Immuno-Hematological Complications of Transfusion in Thalassemia Patients: First Report in the Marrakech Region (Morocco)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- -
- Sociodemographic data: age, sex.
- -
- Clinical data: types of thalassemia.
- -
- Transfusion data: frequency of transfusions per year, number of bags of packed red blood cells per year, average interval between transfusions per week.
- -
- Immunohematological data: ABO/Rh group, RAI status, TCD, and alloantibody types.
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patients
3.2. Characteristics of Transfusion in the Study Population
3.3. Comparison Between Alloimmunized and Non-Alloimmunized Thalassemia Patients
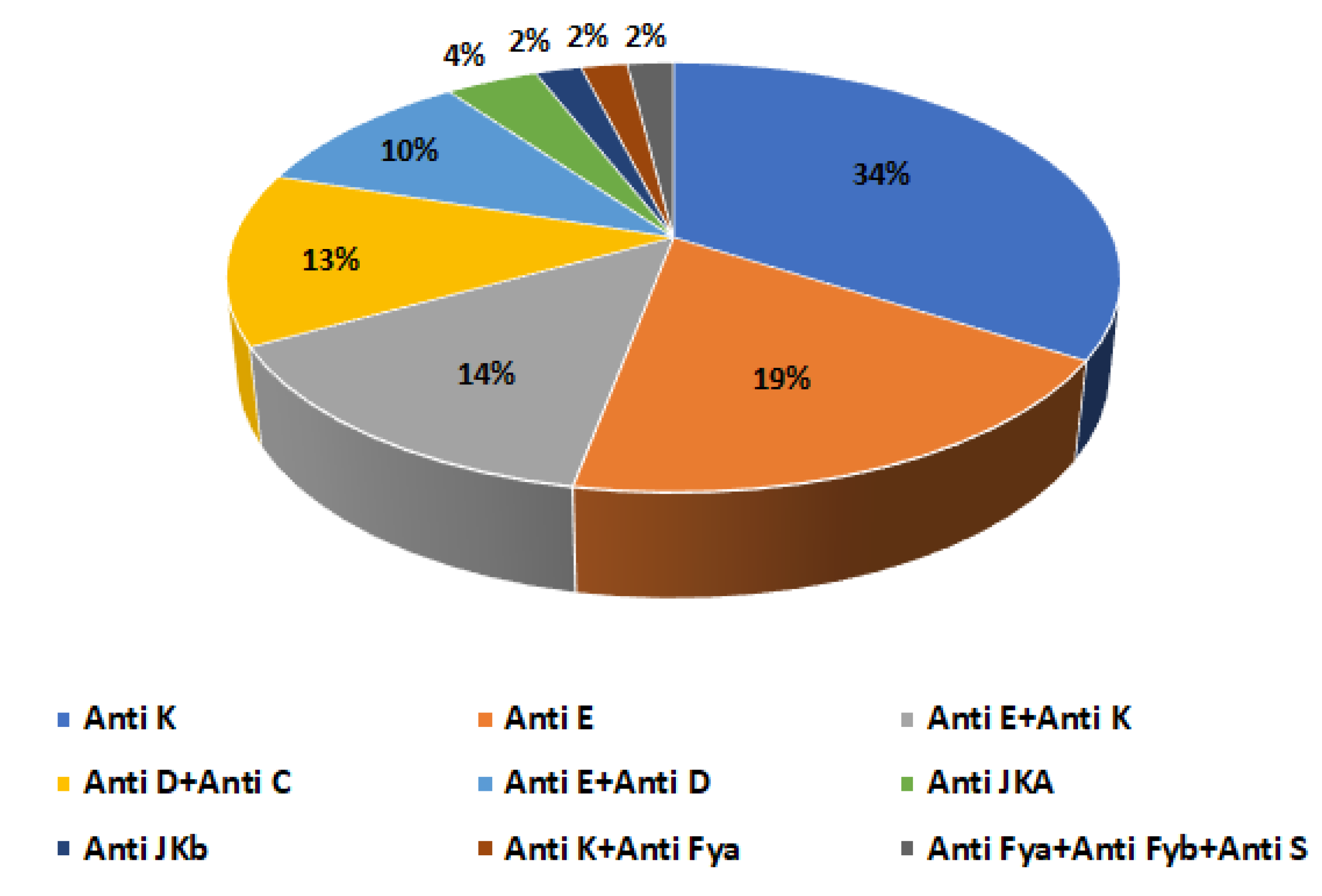
3.4. Alloantibodies in Alloimmunized Thalassemic Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kattamis, A.; Kwiatkowski, J.L.; Aydinok, Y. Thalassaemia. Lancet 2022, 18, 2310–2324. [Google Scholar] [CrossRef] [PubMed]
- Elghetany, M.T.; Banki, K. Erythrocytic disorders. In Henry’s Clinical Diagnosis and Management by Laboratory Methods, 21st ed.; McPherson, R.A., Pincus, M.R., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2007; pp. 528–529. [Google Scholar]
- Baird, D.C.; Batten, S.H.; Sparks, S.K. Alpha- and Beta-thalassemia: Rapid Evidence Review. Am. Fam. Physician 2022, 105, 272–280. [Google Scholar] [PubMed]
- Borgna-Pignatti, C.; Galanello, R. Thalassemias and related disorders: Quantitative disorders of hemoglobin synthesis. In Wintrobe’s Clinical Hematology, 11th ed.; Greer, J.P., Rodgers, G.M., Paraskevas, F., Foerster, J., Lukens, J.N., Glader, B., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2004; pp. 1332–1335. [Google Scholar]
- Rebulla, P. Blood transfusion in beta thalassaemia major. Transfus. Med. 1995, 5, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Prati, D. Benefits and complications of regular blood transfusion in patients with beta-thalassemia major. Vox Sang. 2000, 79, 129–137. [Google Scholar] [CrossRef]
- Bhatti, F.A.; Salamat, N.; Nadeem, A.; Shabbir, N. Red cell immunization in beta thalassemia major. J. Coll. Physicians Surg. Pak. 2004, 14, 657–660. [Google Scholar]
- Salama, M.A.; Sadek, N.A.; Hassab, H.M.; Abadeer, A.F.; Mikhael, I.L. Erythrocyte autoantibodies and expression of CD59 on the surface of red blood cells of polytransfused patients with beta-thalassemia major. Br. J. Biomed. Sci. 2004, 61, 88–92. [Google Scholar] [CrossRef]
- Singer, S.T.; Wu, V.; Mignacca, R.; Kuypers, F.A.; Morel, P.; Vichinsky, E.P. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood 2000, 96, 3369–3373. [Google Scholar] [CrossRef]
- Franchini, M.; Forni, G.L.; Marano, G.; Cruciani, M.; Mengoli, C.; Pinto, V.; Liumbruno, G.M. Red blood cell alloimmunisation in transfusion-dependent thalassaemia: A systematic review. Blood Transfus. 2019, 17, 4. [Google Scholar]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef]
- Khan, M.S. Consanguinity ratio in β-thalassemia major patients in District Bannu. J. Pak. Med. Assoc. 2015, 65, 1161–1163. [Google Scholar]
