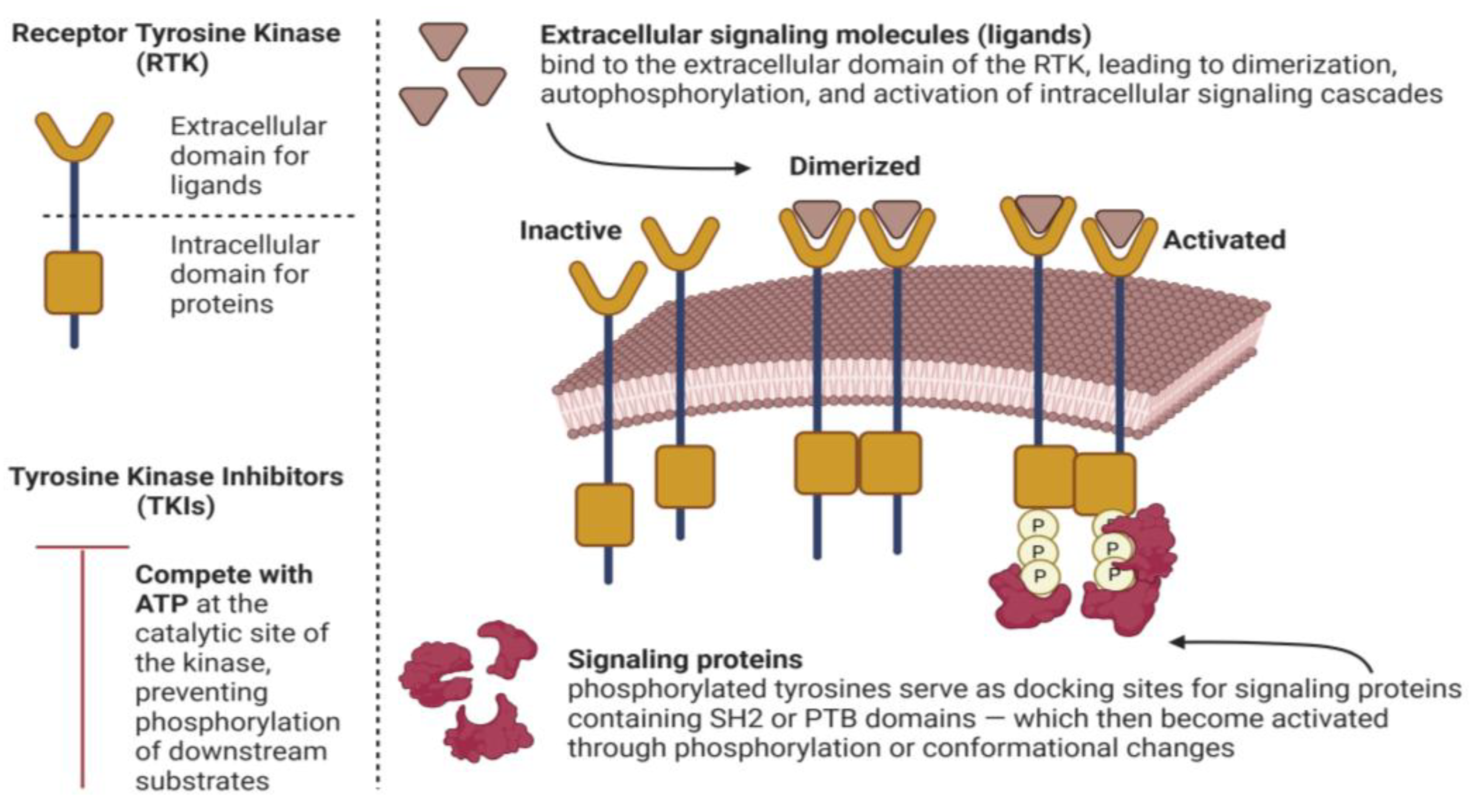
3.1. Mechanism of Action
3.2. Pharmacokinetics and Pharmacodynamics
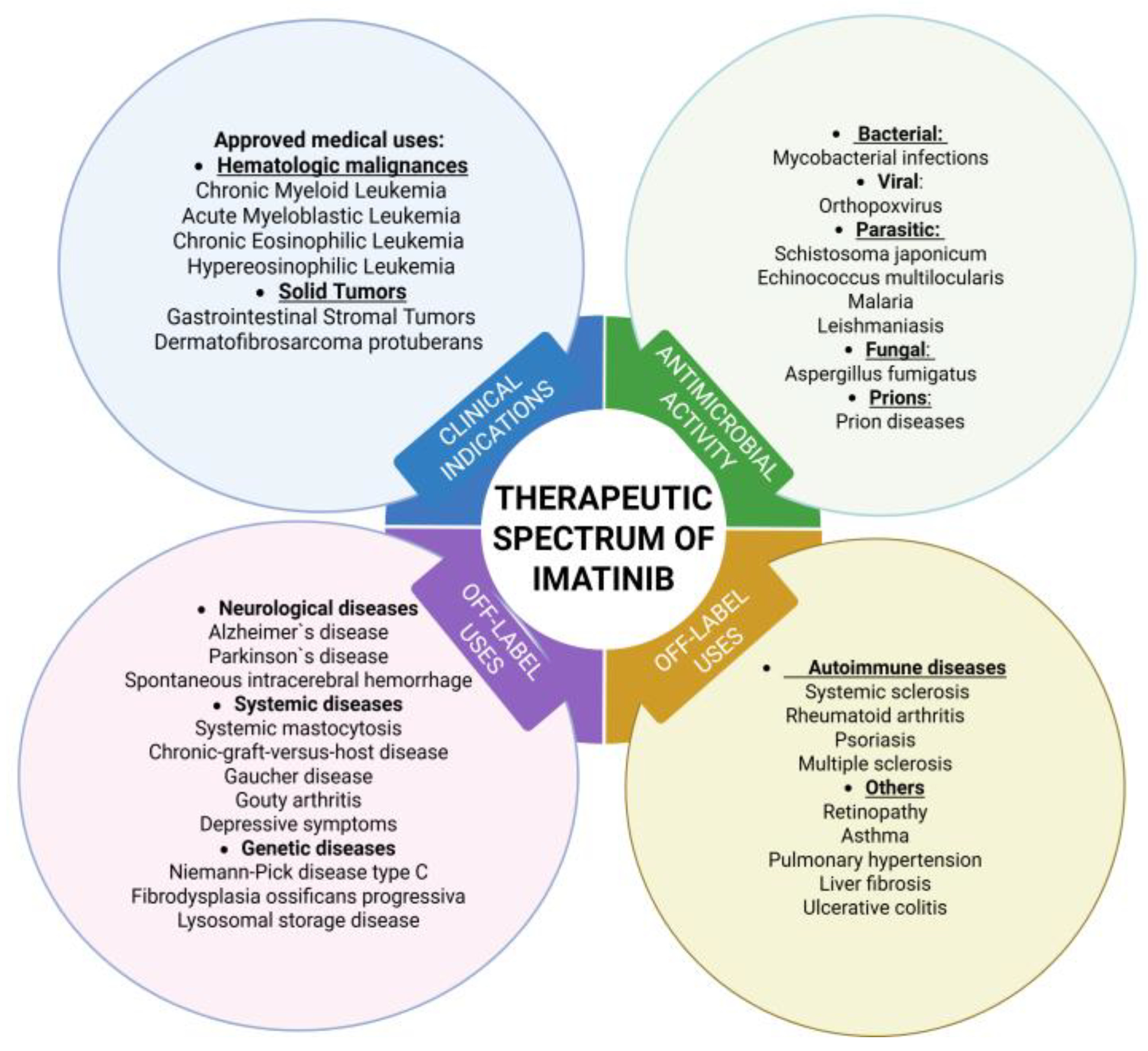
3.3. Clinical Indications
3.4. Possible Interactions
4. Biomedical Applications Beyond Cancer
4.1. Neurological Diseases
4.1.1. Alzheimer’s Disease
4.1.2. Parkinson’s Disease
4.1.3. Ischemic Stroke
4.1.4. Spontaneous Intracerebral Hemorrhage (ICH)
4.1.5. Spinal Cord Injury
4.2. Genetic Diseases
4.2.1. Niemann–Pick Type C (NPC)
4.2.2. Fibrodysplasia Ossificans Progressiva
4.3. Autoimmune Diseases
4.3.1. Chronic Graft-Versus-Host Disease (cGvHD)
4.3.2. Multiple Sclerosis (MS)
4.4. Others
4.4.1. Pulmonary Arterial Hypertension
4.4.2. Asthma
4.4.3. Ulcerative Colitis (UC)
4.5. Skin Disorders
5. Antimicrobial and Antiparasitic Activity
6. New Indications for IMT Application
6.1. Systemic Diseases
6.2. Genetic Diseases
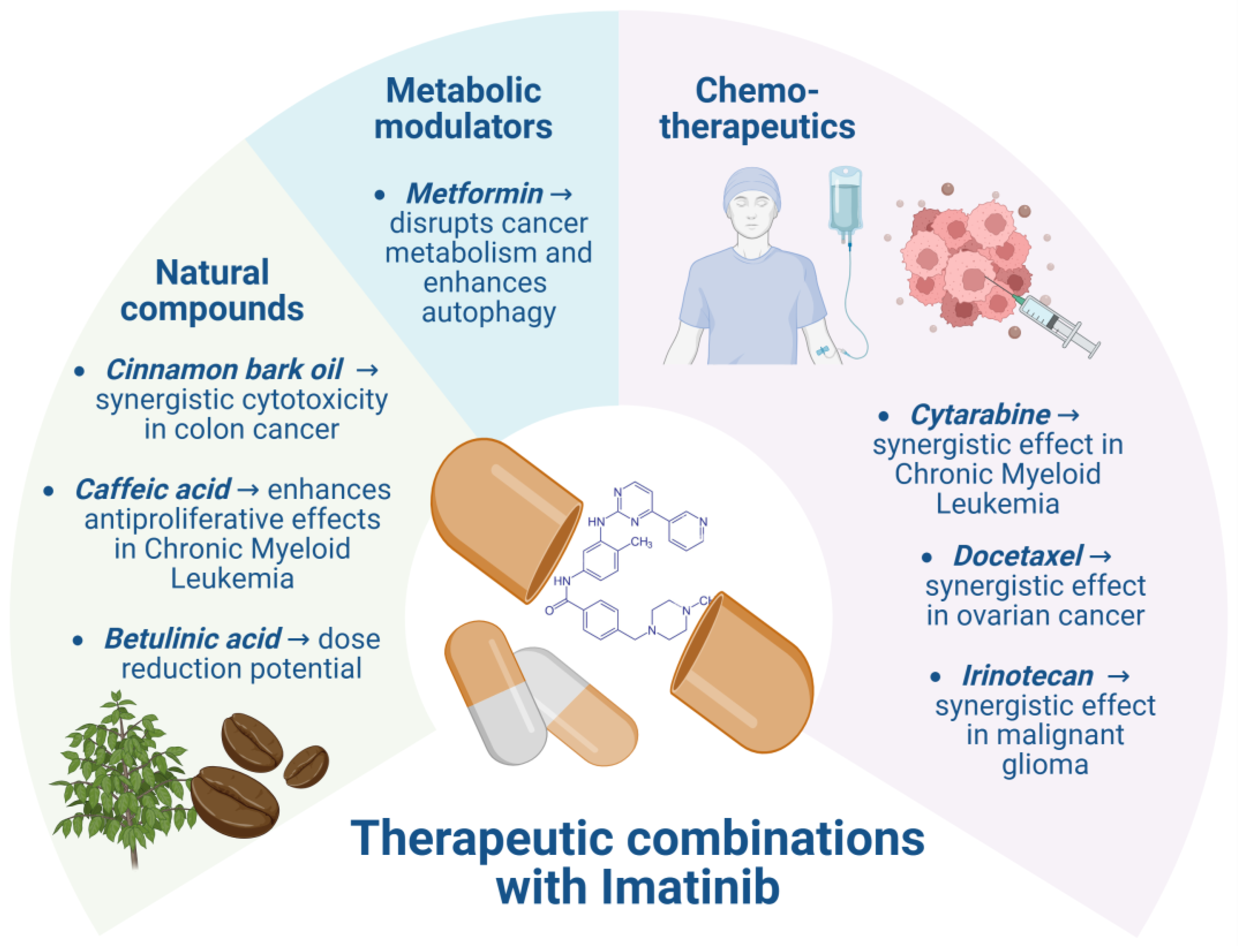
7. Therapeutic Combinations of IMT
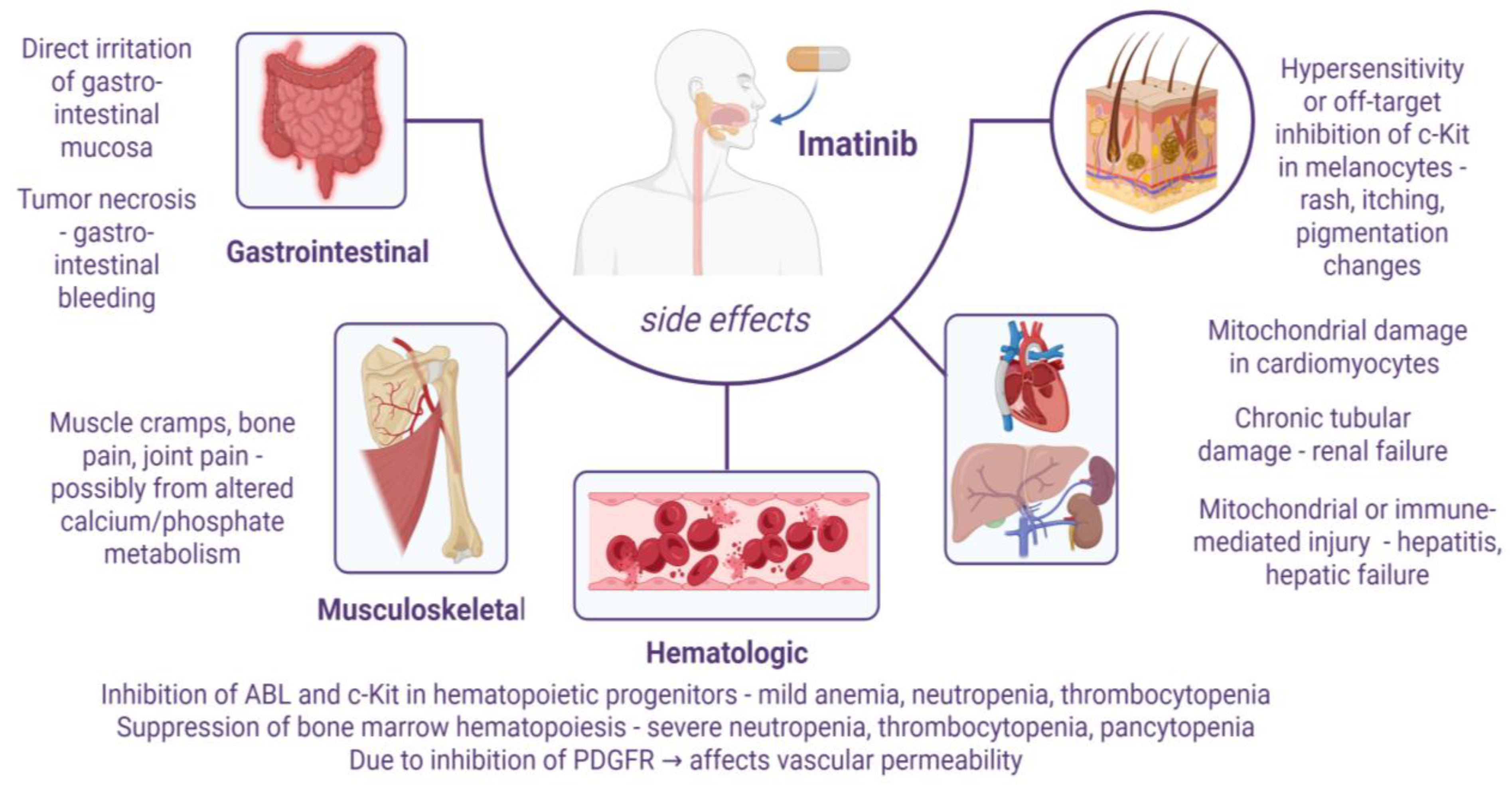
8. Limitations and Challenges
9. Advances in Drug Delivery Systems
9.1. Polymeric NPs
9.2. Lipid-Based Nanocarriers
9.3. Inorganic NPs
9.4. “Smart” Systems
9.5. Hydrogels
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FDA | U.S. Food and Drug Administration |
| IMT | Imatinib |
| IMTM | Imatinib mesylate |
| PTKs | Protein tyrosine kinases |
| RTKs | Receptor tyrosine kinases |
| TKIs | Tyrosine kinase inhibitors |
| CML | Chronic myeloid leukemia |
| PDGFR | Platelet-derived growth factor receptor |
| GIST | Gastrointestinal stromal tumors |
| NPC | Niemann–Pick type C |
| ICH | Intracerebral hemorrhage |
| cGvHD | Chronic graft-versus-host disease |
| MS | Multiple sclerosis |
| CNS | Central nervous system |
| SSc | Systemic sclerosis |
| CGCJ | Central giant cell granuloma of the jaw |
| GD | Gaucher disease |
| T1D | Type 1 Diabetes |
| T2D | Type 2 Diabetes |
| CO | Cinnamon bark oil |
| DDSs | Drug delivery systems |
| FR | Folate receptor |
| NLCs | Nanostructured lipid carriers |
| CS | Chitosan |
| NPs | Nanoparticles |
| OS | Overall survival |
| PFS | Progression-free survival |
| MDR | Multidrug resistance |
References
- Colonna, G. Overcoming Barriers in Cancer Biology Research: Current Limitations and Solutions. Cancers 2025, 17, 2102. [Google Scholar] [CrossRef]
- Ardasheva, R.; Prissadova, N.; Turiyski, V.; Tolekova, A.; Krastev, A.; Pencheva, M.; Popov, V. Effects of Electron Radiation on Serotonin Signaling and Reactivity of Rat Gastric Smooth Muscle. Toxics 2023, 11, 603. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Mongre, R.K.; Mishra, C.B.; Shukla, A.K.; Prakash, A.; Jung, S.; Ashraf-Uz-Zaman, M.; Lee, M.-S. Emerging Importance of Tyrosine Kinase Inhibitors against Cancer: Quo Vadis to Cure? Int. J. Mol. Sci. 2021, 22, 11659. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.G.; Kokova, V.; Peychev, Z.; Apostolov, A. Effect of Fucoidan, Haberlea Rhodopensis and Propolis on Mobilization of The CD34+ Stem Cells in Rats. Farmacia 2017, 65, 567–570. [Google Scholar]
- Kim, M.; Baek, M.; Kim, D.J. Protein Tyrosine Signaling and Its Potential Therapeutic Implications in Carcinogenesis. Curr. Pharm. Des. 2017, 23, 4226–4246. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Ojo, D.A.; Okeowo, O.T.; Omotuyi, I.O. Exploring Receptor Tyrosine Kinases-Inhibitors in Cancer Treatments. Egypt. J. Med. Hum. Genet. 2019, 20, 35. [Google Scholar] [CrossRef]
- Hussain, S.; Mursal, M.; Verma, G.; Hasan, S.M.; Khan, M.F. Targeting Oncogenic Kinases: Insights on FDA Approved Tyrosine Kinase Inhibitors. Eur. J. Pharmacol. 2024, 970, 176484. [Google Scholar] [CrossRef]
- Das, R.; Tambe, G.; Shard, A. Sulfonamides as Tyrosine Kinase Modulators—A Promising Class of Anticancer Agents. Results Chem. 2023, 5, 100950. [Google Scholar] [CrossRef]
- Orrico, K.B. Basic Concepts of Cancer Genetics and Receptor Tyrosine Kinase Inhibition for Pharmacists. A Narrative Review. J. Oncol. Pharm. Pract. 2023, 29, 1187–1195. [Google Scholar] [CrossRef]
- Mhaibes, R.M. The Impact of Tyrosine Kinase Inhibitors on Cancer Therapy: A Comprehensive Review. Med. Med. Chem. 2024, 1, 36–42. [Google Scholar]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A Breakthrough of Targeted Therapy in Cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.P.; Gerriets, V. Imatinib. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551676/ (accessed on 28 August 2025).
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Druker, B.J.; Tamura, S.; Buchdunger, E.; Ohno, S.; Segal, G.M.; Fanning, S.; Zimmermann, J.; Lydon, N.B. Effects of a Selective Inhibitor of the Abl Tyrosine Kinase on the Growth of Bcr–Abl Positive Cells. Nat. Med. 1996, 2, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Napier, R.J.; Norris, B.A.; Swimm, A.; Giver, C.R.; Harris, W.A.C.; Laval, J.; Napier, B.A.; Patel, G.; Crump, R.; Peng, Z.; et al. Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-Microbial Immunity. PLoS Pathog. 2015, 11, e1004770. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-Approved Small Molecule Protein Kinase Inhibitors: A 2023 Update. Pharmacol. Res. 2023, 187, 106552. [Google Scholar] [CrossRef]
- Sobierajska, P.; Wiatrak, B.; Jawien, P.; Janeczek, M.; Wiglusz, K.; Szeląg, A.; Wiglusz, R.J. Imatinib-Functionalized Galactose Hydrogels Loaded with Nanohydroxyapatite as a Drug Delivery System for Osteosarcoma: In Vitro Studies. ACS Omega 2023, 8, 17891–17900. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 123596, Imatinib Mesylate” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/imatinib-mesylate (accessed on 28 August 2025).