- Bejaoui, M.; Guirat, N. Beta thalassemia major in a developing country: Epidemiological, clinical and evolutionary aspects. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013002. [Google Scholar] [CrossRef]
- Laghari, Z.; Baig, N.; Charan, T.; Lashari, K.; Suhag, R. Distribution of ABO Blood Groups and Rhesus Factor in ß-Thalassemia Patients at Thalassemia Care Center NawabShah, Pakistan. Sindh Univ. Res. J. 2018, 50, 123–128. [Google Scholar] [CrossRef]
- Agouzal, M.; Quyou, A.; Benchekroune, K.; Khattab, M. Aspects épidémiologiques et économiques des traitements chélateurs au centre thérapeutique de la thalassémie au Maroc. Rev. Méd. Bruxelles 2010, 31, 73–144. [Google Scholar]
- Romdhane, H.; Amara, H.; Abdelkefi, S.; Souyeh, N.; Chakroun, T.; Jarrey, I.; Yacoub, S.J. Profil clinico-biologique et immunohématologique des patients atteints de β-thalassémie en Tunisie: À propos de 26 cas. Transfus. Clin. Biol. 2014, 21, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef]
- Hamani, F.; Oribi, C. La prévalence de la bêta-thalassémie au niveau de l’EPH Ain Tadless. DSpace 2018, 2, 181. [Google Scholar]
- Zahir, H.; Chakour, M.; Mouhib, H.; Yahyaoui, H.; Ameur, M.A. Aspect épidémiologique, clinico-biologique, thérapeutique et évolutif de la ß-thalassémie au Maroc. Ann. Biol. Clin. 2019, 77, 169–173. [Google Scholar]
- Ouadghiri, S.; Morabit, K.E.; Elansari, N.; Atouf, O.; Elkababri, M.; Hessissen, L.; Essakalli, M. Human leukocyte antigen immunization in transfusiondependent Moroccan patients with beta-thalassemia major: Prevalence and risk factors. Hemato. Trans. & Cell Th. 2024, 46, 360–365. [Google Scholar]
- Laghmami, R. Les thalassémies en région de Marrakech, Haouz et Sud du Maroc. Ph.D. Dissertation, Cadi Ayyad University, Marrakech, Morocco, 2018. [Google Scholar]
- Agouzal, M.; Arfaoui, A.; Quyou, A.; Khattab, M. Beta thalassemia major: The Moroccan experience. J. Public Health Epidemiol. 2010, 2, 25–28. [Google Scholar]
- Almorish, M.A.; Al-Absi, B.; Elkhalifa, A.M.; Alhamidi, A.H.; Abdelrahman, M. Red blood cell alloimmunization in blood transfusion-dependent β thalassemia major patients in Sana’a City-Yemen. Sci. Rep. 2024, 14, 1005. [Google Scholar] [CrossRef]
- Debele, G.J.; Fita, F.U.; Tibebu, M. Prevalence of ABO and Rh blood group among volunteer blood donors at the blood and tissue bank service in Addis Ababa, Ethiopia. J. Blood Med. 2023, 14, 19–24. [Google Scholar] [CrossRef]
- Benahadi, A.; Alami, R.; Boulahdid, S.; Adouani, B.; Laouina, A.; Mokhtari, A.; Benajiba, M. Distribution of ABO and Rhesus D blood antigens in Morocco. Internet J. Biol. Anthropol. 2013, 6, 1–6. [Google Scholar]
- Benalla, A.; Trougouty, N.; Sidqi, Z.; Mekhfi, H.; Benajiba, M. Distribution of ABO and Rh blood groups in the oriental region of Morocco. Mintage J. Pharm. Med. Sci. 2017, 6, 5–7. [Google Scholar]