- Akagi, S.; Nakamura, K.; Miura, D.; Saito, Y.; Matsubara, H.; Ogawa, A.; Matoba, T.; Egashira, K.; Ito, H. Delivery of Imatinib-Incorporated Nanoparticles into Lungs Suppresses the Development of Monocrotaline-Induced Pulmonary Arterial Hypertension. Int. Heart J. 2015, 56, 354–359. [Google Scholar] [CrossRef]
- Bhullar, S.; Goyal, N.; Gupta, S. In-Vitro pH-Responsive Release of Imatinib from Iron-Supplement Coated Anatase TiO2 Nanoparticles. Sci. Rep. 2022, 12, 4600. [Google Scholar] [CrossRef]
- Ghadami, A.; Fathi-karkan, S.; Siddiqui, B.; Gondal, S.A.; Rahdar, A.; Garousi, N.A.; Kharaba, Z.; Ghotekar, S. Nanotechnology in Imatinib Delivery: Advancing Cancer Treatment through Innovative Nanoparticles. Med. Oncol. 2025, 42, 116. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5291, Imatinib” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/imatinib (accessed on 24 August 2025).
- Vandyke, K.; Fitter, S.; Dewar, A.L.; Hughes, T.P.; Zannettino, A.C.W. Dysregulation of Bone Remodeling by Imatinib Mesylate. Blood 2010, 115, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Arora, B.; Gota, V.; Menon, H.; Sengar, M.; Nair, R.; Patial, P.; Banavali, S.D. Therapeutic Drug Monitoring for Imatinib: Current Status and Indian Experience. Indian J. Med. Paediatr. Oncol. 2013, 34, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Dutreix, C.; Mehring, G.; Hayes, M.J.; Ben-Am, M.; Seiberling, M.; Pokorny, R.; Capdeville, R.; Lloyd, P. Absolute Bioavailability of Imatinib (Glivec®) Orally versus Intravenous Infusion. J. Clin. Pharmacol. 2004, 44, 158–162. [Google Scholar] [CrossRef]
- Barratt, D.T.; Somogyi, A.A. Role of Pharmacogenetics in Personalised Imatinib Dosing. Transl. Cancer Res. 2017, 6, S1541–S1557. [Google Scholar] [CrossRef]
- Di Gion, P.; Kanefendt, F.; Lindauer, A.; Scheffler, M.; Doroshyenko, O.; Fuhr, U.; Wolf, J.; Jaehde, U. Clinical Pharmacokinetics of Tyrosine Kinase Inhibitors: Focus on Pyrimidines, Pyridines and Pyrroles. Clin. Pharmacokinet. 2011, 50, 551–603. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Wang, Q.; Liu, Z.; Dong, X.; Shi, S.; Xiao, H. Factors Influencing the Steady-State Plasma Concentration of Imatinib Mesylate in Patients With Gastrointestinal Stromal Tumors and Chronic Myeloid Leukemia. Front. Pharmacol. 2020, 11, 569843. [Google Scholar] [CrossRef]
- Omran, M.M.; Ibrahim, A.B.; Abdelfattah, R.; Shouman, S.A.; Hamza, M.S. Imatinib Pharmacokinetics and Creatine Kinase Levels in Chronic Myeloid Leukemia Patients: Implications for Therapeutic Response and Monitoring. Eur. J. Clin. Pharmacol. 2024, 80, 1061–1068. [Google Scholar] [CrossRef]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: A Review. JAMA 2025, 333, 1618. [Google Scholar] [CrossRef]
- El-Tanani, M.; Nsairat, H.; Matalka, I.I.; Lee, Y.F.; Rizzo, M.; Aljabali, A.A.; Mishra, V.; Mishra, Y.; Hromić-Jahjefendić, A.; Tambuwala, M.M. The Impact of the BCR-ABL Oncogene in the Pathology and Treatment of Chronic Myeloid Leukemia. Pathol.-Res. Pract. 2024, 254, 155161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kulshrestha, R.; Singh, N.; Jaggi, A.S. Expanding Spectrum of Anticancer Drug, Imatinib, in the Disorders Affecting Brain and Spinal Cord. Pharmacol. Res. 2019, 143, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Suttorp, M.; Bornhäuser, M.; Metzler, M.; Millot, F.; Schleyer, E. Pharmacology and Pharmacokinetics of Imatinib in Pediatric Patients. Expert Rev. Clin. Pharmacol. 2018, 11, 219–231. [Google Scholar] [CrossRef]
- Di Vito, A.; Ravegnini, G.; Gorini, F.; Aasen, T.; Serrano, C.; Benuzzi, E.; Coschina, E.; Monesmith, S.; Morroni, F.; Angelini, S.; et al. The Multifaceted Landscape behind Imatinib Resistance in Gastrointestinal Stromal Tumors (GISTs): A Lesson from Ripretinib. Pharmacol. Ther. 2023, 248, 108475. [Google Scholar] [CrossRef]
- Masucci, M.T.; Motti, M.L.; Minopoli, M.; Di Carluccio, G.; Carriero, M.V. Emerging Targeted Therapeutic Strategies to Overcome Imatinib Resistance of Gastrointestinal Stromal Tumors. Int. J. Mol. Sci. 2023, 24, 6026. [Google Scholar] [CrossRef]
- Adiwidjaja, J.; Boddy, A.V.; McLachlan, A.J. Implementation of a Physiologically Based Pharmacokinetic Modeling Approach to Guide Optimal Dosing Regimens for Imatinib and Potential Drug Interactions in Paediatrics. Front. Pharmacol. 2020, 10, 1672. [Google Scholar] [CrossRef]
- Teo, Y.L.; Ho, H.K.; Chan, A. Metabolism-related Pharmacokinetic Drug−drug Interactions with Tyrosine Kinase Inhibitors: Current Understanding, Challenges and Recommendations. Br. J. Clin. Pharmacol. 2015, 79, 241–253. [Google Scholar] [CrossRef]
- Reichardt, P. The Story of Imatinib in GIST—A Journey through the Development of a Targeted Therapy. Oncol. Res. Treat. 2018, 41, 472–477. [Google Scholar] [CrossRef]
- Copyright Northumbria Healthcare NHS Trust & North of England Cancer Network Systemic Anticancer Therapy Drug Interactions Table. 2012. Available online: https://northerncanceralliance.nhs.uk/wp-content/uploads/2018/10/SACT-Interactions-Table1.pdf (accessed on 24 August 2025).
- Accord Healthcare Limited. ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS Imatinib Accord 100 Mg Film-Coated Tablets. Available online: https://www.accord-healthcare.com/ie/system/files/spc/ema-spc-h-2681-en-var%2039.G.pdf (accessed on 24 August 2025).
- De Diego García, P.; Trincado Aznar, P.; Playán Usón, J.; Albero Gamboa, R. Tratamiento con levotiroxina e imatinib. Endocrinol. Nutr. 2008, 55, 304–307. [Google Scholar] [CrossRef]
- Chan, W.-J.J.; Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V.; Harnett, J.E. Interactions between Natural Products and Cancer Treatments: Underlying Mechanisms and Clinical Importance. Cancer Chemother. Pharmacol. 2023, 91, 103–119. [Google Scholar] [CrossRef]
- Escudero-Vilaplana, V.; Collado-Borrell, R.; Villanueva-Bueno, C.; Álvarez, R.; Herranz, A.; Sanjurjo, M. Acute Pancreatitis in a Patient Treated with Imatinib and Gefitinib. J. Oncol. Pharm. Pract. 2021, 27, 980–983. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, J.; Baruchel, A.; Biondi, A.; De Bont, E.; Dresse, M.; Suttorp, M.; Millot, F.; International BFM Group (iBFM) Study Group Chronic Myeloid Leukaemia Committee. Managing Children with Chronic Myeloid Leukaemia (CML): Recommendations for the Management of CML in Children and Young People up to the Age of 18 Years. Br. J. Haematol. 2014, 167, 33–47. [Google Scholar] [CrossRef] [PubMed]
- León, R.; Gutiérrez, D.A.; Pinto, C.; Morales, C.; De La Fuente, C.; Riquelme, C.; Cortés, B.I.; González-Martin, A.; Chamorro, D.; Espinosa, N.; et al. C-Abl Tyrosine Kinase down-Regulation as Target for Memory Improvement in Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1180987. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.D.; Chamorro, D.; Yañez, M.J.; Gonzalez, M.; Leal, N.; Von Bernhardi, R.; Dulcey, A.E.; Marugan, J.; Ferrer, M.; Soto, C.; et al. Reduction of Blood Amyloid-β Oligomers in Alzheimer’s Disease Transgenic Mice by c-Abl Kinase Inhibition. J. Alzheimers Dis. 2016, 54, 1193–1205. [Google Scholar] [CrossRef]
- Cancino, G.I.; Perez De Arce, K.; Castro, P.U.; Toledo, E.M.; Von Bernhardi, R.; Alvarez, A.R. C-Abl Tyrosine Kinase Modulates Tau Pathology and Cdk5 Phosphorylation in AD Transgenic Mice. Neurobiol. Aging 2011, 32, 1249–1261. [Google Scholar] [CrossRef]
- Cancino, G.I.; Toledo, E.M.; Leal, N.R.; Hernandez, D.E.; Yévenes, L.F.; Inestrosa, N.C.; Alvarez, A.R. STI571 Prevents Apoptosis, Tau Phosphorylation and Behavioural Impairments Induced by Alzheimer’s β-Amyloid Deposits. Brain 2008, 131, 2425–2442. [Google Scholar] [CrossRef]
- Marín, T.; Valls, C.; Jerez, C.; Huerta, T.; Elgueta, D.; Vidal, R.L.; Alvarez, A.R.; Cancino, G.I. The C-Abl/P73 Pathway Induces Neurodegeneration in a Parkinson’s Disease Model. IBRO Neurosci. Rep. 2022, 13, 378–387. [Google Scholar] [CrossRef]
- Lastovetskyi, I.; Cytlau, B.; Marczyk, Ł.; Zdrojewska, K.; Łach, A.; Krupa, J.; Lorkowska-Zawicka, B.; Giżycka, B.B. Modern Pharmacological Treatment of Parkinson’s Disease: Reviving Known Drugs and New Perspectives. Int. J. Pharm. Res. Allied Sci. 2024, 13, 29–39. [Google Scholar] [CrossRef]
- Zeitelhofer, M.; Stefanitsch, C.; Protzmann, J.; Torrente, D.; Adzemovic, M.Z.; Lewandowski, S.A.; Muhl, L.; Eriksson, U.; Nilsson, I.; Su, E.; et al. Reduced Myofibroblast Transdifferentiation and Fibrotic Scarring in Ischemic Stroke after Imatinib Treatment. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wahlgren, N.; Thorén, M.; Höjeberg, B.; Käll, T.-B.; Laska, A.-C.; Sjöstrand, C.; Höijer, J.; Almqvist, H.; Holmin, S.; Lilja, A.; et al. Randomized Assessment of Imatinib in Patients with Acute Ischaemic Stroke Treated with Intravenous Thrombolysis. J. Intern. Med. 2017, 281, 273–283. [Google Scholar] [CrossRef]
- Pearce, W.J.; Doan, C.; Carreon, D.; Kim, D.; Durrant, L.M.; Manaenko, A.; McCoy, L.; Obenaus, A.; Zhang, J.H.; Tang, J. Imatinib Attenuates Cerebrovascular Injury and Phenotypic Transformation after Intracerebral Hemorrhage in Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R1093–R1104. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, B.; Khatibi, N.; Rolland, W.; Suzuki, H.; Zhang, J.H.; Tang, J. PDGFR-α Inhibition Preserves Blood-brain Barrier after Intracerebral Hemorrhage. Ann. Neurol. 2011, 70, 920–931. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, J.Y.; Yune, T.Y. Imatinib Prevents Blood-Spinal Cord Barrier Disruption by Inhibiting PDGFR-Mediated JMJD3 Expression and Activation after Spinal Cord Injury. Fluids Barriers CNS 2025, 22, 76. [Google Scholar] [CrossRef]
- Yáñez, M.J.; Belbin, O.; Estrada, L.D.; Leal, N.; Contreras, P.S.; Lleó, A.; Burgos, P.V.; Zanlungo, S.; Alvarez, A.R. C-Abl Links APP-BACE1 Interaction Promoting APP Amyloidogenic Processing in Niemann-Pick Type C Disease. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2016, 1862, 2158–2167. [Google Scholar] [CrossRef]
- Alvarez, A.R.; Klein, A.; Castro, J.; Cancino, G.I.; Amigo, J.; Mosqueira, M.; Vargas, L.M.; Yevenes, L.F.; Bronfman, F.C.; Zanlungo, S. Imatinib Therapy Blocks Cerebellar Apoptosis and Improves Neurological Symptoms in a Mouse Model of Niemann-Pick Type C Disease. FASEB J. 2008, 22, 3617–3627. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Andolina, J.R.; Adamson, P.C.; Teachey, D.T.; Finklestein, J.Z.; Ebb, D.H.; Whitehead, B.; Jacobs, B.; Siegel, D.M.; Keen, R.; et al. Early Clinical Observations on the Use of Imatinib Mesylate in FOP: A Report of Seven Cases. Bone 2018, 109, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Mohty, M. Updates in Chronic Graft-versus-host Disease Management. Am. J. Hematol. 2023, 98, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.M.; Zeiser, R. Kinase Inhibition as Treatment for Acute and Chronic Graft-Versus-Host Disease. Front. Immunol. 2021, 12, 760199. [Google Scholar] [CrossRef]
- Crespo, O.; Kang, S.C.; Daneman, R.; Lindstrom, T.M.; Ho, P.P.; Sobel, R.A.; Steinman, L.; Robinson, W.H. Tyrosine Kinase Inhibitors Ameliorate Autoimmune Encephalomyelitis in a Mouse Model of Multiple Sclerosis. J. Clin. Immunol. 2011, 31, 1010–1020. [Google Scholar] [CrossRef]
- Fawzy, M.G.; Selim, M.S.; Khorshid, O.A.; Rashed, L.A.; Elkordy, M.A.; Abdel Latif, N.S. Comparative Study on the Possible Ameliorative Effects of Imatinib and Ibudilast in a Rat Model of Multiple Sclerosis. Future J. Pharm. Sci. 2025, 11, 47. [Google Scholar] [CrossRef]
- Yotov, V.; Ardasheva, R.; Mihaylova, A.; Doncheva, N.; Kostadinov, I.; Turiyski, V. Simulated Microgravity Affects Carrageenan-Induced Inflammation Process in Rats. Pharmacia 2023, 70, 1531–1538. [Google Scholar] [CrossRef]
- Azizi, G.; Haidari, M.R.; Khorramizadeh, M.; Naddafi, F.; Sadria, R.; Javanbakht, M.H.; Sedaghat, R.; Tofighi Zavareh, F.; Mirshafiey, A. Effects of Imatinib Mesylate in Mouse Models of Multiple Sclerosis and in Vitro Determinants. Iran J. Allergy Asthma Immunol. 2014, 13, 198–206. [Google Scholar]
- Speich, R.; Ulrich, S.; Domenighetti, G.; Huber, L.C.; Fischler, M.; Treder, U.; Breitenstein, A. Efficacy and Safety of Long-Term Imatinib Therapy for Pulmonary Arterial Hypertension. Respiration 2015, 89, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.N.; Katz, H.R.; Cui, J.; Lai, J.; Kazani, S.; Crosby-Thompson, A.; Garofalo, D.; Castro, M.; Jarjour, N.; DiMango, E.; et al. KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma. N. Engl. J. Med. 2017, 376, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Foer, D.; Cahill, K.N.; Israel, E.; Maiorino, E.; Röhl, A.; Boyce, J.A.; Weiss, S.T. Systems Approaches to Treatment Response to Imatinib in Severe Asthma: A Pilot Study. J. Pers. Med. 2021, 11, 240. [Google Scholar] [CrossRef]
- Khodir, A.E.; Said, E.; Atif, H.; ElKashef, H.A.; Salem, H.A. Targeting Nrf2/HO-1 Signaling by Crocin: Role in Attenuation of AA-Induced Ulcerative Colitis in Rats. Biomed. Pharmacother. 2019, 110, 389–399. [Google Scholar] [CrossRef]
- Shalaby, M.; Abdelaziz, R.R.; Ghoneim, H.A.; Suddek, G.M. Imatinib Mitigates Experimentally-Induced Ulcerative Colitis: Possible Contribution of NF-kB/JAK2/STAT3/COX2 Signaling Pathway. Life Sci. 2023, 321, 121596. [Google Scholar] [CrossRef]
- Hayakawa, K.; Maeda, T.; Egusa, C.; Okubo, Y.; Harada, K. Topical Application of Imatinib Mesylate Ameliorated Psoriasis-like Skin Lesions in Imiquimod-induced Murine Model via Angiogenesis Inhibition. Exp. Dermatol. 2023, 32, 878–888. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Venalis, P.; Dees, C.; Busch, N.; Zwerina, J.; Schett, G.; Distler, O.; Distler, J.H.W. Treatment with Imatinib Prevents Fibrosis in Different Preclinical Models of Systemic Sclerosis and Induces Regression of Established Fibrosis. Arthritis Rheum. 2009, 60, 219–224. [Google Scholar] [CrossRef]
- Horton, J.A.; Chung, E.J.; Hudak, K.E.; Sowers, A.; Thetford, A.; White, A.O.; Mitchell, J.B.; Citrin, D.E. Inhibition of Radiation-Induced Skin Fibrosis with Imatinib. Int. J. Radiat. Biol. 2013, 89, 162–170. [Google Scholar] [CrossRef]
- Waheed, A.; Buckingham, C.; Brar, G. AB0626 Imatinib for the Treatment of Systemic Sclerosis: Rationale, Clinical Evidence and Future Development. Ann. Rheum. Dis. 2020, 79, 1608–1609. [Google Scholar] [CrossRef]
- Sassetti, C.; Borrelli, C.; Mazuy, M.; Guerriero, C.; Rigante, D.; Esposito, S. New Challenging Systemic Therapies for Juvenile Scleroderma: A Comprehensive Review. Pharmaceuticals 2025, 18, 643. [Google Scholar] [CrossRef]
- Inamo, Y.; Ochiai, T. Successful Combination Treatment of a Patient with Progressive Juvenile Localized Scleroderma (Morphea) Using Imatinib, Corticosteroids, and Methotrexate. Pediatr. Dermatol. 2013, 30, e191–e193. [Google Scholar] [CrossRef]
- Spiera, R.F.; Gordon, J.K.; Mersten, J.N.; Magro, C.M.; Mehta, M.; Wildman, H.F.; Kloiber, S.; Kirou, K.A.; Lyman, S.; Crow, M.K. Imatinib Mesylate (Gleevec) in the Treatment of Diffuse Cutaneous Systemic Sclerosis: Results of a 1-Year, Phase IIa, Single-Arm, Open-Label Clinical Trial. Ann. Rheum. Dis. 2011, 70, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Banks, T.; Steen, V.; Katona, I.; Jones, O. Successful Treatment of Pansclerotic Morphea with Imatinib Mesylate in a Pediatric Patient. Ann. Paediatr. Rheumatol. 2013, 2, 43. [Google Scholar] [CrossRef]
- Cleverley, T.L.; Peddineni, S.; Guarner, J.; Cingolani, F.; Garcia, P.K.; Koehler, H.; Mocarski, E.S.; Kalman, D. The Host-Directed Therapeutic Imatinib Mesylate Accelerates Immune Responses to Mycobacterium Marinum Infection and Limits Pathology Associated with Granulomas. PLoS Pathog. 2023, 19, e1011387. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, S.; Girigoswami, A.; Girigoswami, K. A Review on Current Theories and Potential Therapies for Prion Diseases. Mol. Biol. Rep. 2025, 52, 674. [Google Scholar] [CrossRef]
- Yun, S.-W.; Ertmer, A.; Flechsig, E.; Gilch, S.; Riederer, P.; Gerlach, M.; Schätzl, H.M.; Klein, M.A. The Tyrosine Kinase Inhibitor Imatinib Mesylate Delays Prion Neuroinvasion by Inhibiting Prion Propagation in the Periphery. J. Neurovirol. 2007, 13, 328–337. [Google Scholar] [CrossRef]
- Kato, H. Epidemiology of Leishmaniasis: Risk Factors for Its Pathology and Infection. Parasitol. Int. 2025, 105, 102999. [Google Scholar] [CrossRef]
- Moslehi, M.; Namdar, F.; Esmaeilifallah, M.; Hejazi, S.; Sokhanvari, F.; Siadat, A.; Hosseini, S.; Iraji, F. Evaluation of Different Concentrations of Imatinib on the Viability of Leishmania Major: An In Vitro Study. Adv. Biomed. Res. 2019, 8, 61. [Google Scholar] [CrossRef]
- Zubair Ahmed, F.; Shaifulla, P. Assessment of Efficacy and Safety of Imatinib as an Adjunct Host-Targeted Therapy for Parasite Clearance in Chloroquine-Resistant Malaria: A Prospective Case Control Study. Egypt. J. Intern. Med. 2025, 37, 11. [Google Scholar] [CrossRef]
- Subroto, E.; Van Neer, J.; Valdes, I.; De Cock, H. Growth of Aspergillus Fumigatus in Biofilms in Comparison to Candida Albicans. J. Fungi 2022, 8, 48. [Google Scholar] [CrossRef]
- Seegers, C.I.I.; Lee, D.J.; Zarnovican, P.; Kirsch, S.H.; Müller, R.; Haselhorst, T.; Routier, F.H. Identification of Compounds Preventing A. Fumigatus Biofilm Formation by Inhibition of the Galactosaminogalactan Deacetylase Agd3. Int. J. Mol. Sci. 2023, 24, 1851. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (Formerly Monkeypox): Pathogenesis, Prevention and Treatment. Signal Transduct. Target. Ther. 2023, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Autier, B.; Robert-Gangneux, F.; Dion, S. Chemotherapy for the Treatment of Alveolar Echinococcosis: Where Are We? Parasite 2024, 31, 56. [Google Scholar] [CrossRef] [PubMed]
- Hemer, S.; Brehm, K. In Vitro Efficacy of the Anticancer Drug Imatinib on Echinococcus Multilocularis Larvae. Int. J. Antimicrob. Agents 2012, 40, 458–462. [Google Scholar] [CrossRef]
- Giannetti, M.P. Treatment of Systemic Mastocytosis. Ann. Allergy Asthma Immunol. 2021, 127, 412–419. [Google Scholar] [CrossRef]
- Buonomo, A.; Nucera, E.; Criscuolo, M. Treatment of Indolent and Advanced Systemic Mastocytosis: Systemic Mastocytosis Treatment. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022040. [Google Scholar] [CrossRef]
- Tallent, B.; Padilla, R.J.; McKay, C.; Foreman, A.K.M.; Fan, Z.; Blatt, J. Response of Central Giant Cell Granuloma of the Jaw to Imatinib. J. Pediatr. Hematol. Oncol. 2023, 45, 278–280. [Google Scholar] [CrossRef]
- Reber, L.L.; Starkl, P.; Balbino, B.; Sibilano, R.; Gaudenzio, N.; Rogalla, S.; Sensarn, S.; Kang, D.; Raghu, H.; Sokolove, J.; et al. The Tyrosine Kinase Inhibitor Imatinib Mesylate Suppresses Uric Acid Crystal-Induced Acute Gouty Arthritis in Mice. PLoS ONE 2017, 12, e0185704. [Google Scholar] [CrossRef]
- Carvajal, R.D. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA 2011, 305, 2327. [Google Scholar] [CrossRef]
- Wei, X.; Mao, L.; Chi, Z.; Sheng, X.; Cui, C.; Kong, Y.; Dai, J.; Wang, X.; Li, S.; Tang, B.; et al. Efficacy Evaluation of Imatinib for the Treatment of Melanoma: Evidence From a Retrospective Study. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 495–501. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Available online: https://clinicaltrials.gov/search?cond=metastatic%20melanoma&intr=imatinib (accessed on 29 October 2025).