- Ben Salah, N.; El Borgi, W.; Lakhal, F.B.; Mansour, M.B.; Gouider, E.; Gorgi, Y.; Hafsia, R. Immunisation anti-érythrocytaire et anti-HLA au cours des hémoglobinopathies. Transfus. Clin. Biol. 2014, 21, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, F.; Benkirane, S.; Kouzih, J.; Woumki, A.; Mamad, H.; Masrar, A. Profil épidémiologique des hémoglobinopathies: Étude transversale descriptive autour du cas index. Pan Afr. Med. J. 2017, 27, 1. [Google Scholar] [CrossRef]
- Cao, A.; Galanello, R. Beta-thalassemia. Genet. Med. 2010, 12, 61–76. [Google Scholar] [CrossRef]
- Ameen, R.; Al-Shemmari, S.; Al-Humood, S.; Chowdhury, R.I.; Al-Eyaadi, O.; Al-Bashir, A. RBC alloimmunization and autoimmunization among transfusion-dependent Arab thalassemia patients. Transfusion 2003, 43, 1604–1610. [Google Scholar] [CrossRef]
- Azarkeivan, A.; Ansari, S.; Ahmadi, M.H.; Hajibeigy, B.; Maghsudlu, M.; Nasizadeh, S.; Shaigan, M.; Toolabi, A.; Salahmand, M. Blood Transfusion and Alloimmunization in Patients with Thalassemia: Multicenter Study. Pediatr. Hematol. Oncol. 2011, 28, 479–485. [Google Scholar] [CrossRef]
- Yadav, B.K.; Chaudhary, R.K.; Elhence, P.; Phadke, S.R.; Mandal, K.; Saxena, D.; Moirangthem, A. Red cell alloimmunization and associated risk factors in multiply transfused thalassemia patients: A prospective cohort study conducted at a tertiary care center in Northern India. Asian J. Transfus. Sci. 2023, 17, 145–150. [Google Scholar] [CrossRef]
- El Kababi, S.; Benajiba, M.; El Khalfi, B.; Hachim, J.; Soukri, A. Red blood cell alloimmunizations in beta-thalassemia patients in Casablanca/Morocco: Prevalence and risk factors. Transfus. Clin. Biol. 2019, 26, 240–248. [Google Scholar] [CrossRef]
- Wilson, M.M.; El Masry, M.M.; El-Ghamrawy, M.K.; El-Hadi, N.A.; Abou-Elalla, A.A. Study of the frequency and specificity of red cell antibodies in patients with hemoglobinopathies. Indian J. Hematol. Blood Transfus. 2023, 39, 579–585. [Google Scholar] [CrossRef]
- Al-Riyami, A.Z.; Daar, S. Red cell alloimmunization in transfusion-dependent and transfusion-independent beta thalassemia: A review from the Eastern Mediterranean Region (EMRO). Transfus. Apher. Sci. 2019, 58, 102678. [Google Scholar] [CrossRef]
- Romphruk, A.V.; Simtong, P.; Butryojantho, C.; Pimphumee, R.; Junta, N.; Srichai, S.; Puapairoj, C. The prevalence, alloimmunization risk factors, antigenic exposure, and evaluation of antigen-matched red blood cells for thalassemia transfusions: A 10-year experience at a tertiary care hospital. Transfusion 2019, 59, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Valle Neto, O.G.D.; Alves, V.M.; Pereira, G.D.A.; Moraes-Souza, H.; Martins, P.R.J. Clinical and epidemiological profile of alloimmunized and autoimmunized multi-transfused patients against red blood cell antigens in a blood center of Minas Gerais. Hematol. Transfus. Cell Ther. 2018, 40, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hoeltge, G.A.; Domen, R.E.; Rybicki, L.A.; Schaffer, P.A. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Arch. Pathol. Lab. Med. 1995, 119, 42–45. [Google Scholar] [PubMed]