- Zhou, M.; Shen, Q.; Li, B. JAK Inhibitors: A New Choice for Diabetes Mellitus? Diabetol. Metab. Syndr. 2025, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Salaroli, A.; Loglisci, G.; Serrao, A.; Alimena, G.; Breccia, M. Fasting Glucose Level Reduction Induced by Imatinib in Chronic Myeloproliferative Disease with TEL-PDGFRβ Rearrangement and Type 1 Diabetes. Ann. Hematol. 2012, 91, 1823–1824. [Google Scholar] [CrossRef] [PubMed]
- Althubiti, M. Tyrosine Kinase Targeting: A Potential Therapeutic Strategy for Diabetes. Saudi J. Med. Med. Sci. 2022, 10, 183–191. [Google Scholar] [CrossRef]
- Box, C.V.J.; Sandhu, A.K.; Turaihi, A.H.; Xiaoké, P.; Dallinga-Thie, G.; Aman, J.; Eringa, E.C. Effects of Imatinib on Vascular Insulin Sensitivity and Free Fatty Acid Transport in Early Weight Gain. PLoS ONE 2021, 16, e0250442. [Google Scholar] [CrossRef]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and Cardiovascular Disease Risk among the MASHAD Study Population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef]
- Sucharski, H.C.; Dudley, E.K.; Williams, J.; Dewal, R.; Stanford, K.I.; Mohler, P.J.; Koenig, S.N. Abstract 423: Imatinib Promotes Reverse Cholesterol Transport And Elevates Sr-Bi. Arterioscler. Thromb. Vasc. Biol. 2022, 42, A423. [Google Scholar] [CrossRef]
- Wang-Rosenke, Y.; Khadzhynov, D.; Loof, T.; Mika, A.; Kawachi, H.; Neumayer, H.-H.; Peters, H. Tyrosine Kinases Inhibition by Imatinib Slows Progression in Chronic Anti-Thy1 Glomerulosclerosis of the Rat. BMC Nephrol. 2013, 14, 223. [Google Scholar] [CrossRef]
- Zoja, C.; Corna, D.; Rottoli, D.; Zanchi, C.; Abbate, M.; Remuzzi, G. Imatinib Ameliorates Renal Disease and Survival in Murine Lupus Autoimmune Disease. Kidney Int. 2006, 70, 97–103. [Google Scholar] [CrossRef]
- Buhl, E.M.; Djudjaj, S.; Klinkhammer, B.M.; Ermert, K.; Puelles, V.G.; Lindenmeyer, M.T.; Cohen, C.D.; He, C.; Borkham-Kamphorst, E.; Weiskirchen, R.; et al. Dysregulated Mesenchymal PDGFR-β Drives Kidney Fibrosis. EMBO Mol. Med. 2020, 12, e11021. [Google Scholar] [CrossRef] [PubMed]
- Yañez, M.J.; Campos, F.; Marín, T.; Klein, A.D.; Futerman, A.H.; Alvarez, A.R.; Zanlungo, S. C-Abl Activates RIPK3 Signaling in Gaucher Disease. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2021, 1867, 166089. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.K.S. Schistosoma Japonicum–Associated Colorectal Cancer: A Review. Am. J. Trop. Med. Hyg. 2019, 100, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhai, X.; Huang, S.; Jiang, L.; Yu, Z.; Huang, J. Protein Kinases: Potential Drug Targets Against Schistosoma Japonicum. Front. Cell. Infect. Microbiol. 2021, 11, 691757. [Google Scholar] [CrossRef]
- Weintraub, M.K.; Bisson, C.M.; Nouri, J.N.; Vinson, B.T.; Eimerbrink, M.J.; Kranjac, D.; Boehm, G.W.; Chumley, M.J. Imatinib Methanesulfonate Reduces Hippocampal Amyloid-Beta and Restores Cognitive Function Following Repeated Endotoxin Exposure. Brain. Behav. Immun. 2013, 33, 24–28. [Google Scholar] [CrossRef]
- Merali, Z.; Leung, J.; Mikulis, D.; Silver, F.; Kassner, A. Longitudinal Assessment of Imatinib’s Effect on the Blood–Brain Barrier After Ischemia/Reperfusion Injury with Permeability MRI. Transl. Stroke Res. 2015, 6, 39–49. [Google Scholar] [CrossRef]
- Magro, F.; Costa, C. Long-Standing Remission of Crohn’s Disease under Imatinib Therapy in a Patient with Crohn’s Disease. Inflamm. Bowel Dis. 2006, 12, 1087–1089. [Google Scholar] [CrossRef]
- Boctor, A.; Hugot, J.-P.; Leblanc, T.; Martinez-Vinson, C.; Allez, M.; Bellaïche, M. Imatinib in Refractory Crohn Disease: A Series of 6 Cases. Crohns Colitis 360 2019, 1, otz034. [Google Scholar] [CrossRef]
- Samei, L.; Yaling, P.; Lihua, Y.; Yan, Z.; Shuyan, J. Effects and Mechanism of Imatinib in Inhibiting Colon Cancer Cell Proliferation. Med. Sci. Monit. 2016, 22, 4126–4131. [Google Scholar] [CrossRef]
- Olivieri, A.; Locatelli, F.; Zecca, M.; Sanna, A.; Cimminiello, M.; Raimondi, R.; Gini, G.; Mordini, N.; Balduzzi, A.; Leoni, P.; et al. Imatinib for Refractory Chronic Graft-versus-Host Disease with Fibrotic Features. Blood 2009, 114, 709–718. [Google Scholar] [CrossRef]
- Baird, K.; Comis, L.E.; Joe, G.O.; Steinberg, S.M.; Hakim, F.T.; Rose, J.J.; Mitchell, S.A.; Pavletic, S.Z.; Figg, W.D.; Yao, L.; et al. Imatinib Mesylate for the Treatment of Steroid-Refractory Sclerotic-Type Cutaneous Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Magro, L.; Mohty, M.; Catteau, B.; Coiteux, V.; Chevallier, P.; Terriou, L.; Jouet, J.-P.; Yakoub-Agha, I. Imatinib Mesylate as Salvage Therapy for Refractory Sclerotic Chronic Graft-versus-Host Disease. Blood 2009, 114, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Belle, L.; Fransolet, G.; Somja, J.; Binsfeld, M.; Delvenne, P.; Drion, P.; Hannon, M.; Beguin, Y.; Ehx, G.; Baron, F. Limited Impact of Imatinib in a Murine Model of Sclerodermatous Chronic Graft-versus-Host Disease. PLoS ONE 2016, 11, e0167997. [Google Scholar] [CrossRef] [PubMed]
- Samaha, M.M.; Said, E.; Salem, H.A. Modulatory Role of Imatinib Mesylate on Pancreatic β-Cells’ Secretory Functions in an STZ Rat Model of Diabetes Mellitus. Chem. Biol. Interact. 2020, 328, 109197. [Google Scholar] [CrossRef]
- Gitelman, S.E.; Bundy, B.N.; Ferrannini, E.; Lim, N.; Blanchfield, J.L.; DiMeglio, L.A.; Felner, E.I.; Gaglia, J.L.; Gottlieb, P.A.; Long, S.A.; et al. Imatinib Therapy for Patients with Recent-Onset Type 1 Diabetes: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Diabetes Endocrinol. 2021, 9, 502–514. [Google Scholar] [CrossRef]
- Lassila, M.; Jandeleit-Dahm, K.; Seah, K.K.; Smith, C.M.; Calkin, A.C.; Allen, T.J.; Cooper, M.E. Imatinib Attenuates Diabetic Nephropathy in Apolipoprotein E-Knockout Mice. J. Am. Soc. Nephrol. 2005, 16, 363–373. [Google Scholar] [CrossRef]
- Savikko, J.; Taskinen, E.; Von Willebrand, E. Chronic Allograft Nephropathy Is Prevented by Inhibition of Platelet-Derived Growth Factor Receptor: Tyrosine Kinase Inhibitors as a Potential Therapy. Transplantation 2003, 75, 1147–1153. [Google Scholar] [CrossRef]
- Schellings, M.W.M.; Baumann, M.; Van Leeuwen, R.E.W.; Duisters, R.F.J.J.; Janssen, S.H.P.; Schroen, B.; Peutz-Kootstra, C.J.; Heymans, S.; Pinto, Y.M. Imatinib Attenuates End-Organ Damage in Hypertensive Homozygous TGR(mRen2)27 Rats. Hypertension 2006, 47, 467–474. [Google Scholar] [CrossRef]
- Zhan, Y.; Krafft, P.R.; Lekic, T.; Ma, Q.; Souvenir, R.; Zhang, J.H.; Tang, J. Imatinib Preserves Blood–Brain Barrier Integrity Following Experimental Subarachnoid Hemorrhage in Rats. J. Neurosci. Res. 2015, 93, 94–103. [Google Scholar] [CrossRef]
- Chien, H.D.; Pantaleo, A.; Kesely, K.R.; Noomuna, P.; Putt, K.S.; Tuan, T.A.; Low, P.S.; Turrini, F.M. Imatinib Augments Standard Malaria Combination Therapy without Added Toxicity. J. Exp. Med. 2021, 218, e20210724. [Google Scholar] [CrossRef]
- Guo, J.; Si, L.; Kong, Y.; Flaherty, K.T.; Xu, X.; Zhu, Y.; Corless, C.L.; Li, L.; Li, H.; Sheng, X.; et al. Phase II, Open-Label, Single-Arm Trial of Imatinib Mesylate in Patients With Metastatic Melanoma Harboring c-Kit Mutation or Amplification. J. Clin. Oncol. 2011, 29, 2904–2909. [Google Scholar] [CrossRef]
- Jung, S.; Armstrong, E.; Wei, A.Z.; Ye, F.; Lee, A.; Carlino, M.S.; Sullivan, R.J.; Carvajal, R.D.; Shoushtari, A.N.; Johnson, D.B. Clinical and Genomic Correlates of Imatinib Response in Melanomas with KIT Alterations. Br. J. Cancer 2022, 127, 1726–1732. [Google Scholar] [CrossRef]
- Coelho-Macias, V.; Mendes-Bastos, P.; Assis-Pacheco, F.; Cardoso, J. Imatinib: A Novel Treatment Approach for Generalized Morphea. Int. J. Dermatol. 2014, 53, 1299–1302. [Google Scholar] [CrossRef]
- Alcántara-Reifs, C.M.; Garnacho-Saucedo, G.M.; Salido-Vallejo, R.; De La Corte-Sánchez, S.; García-Nieto, A.V. Imatinib Treatment of Therapy Resistant Generalized Deep Morphea: Imatinib in Resistant Generalized Deep Morphea. Dermatol. Ther. 2015, 28, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Adzemovic, M.Z.; Zeitelhofer, M.; Eriksson, U.; Olsson, T.; Nilsson, I. Imatinib Ameliorates Neuroinflammation in a Rat Model of Multiple Sclerosis by Enhancing Blood-Brain Barrier Integrity and by Modulating the Peripheral Immune Response. PLoS ONE 2013, 8, e56586. [Google Scholar] [CrossRef]
- Jain, A. Imatinib Induced Complete Remission of Psoriasis in a Patient with Chronic Myeloid Leukemia. Indian J. Hematol. Blood Transfus. 2020, 36, 198–199. [Google Scholar] [CrossRef]
- Rothman, A.; Villar, S.; Middleton, J.; Law, M.; Varian, F.; Zafar, H.; Kiely, D.G.; Roussakis, A.; Howard, L.; Toshner, M.; et al. Positioning Imatinib for Pulmonary Arterial Hypertension (PIPAH) Study. Eur. Heart J. 2024, 45, ehae666.2182. [Google Scholar] [CrossRef]
- Wang, S.; Wilkes, M.C.; Leof, E.B.; Hirschberg, R. Imatinib Mesylate Blocks a non-Smad TGF-β Pathway and Reduces Renal Fibrogenesis in Vivo. FASEB J. 2005, 19, 1–11. [Google Scholar] [CrossRef]
- Khanna, D.; Saggar, R.; Mayes, M.D.; Abtin, F.; Clements, P.J.; Maranian, P.; Assassi, S.; Saggar, R.; Singh, R.R.; Furst, D.E. A One-year, Phase I/IIa, Open-label Pilot Trial of Imatinib Mesylate in the Treatment of Systemic Sclerosis–Associated Active Interstitial Lung Disease. Arthritis Rheum. 2011, 63, 3540–3546. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Gorgoulis, V.G.; Katsiari, C.G.; Evangelou, K.; Kostopoulos, C.; Black, C.M. Imatinib for the Treatment of Refractory, Diffuse Systemic Sclerosis. Rheumatology 2008, 47, 735–737. [Google Scholar] [CrossRef]
- Vega-Ruiz, A.; Cortes, J.E.; Sever, M.; Manshouri, T.; Quintás-Cardama, A.; Luthra, R.; Kantarjian, H.M.; Verstovsek, S. Phase II Study of Imatinib Mesylate as Therapy for Patients with Systemic Mastocytosis. Leuk. Res. 2009, 33, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Droogendijk, H.J.; Kluin-Nelemans, H.J.C.; Van Doormaal, J.J.; Oranje, A.P.; Van De Loosdrecht, A.A.; Van Daele, P.L.A. Imatinib Mesylate in the Treatment of Systemic Mastocytosis: A Phase II Trial. Cancer 2006, 107, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Pardanani, A.; Butterfield, J.H.; Li, C.; Tefferi, A. Cytoreductive Therapy in 108 Adults with Systemic Mastocytosis: Outcome Analysis and Response Prediction during Treatment with Interferon-alpha, Hydroxyurea, Imatinib Mesylate or 2-chlorodeoxyadenosine. Am. J. Hematol. 2009, 84, 790–794. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; Matito, A.; Morgado, J.M.; Sánchez-Muñoz, L.; Jara-Acevedo, M.; García-Montero, A.; Mayado, A.; Caldas, C.; Teodósio, C.; Muñoz-González, J.I.; et al. Imatinib in Systemic Mastocytosis: A Phase IV Clinical Trial in Patients Lacking Exon 17 KIT Mutations and Review of the Literature. Oncotarget 2017, 8, 68950–68963. [Google Scholar] [CrossRef] [PubMed]
- Sadanaga, A.; Nakashima, H.; Masutani, K.; Miyake, K.; Shimizu, S.; Igawa, T.; Sugiyama, N.; Niiro, H.; Hirakata, H.; Harada, M. Amelioration of Autoimmune Nephritis by Imatinib in MRL/Lpr Mice. Arthritis Rheum. 2005, 52, 3987–3996. [Google Scholar] [CrossRef]
- Stegmeier, F.; Warmuth, M.; Sellers, W.R.; Dorsch, M. Targeted Cancer Therapies in the Twenty-First Century: Lessons from Imatinib. Clin. Pharmacol. Ther. 2010, 87, 543–552. [Google Scholar] [CrossRef]
- Qian, S.; Zheng, C.; Wu, Y.; Huang, H.; Wu, G.; Zhang, J. Targeted Therapy for Leukemia Based on Nanomaterials. Heliyon 2024, 10, e34951. [Google Scholar] [CrossRef]
- Paz, G.S.; Fernandes, J. Metformin as a Therapeutic Tool for Resistant Hematological Malignancies: A Literature Review. Chin. Clin. Oncol. 2024, 13, 39. [Google Scholar] [CrossRef]
- Lee, J.; Park, D.; Lee, Y. Metformin Synergistically Potentiates the Antitumor Effects of Imatinib in Colorectal Cancer Cells. Dev. Reprod. 2017, 21, 139–150. [Google Scholar] [CrossRef]
- Glamoclija, U.; Mahmutovic, L.; Bilajac, E.; Soljic, V.; Vukojevic, K.; Suljagic, M. Metformin and Thymoquinone Synergistically Inhibit Proliferation of Imatinib-Resistant Human Leukemic Cells. Front. Pharmacol. 2022, 13, 867133. [Google Scholar] [CrossRef]
- Kim, D.S.; Na, Y.J.; Kang, M.H.; Yoon, S.-Y.; Choi, C.W. Use of Deferasirox, an Iron Chelator, to Overcome Imatinib Resistance of Chronic Myeloid Leukemia Cells. Korean J. Intern. Med. 2016, 31, 357–366. [Google Scholar] [CrossRef]
- Matei, D.; Emerson, R.E.; Schilder, J.; Menning, N.; Baldridge, L.A.; Johnson, C.S.; Breen, T.; McClean, J.; Stephens, D.; Whalen, C.; et al. Imatinib Mesylate in Combination with Docetaxel for the Treatment of Patients with Advanced, Platinum-resistant Ovarian Cancer and Primary Peritoneal Carcinomatosis: A Hoosier Oncology Group Trial. Cancer 2008, 113, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Khaing, E.M.; Phaechamud, T.; Intaraphairot, T. Synergistic Anticancer Activity of Cinnamon Bark Oil and Imatinib Mesylate Combination on Colorectal Cancer Cell Lines. Key Eng. Mater. 2022, 914, 93–98. [Google Scholar] [CrossRef]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Willig, J.B.; De Couto, N.M.G.; Vianna, D.R.B.; Mariot, C.D.S.; Gnoatto, S.C.B.; Buffon, A.; Pilger, D.A. Betulinic Acid-Brosimine B Hybrid Compound Has a Synergistic Effect with Imatinib in Chronic Myeloid Leukemia Cell Line, Modulating Apoptosis and Autophagy. Pharmaceuticals 2023, 16, 586. [Google Scholar] [CrossRef]
- Danışman Kalındemirtaş, F.; Birman, H.; Candöken, E.; Bilgiş Gazioğlu, S.; Melikoğlu, G.; Kuruca, S. Cytotoxic Effects of Some Flavonoids and Imatinib on the K562 Chronic Myeloid Leukemia Cell Line: Data Analysis Using the Combination Index Method. Balk. Med. J. 2019, 36, 96–105. [Google Scholar] [CrossRef]
- Kampers, L.F.C.; Metselaar, D.S.; Vinci, M.; Scirocchi, F.; Veldhuijzen Van Zanten, S.; Eyrich, M.; Biassoni, V.; Hulleman, E.; Karremann, M.; Stücker, W.; et al. The Complexity of Malignant Glioma Treatment. Cancers 2025, 17, 879. [Google Scholar] [CrossRef]
- Lu, J.; Hu, Y.; Qian, R.; Zhang, Y.; Yang, X.; Luo, P. Enhanced Proliferation Inhibition and Apoptosis in Glioma Cells Elicited by Combination of Irinotecan and Imatinib. Eur. J. Pharmacol. 2020, 874, 173022. [Google Scholar] [CrossRef]
- Stavrakeva, K.; Popova, M.; Esad, M.; Apostolova, E.; Kokova, V.; Bacelova, M.; Alakidi, A.; Bivolarska, A. Drug-Induced Liver Toxicity. Acta Medica Bulg. 2024, 51, 77–85. [Google Scholar] [CrossRef]
- Cortes, J.; Goldman, J.M.; Hughes, T. Current Issues in Chronic Myeloid Leukemia: Monitoring, Resistance, and Functional Cure. J. Natl. Compr. Canc. Netw. 2012, 10, S-1–S-13. [Google Scholar] [CrossRef]
- Knight, G.W.A.; Mclellan, D. Use and Limitations of Imatinib Mesylate (Glivec), a Selective Inhibitor of the Tyrosine kinaseAbl Transcript in the Treatment of Chronic Myeloid Leukaemia. Br. J. Biomed. Sci. 2004, 61, 103–111. [Google Scholar] [CrossRef]
- Amir, M.; Javed, S. A Review on the Therapeutic Role of TKIs in Case of CML in Combination With Epigenetic Drugs. Front. Genet. 2021, 12, 742802. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. A Discussion of Chronic Myeloid Leukemia. J. Innov. Med. Res. 2023, 2, 11–22. [Google Scholar] [CrossRef]
- Cohen, M.H.; Johnson, J.R.; Pazdur, R.U.S. Food and Drug Administration Drug Approval Summary: Conversion of Imatinib Mesylate (STI571; Gleevec) Tablets from Accelerated Approval to Full Approval. Clin. Cancer Res. 2005, 11, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Sutter, B.; BÜRGER, H.M. Crystal Modification of a N-Phenyl-2-Pyrimidineamine Derivative, Processes for Its Manufacture and Its Use. WO1999003854A1, 28 January 1999. [Google Scholar]
- Musumeci, F.; Schenone, S.; Grossi, G.; Brullo, C.; Sanna, M. Analogs, Formulations and Derivatives of Imatinib: A Patent Review. Expert Opin. Ther. Pat. 2015, 25, 1411–1421. [Google Scholar] [CrossRef]
- Latagliata, R.; Breccia, M.; Carmosino, I.; Cannella, L.; De Cuia, R.; Diverio, D.; Frustaci, A.; Loglisci, G.; Mancini, M.; Santopietro, M.; et al. “Real-Life” Results of Front-Line Treatment with Imatinib in Older Patients (≥65 Years) with Newly Diagnosed Chronic Myelogenous Leukemia. Leuk. Res. 2010, 34, 1472–1475. [Google Scholar] [CrossRef]
- Cervantes, F.; Correa, J.-G.; Pérez, I.; García-Gutiérrez, V.; Redondo, S.; Colomer, D.; Jiménez-Velasco, A.; Steegmann, J.-L.; Sánchez-Guijo, F.; Ferrer-Marín, F.; et al. Imatinib Dose Reduction in Patients with Chronic Myeloid Leukemia in Sustained Deep Molecular Response. Ann. Hematol. 2017, 96, 81–85. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology of Tyrosine Kinase Inhibitors in Cancer Therapy: A Perspective. Int. J. Mol. Sci. 2021, 22, 6538. [Google Scholar] [CrossRef]
- Kalaydina, R.-V.; Bajwa, K.; Qorri, B.; DeCarlo, A.; Szewczuk, M.R. Recent Advances in “Smart” Delivery Systems for Extended Drug Release in Cancer Therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef]
- Svenson, S. What Nanomedicine in the Clinic Right Now Really Forms Nanoparticles? WIREs Nanomed. Nanobiotechnology 2014, 6, 125–135. [Google Scholar] [CrossRef]
- Hawthorne, D.; Pannala, A.; Sandeman, S.; Lloyd, A. Sustained and Targeted Delivery of Hydrophilic Drug Compounds: A Review of Existing and Novel Technologies from Bench to Bedside. J. Drug Deliv. Sci. Technol. 2022, 78, 103936. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Fardshouraki, S.; Mirian, M.; Safaeian, L.; Jandaghian, S.; Taymouri, S. Encapsulation of Imatinib in Targeted KIT-5 Nanoparticles for Reducing Its Cardiotoxicity and Hepatotoxicity. Anticancer Agents Med. Chem. 2020, 20, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Fabrication and Characterization of Chitosan-Based Polymeric Nanoparticles of Imatinib for Colorectal Cancer Targeting Application. Int. J. Biol. Macromol. 2020, 151, 104–115. [Google Scholar] [CrossRef]