- Davari, K.; Soltanpour, M.S. Study of alloimmunization and autoimmunization in Iranian β-thalassemia major patients. Asian J. Transfus. Sci. 2016, 10, 88–92. [Google Scholar] [CrossRef]
- Vichinsky, E.; Neumayr, L.; Trimble, S.; Giardina, P.J.; Cohen, A.R.; Coates, T.; Boudreaux, J.; Neufeld, E.J.; Kenney, K.; Grant, A.; et al. Transfusion complications in thalassemia patients: A report from the Centers for Disease Control and Prevention (CME): Transfusion Complications in Thalassemia. Transfusion 2014, 54, 972–981. [Google Scholar] [CrossRef]
- Indriani, V.; Mulyono, B.; Triyono, T.; Handayaningsih, A.E.; Chandra, L.A. Prevalence of alloimmunization events in thalassemia patients with repeated transfusions in the Rhesus blood group system: A systematic review and meta-analysis. J. Clin. Med. Res. 2025, 17, 106. [Google Scholar] [CrossRef]
- Achargui, S.; Zidouh, A.; Abirou, S.; Merhfour, F.Z.; Monsif, S.; Amahrouch, S.; El Ghobre, A.; El Halhali, M.; Temmara, H.; El Hryfy, A. Identification des allo-anticorps seuls et associés: Bilan de trois années au centre régional de transfusion sanguine de Rabat/Maroc et difficultés de prise en charge transfusionnelle. Transfus. Clin. Biol. 2017, 24, 422–430. [Google Scholar] [CrossRef]
| Characteristics | Number of Patients n = 89 (%) | p Value |
|---|---|---|
| Sex | 0.004 | |
| Male | 46 (51.7%) | |
| Female | 43 (48.3%) | |
| Age (years) | 0.001 | |
| 0–18 | 47 (52.8%) | |
| 19–30 | 37 (41.6%) | |
| 31–70 | 5 (5.6%) | |
| ABO/Rh blood type | 0.001 | |
| A- | 1 (1.12%) | |
| A+ | 19 (21.34%) | |
| AB- | 1 (1.12%) | |
| AB+ | 5 (5.6%) | |
| B- | 3 (3.4%) | |
| B+ | 10 (11.23%) | |
| O- | 6 (6.79%) | |
| O+ | 44 (49.4%) | |
| Types of Thalassemia | 0.001 | |
| β-Th Major | 73 (67%) | |
| β-Th Intermediate | 9 (8.3%) | |
| β-Th Minor | 3 (2.8%) | |
| Th-Sickle Cell Disease Association | 4 (3.7%) |
| Parameter | β-Thalassemia Major | β-Thalassemia Intermediate | β-Thalassemia Minor | Thalassemia-Sickle Cell Association | p Value |
|---|---|---|---|---|---|
| Percentage of transfused cases (%) | 67 | 8.3 | 2.8 | 3.7 | 0.001 |
| Number of transfusions/year | 12–26 | 3–6 | 1 | 6–12 | 0.031 |
| Number of packed red blood cell units/year | 12–60 | 2–12 | 1–2 | 4–36 | 0.001 |
| Average interval between transfusions (week) | 0.5–4 | 8–16 | 50 | 2–6 | 0.047 |
| Characteristics | Alloimmunized Patients n = 42 (47.2%) | Non-Alloimmunized Patients n = 47 (52.8%) | p Value |
|---|---|---|---|
| Sex | ˂0.01 | ||
| Males | 26.9% | 24.7% | |
| Females | 20.3% | 28.1% | |
| Age (years) | 0.50 | ||
| 0–18 | 28% | 24.7% | |
| 19–30 | 16.8% | 24.7% | |
| 31–70 | 2.3% | 3.5% | |
| Number of transfusions/year | ˂0.01 | ||
| ˂12 | 2.3% | 52.8% | |
| ≥12 | 49.9% | 0% | |
| Number of bags of packed red blood cells/year (min–max) | 24–60 | 12–24 | ˂0.01 |
| Types of Thalassemia | ˂0.01 | ||
| β-Thalassemia Major | 46% | 36% | |
| β-Thalassemia Intermediate | 0% | 10.1% | |
| β-Thalassemia Minor | 0% | 3.4% | |
| Thalassemia-Sickle