- Hasandoost, L.; Akbarzadeh, A.; Attar, H.; Heydarinasab, A. In Vitro Effect of Imatinib Mesylate Loaded on Polybutylcyanoacrylate Nanoparticles on Leukemia Cell Line K562. Artif. Cells Nanomed. Biotechnol. 2017, 45, 665–669. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, X. Oral Delivery of Imatinib through Galactosylated Polymeric Nanoparticles to Explore the Contribution of a Saccharide Ligand to Absorption. Int. J. Pharm. 2019, 568, 118508. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Shinde, P.; Page, A.; Prajapati, B.G. Poly Lactic Co-Glycolic Acid d-α-Tocopheryl Polyethylene Glycol 1000 Succinate Fabricated Polyethylene Glycol Hybrid Nanoparticles of Imatinib Mesylate for the Treatment of Glioblastoma Multiforme. Curr. Med. Chem. 2025, 32, 8350–8370. [Google Scholar] [CrossRef]
- El-Mezayen, N.S.; El-Hadidy, W.F.; El-Refaie, W.M.; Shalaby, T.I.; Khattab, M.M.; El-Khatib, A.S. Hepatic Stellate Cell-Targeted Imatinib Nanomedicine versus Conventional Imatinib: A Novel Strategy with Potent Efficacy in Experimental Liver Fibrosis. J. Control. Release 2017, 266, 226–237. [Google Scholar] [CrossRef]
- Molaahmadi, M.R.; Varshosaz, J.; Taymouri, S.; Akbari, V. Lipid Nanocapsules for Imatinib Delivery: Design, Optimization and Evaluation of Anticancer Activity Against Melanoma Cell Line. Iran. J. Pharm. Res. 2019, 18, 1676. [Google Scholar] [CrossRef]
- Siram, K.; Karuppaiah, A.; Gautam, M.; Sankar, V. Fabrication of Hyaluronic Acid Surface Modified Solid Lipid Nanoparticles Loaded with Imatinib Mesylate for Targeting Human Breast Cancer MCF-7 Cells. J. Clust. Sci. 2023, 34, 921–931. [Google Scholar] [CrossRef]
- Labala, S.; Mandapalli, P.K.; Kurumaddali, A.; Venuganti, V.V.K. Layer-by-Layer Polymer Coated Gold Nanoparticles for Topical Delivery of Imatinib Mesylate To Treat Melanoma. Mol. Pharm. 2015, 12, 878–888. [Google Scholar] [CrossRef]
- Naeimipour, B.; Moniri, E.; Vaziri Yazdi, A.; Safaeijavan, R.; Faraji, H. Green Biosynthesis of Magnetic Iron Oxide Nanoparticles Using Mentha Longifolia for Imatinib Mesylate Delivery. IET Nanobiotechnol. 2022, 16, 225–237. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Saeed, A.; Rahman, M.S.U.; Naseer, M.M. Biocompatible, pH-Responsive, and Biodegradable Polyurethanes as Smart Anti-Cancer Drug Delivery Carriers. React. Funct. Polym. 2018, 127, 153–160. [Google Scholar] [CrossRef]
- Mashreghi, M.; Sabeti, B.; Chekin, F. Magnetite Graphene Oxide-Albumin Conjugate: Carrier for the Imatinib Anticancer Drug. J. Mater. Sci. Mater. Med. 2023, 34, 32. [Google Scholar] [CrossRef]
- Aslehashemi, A.; Miralinaghi, M.; Heydarinasab, A. A Magnetic/pH Dual-Sensitive Nanocarrier Based on Biopolymer-Grafted Mesoporous Fe3O4@SiO2 for Imatinib Delivery: Fabrication, Characterization, and Study of the in-Vitro Release Kinetics. Inorg. Chem. Commun. 2025, 174, 113970. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Sun, M.; Fan, Z.; Du, J.-Z. Intestine Enzyme-Responsive Polysaccharide-Based Hydrogel to Open Epithelial Tight Junctions for Oral Delivery of Imatinib against Colon Cancer. Chin. J. Polym. Sci. 2022, 40, 1154–1164. [Google Scholar] [CrossRef]
- Pang, S.; Wu, R.; Lv, W.; Zou, J.; Li, Y.; Li, Y.; Zhang, P.; Ma, X.; Wang, Y.; Liu, S. Use of a pH-Responsive Imatinib Mesylate Sustained-Release Hydrogel for the Treatment of Tendon Adhesion by Inhibiting PDGFRβ/CLDN1 Pathway. Bioact. Mater. 2024, 38, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Zhang, W.; Yang, T.; Lu, Y.; Lu, M.; Gai, Y.; Ma, X.; Xiang, G. Folate Receptor-Targeted Liposomes Enhanced the Antitumor Potency of Imatinib through the Combination of Active Targeting and Molecular Targeting. Int. J. Nanomed. 2014, 9, 2167. [Google Scholar] [CrossRef]
- Sadat Shandiz, S.A.; Shafiee Ardestani, M.; Shahbazzadeh, D.; Assadi, A.; Ahangari Cohan, R.; Asgary, V.; Salehi, S. Novel Imatinib-Loaded Silver Nanoparticles for Enhanced Apoptosis of Human Breast Cancer MCF-7 Cells. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1082–1091. [Google Scholar] [CrossRef]
- Varshosaz, J.; Jandaghian, S.; Mirian, M.; Sajjadi, S.E. Co-Delivery of Rituximab Targeted Curcumin and Imatinib Nanostructured Lipid Carriers in Non-Hodgkin Lymphoma Cells. J. Liposome Res. 2021, 31, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Chang, R.; Peng, A.; Feng, C.; Zhu, W.; Chen, Y.; Tian, X.; Wang, R.; Yan, H.; Jia, D.; et al. Efficient Treatment of Colon Cancer with Codelivery of TRAIL and Imatinib by Liposomes. Pharm. Dev. Technol. 2024, 29, 52–61. [Google Scholar] [CrossRef]
- Vosoughifar, M.; Torabi, Z.; Jalali, H.; Dinari, M. Chitosan-Coated Covalent Triazine Framework-Imatinib Composite Nanofiber Skin Dressing for Melanoma Cancer Treatment. BioNanoScience 2025, 15, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, S.; Zhang, L.; Wang, S.; Qin, H.; Wei, Y.; Wu, X.; Zhang, M. Imatinib@glycymicelles Entrapped in Hydrogel: Preparation, Characterization, and Therapeutic Effect on Corneal Alkali Burn in Mice. Drug Deliv. Transl. Res. 2025, 15, 171–184. [Google Scholar] [CrossRef]
- Ozgenc, E.; Karpuz, M.; Guler, G.; Burak, Z.; Başpınar, Y.; Gundogdu, E.A. Development and Evaluation of 177Lu-Imatinib: Radiolabeling and Cell Culture Studies. Radiochim. Acta 2025, 113, 899–911. [Google Scholar] [CrossRef]
- Shirzad, M.; Salahvarzi, A.; Razzaq, S.; Javid-Naderi, M.J.; Rahdar, A.; Fathi-karkan, S.; Ghadami, A.; Kharaba, Z.; Romanholo Ferreira, L.F. Revolutionizing Prostate Cancer Therapy: Artificial Intelligence—Based Nanocarriers for Precision Diagnosis and Treatment. Crit. Rev. Oncol. Hematol. 2025, 208, 104653. [Google Scholar] [CrossRef]
- Siapoush, S.; Mousazadeh, H.; Rezaei, R.; Hatami, B.; Mazhari, S.; Hashemi, N.; Reza Zali, M.; Baghaei, K. Oral Targeted Delivery of Imatinib by pH Responsive Copolymer Modulates Liver Fibrosis in the Mice Model. Int. J. Pharm. 2023, 641, 123068. [Google Scholar] [CrossRef] [PubMed]


| Disease | Model | IMT Dose | Evidence Level | Outcome |
|---|---|---|---|---|
| Alzheimer’s disease | Mouse model | 20 mg/kg/day i.p. | In vivo | Reduced amyloid-ß and improved cognitive performance [111] |
| Alzheimer’s disease | (1) Mouse model (2) Neuronal cells | 30 mg/kg/day i.p. | (1) In vivo (2) In vitro | Reduced neuronal apoptosis [51] |
| Acute ischaemic stroke | Rat model | 100 mg/kg | In vivo study | 1. Reduced BBB permeability; 2. Reduced cerebral edema; 3. Increased expression of the tight-junction protein ZO-1; 4. Reduced activation of NF-kB [112]. |
| Acute ischaemic stroke | 60 patients 18–85 years old | 400, 600, 800 mg | Phase II clinical study | Redtored BBB, reduced inflammation and edema [55] |
| Asthma | 62 patients aged 18–65 | 200 mg/day for 2 weeks, then 400 mg/day | A randomized double-blind placebo- controlled trial | Decreased airway Hyperresponsiveness [69] |
| Crohn’s disease | Case report | 600 mg/day | Case report | Long-lasting remission [113] |
| Crohn’s disease | Human case series | 300–400 mg | Case series | Remission in 4/6 patients [114] |
| Colon cancer | SW480 cell lines | n/a | Cell culture | Inhibited proliferation of colon cancer cells [115] |
| Early weight gain/insulin resistance | (1) Mouse model; (2) Human endothelial cells | (1) In vivo (2) In vitro | Improved insulin-mediated vasodilatation and reduced free fatty acid transport [102] | |
| Fibrodysplasia ossificans progressiva | 6 children | 340 mg/m2/ day | In vivo study | Decrease intensity and frequency of flare-ups [61] |
| cGVHD | Human clinical pilot study: 19 patients | Starting dose 100 mg/ day | Case series | 7 complete and 8 partial remissions among 19 patients 18-month overall survival~84% [116] |
| Steroid- Refractory cGVHD | Open-label Pilot II trial in children and adults | 100–400 mg daily in adults 65–260 mg/m2 in children | Phase II single-arm trial | Partial response in ~36% and overall clinical benefit in ~86% of patients [117] |
| Refractory sclerotic cGVHD | Retrospective study with 14 patients | 100–400 mg daily | Retrospective study | 50% response rate; Steroid dose reduced; Some patients discontinued treatment [118] |
| Acute and chronic GVHD | Human patients | 100–400 mg/ day/orally | Clinical study | Improved skin/mucosal lesions [63] |
| Sclerodermatous cGVHD | Mouse model | 150 mg/kg/ daily by oral | In vivo | IMT decreased the proliferation of total T cells and of regulatory T cells [119] |
| Diabetes mellitus | Rat model | 10 mg/kg 20 mg/kg | In vivo | Enhanced serum insulin levels Reduced serum glucagon levels [120] |
| Diabetes Type 1 | Randomized, double-blind, placebo-controlled, phase II trial In adults (aged 18–45) | 400 mg/day (4 × 100 mg) for 26 weeks | Randomized, double-blind, placebo-controlled, phase II trial | Enhanced ß-cell function and ß-cell glucose responsiveness [121] |
| Diabetic nephropathy | ApoE-knockout mouse model | 10 mg/kg/day | In vivo | Reduced diabetic nephropathy in apolipoprotein E-knockout mice [122] |
| Radiation- induced skin fibrosis | Mouse model | 0.5 mg/g | Preclinical (in vivo) | Reduced radiation-induced skin fibrosis and collagen deposition [75] |
| Gouty Arthritis | Mouse model | 30/100 mg/kg i.p. | In vivo study | Reduced joint swelling and inflammation in MSU-induced gout [95] |
| Gaucher disease | Mouse model Human fibroblasts from patients | n/a | In vivo study In vitro study | IMT inhibition of c-Abl reduces RIPK3 activation, suggesting therapeutic potential [108] |
| Chronic glomerulo- sclerosis | Rat model | 10 mg/kg/day | In vivo | IMT slowed the progression of Glomerulosclerosis [105] |
| Chronic allograft nephopathy | Rat model | 10 mg/kg/day | In vivo | IMT prevented the progression of chronic allograft [123] |
| Hypertension- induced end- organ damage | Rat model | 30 mg/kg/day | In vivo study | IMT reduced cardiac dysfunction and protected renal microvasculature [124] |
| Spontaneous Intracerebral Hemorrhage | Rat model | 60 mg/kg i.p. | In vivo study | 1. IMT mitigates cerebral vasospasm; 2. IMT helps maintain BBB integrity [56]. |
| Spontaneous Intracerebral Hemorrhage | Mouse model | 30,60,120 mg/kg i.p. | In vivo study | 1. Reduced brain edema; 2. Better neurobehavioral outcomes; 3. Maintained BBB integrity [57]. |
| Subarachnoid hemorrhage | Rat model | 40 mg/kg 120 mg/kg | In vivo study | Maintained BBB integrity and enhanced neurological function [125] |
| Leishmaniasis | In vitro parasite model | Various concentrations tested | In vitro comparative study | Dose-dependent reduction in the viability of Leishmania major, though amphotericin B showed stronger activity [85] |
| Malaria | Clinical trial in humans | 400 mg/day for 3 days | Phase II clinical trial | Accelerated parasite clearance and fever resolution with no increase in adverse events [126] |
| Chloroquine- resistant malaria | Open-label, prospective case–control study in male patients | 400 mg for 3 days | Clinical-prospective, case–control study | Faster fever reduction; More rapid parasite clearance [86] |
| Melanoma | Retrospective control study of 78 patients | 400 mg/day | Retrospective study | Median overall survival (OS) 13.1 months Progression-free survival (PFS) 4.2 months [97] |
| Metastatic melanoma | Phase II study involving 43 patients with c-KIT mutations | 400 mg/day | Phase II open-label, single-arm study | Overall response rate of 23.3%; [127] |
| Melanoma with KIT alternations | Retrospective study of 38 KIT-altered melanoma patients | 400 mg/day | Clinical- multicenter retrospective study | PFS and OS were longer in patients with exon 11/13 mutations compared to exon 17 mutations [128] |
| Metastatic melanoma | A phase II study of patients with metastatic melanoma with KIT mutation | 400 mg twice daily | A single-group, open-label, phase 2 trial | Clinical responses observed in subset of KIT-altered metastatic melanoma patients [96] |
| Morphea | Human case report | 200 mg/day | n/a | Clinical improvement with reduced skin thickening [129] |
| Human therapy-resistant generalized deep morphea | Human case report | 400 mg/day | Case report 2015 | Marked clinical improvement No new lesions during 11-month follow-up [130] |
| Multiple sclerosis | Mouse model and U-87 MG, C6 and WEHI-164 cell lines | 60 mg/kg/day | Preclinical (mouse model and in vitro cell lines) | In vivo reduced disease severity and delayed symptom onset In vitro: decreased cell proliferation, lower pro-inflammatory cytokines [67] |
| Multiple sclerosis | Rat model | Mourine dosing | Preclinical | Reduced blood–brain barrier integrity and reduced neuroinflammation [131] |
| Niemann–Pick Type C | Mouse model | 5 mg/kg in NaCl | In vitro study | 1. Counteract weight loss; 2. Improve neurological function; 3. Enhance Purkinje cells’ survival; 4. Extend lifespan [60]. |
| Psoriasis | Imiquimod-induced psoriasis-like skin in mice | Mourine dosing | Preclinical (mouse model) | Topical IMTM ameliorated psoriasis- like skin lesions by inhibiting angiogenesis [73] |
| Psoriasis | Case study | 400 mg/daily | n/a | Improved skin lesions and complete hematologic remission [132] |
| Pulmonary arterial hypertension | 17 patients | 200 mg starting dose | A phase III study | Lower pulmonary pressure, higher cardiac output, reduced vascular resistance [133] |
| Pulmonary arterial hypertension | 15 patients | 400 mg | Observational study In vivo | Improvement in hemodynamics, quality of life and echocardiographic parameters of right ventricular function [68] |
| Renal fibrosis | Rat model | Days 1 and 2: 50 mg/kg; days 3 and 4: 100 mg/kg; days 5–7: 150 mg/kg) | In vivo | IMT reduced renal fibrogenesis and blocked TGF-ß [134] |
| Spinal cord injury | Rat study | 100 mg/kg i.p. | In vivo | Improved functional recovery and reduced secondary spinal cord damage [58] |
| Systemic sclerosis-assosiated interstitial lung disease | Phase I/IIa one-year, open-label pilot trial | Up to 600 mg/day | Clinical trial Phase I/IIa single-arm | 12/20 patients completed the treatment; 7 withdrew due to adverse effects 1 lost to follow-up [135] |
| Systemic sclerosis | Mouse models of SSc | 150 mg/kg/ day | Preclinical (in vivo) | Prevented fibrosis and induced regression of established fibrosis [74] |
| Human- refractory diffuse systemic sclerosis | Case report | 400 mg/day | Case report | Modest improvement in skin scores; Partial clinical responses [136] |
| Systemic mastocytosis | Clinical study on 20 patients | 400 mg/day | Phase II clinical study | 1 patient with complete remission 6 patients with symptomatic improvement [137] |
| Systemic mastocytosis | Phase II study | 400 mg/day | Phase II clinical study | IMT was effective in treatment, including those who had the D816V mutation [138] |
| Systemic mastocytosis | Adult patients | 100–400 mg/day | n/a | Partial/complete improvement. Response depends on KIT mutation status [139] |
| Systemic mastocytosis (indolent and advanced forms) | Adult patients | Dose depending on disease severity and KIT mutation | Case series | Hematologic and symptomatic improvement observed mainly in patients without KIT D816V mutation [93] |
| Systemic mastocytosis | Adult patients without an exon 17 KIT mutation | 300–400 mg/day | Phase IV clinical study | Well-tolerated safety profile; Partial/complete hematologic and symptomatic improvement in patients without KIT D816V mutation [140] |
| Systemic lupus erythematosus with lupus nephritis | Mouse model | 10 mg/kg 50 mg/kg | In vivo study | IMT (50 mg/kg) prevented glomerular cell proliferation, crescent formation, and reduced mesengial matrix [141] |
| Ulcerative colitis | Rat model | 10 mg/kg/day 20 mg/kg/day (oral pretreatment for 1 week) | Preclinical animal study | Pretreatment with IMT significantly reduced macroscopic and histologic damage, decreased oxidative and inflammatory markers and suppressed COX-2 signaling in the colon [72] |
| System Organ Class Disorders | Adverse Effects |
|---|---|
| Cardiovascular |
|
| Dermatologic |
|
| Gastrointestinal |
|
| General |
|
| Hematologic |
|
| Hepatic |
|
| Infectious |
|
| Metabolic/nutritional |
|
| Musculoskeletal |
|
| Neurological |
|
| Ocular |
|
| Psychiatric |
|
| Renal and urinary |
|
| Respiratory |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdeva, Y.; Georgieva, P.; Katsarov, P. Imatinib in Targeted Therapy: Advances in Biomedical Applications and Drug Delivery Systems. Hemato 2025, 6, 40. https://doi.org/10.3390/hemato6040040
Gvozdeva Y, Georgieva P, Katsarov P. Imatinib in Targeted Therapy: Advances in Biomedical Applications and Drug Delivery Systems. Hemato. 2025; 6(4):40. https://doi.org/10.3390/hemato6040040
Chicago/Turabian StyleGvozdeva, Yana, Petya Georgieva, and Plamen Katsarov. 2025. "Imatinib in Targeted Therapy: Advances in Biomedical Applications and Drug Delivery Systems" Hemato 6, no. 4: 40. https://doi.org/10.3390/hemato6040040
APA StyleGvozdeva, Y., Georgieva, P., & Katsarov, P. (2025). Imatinib in Targeted Therapy: Advances in Biomedical Applications and Drug Delivery Systems. Hemato, 6(4), 40. https://doi.org/10.3390/hemato6040040








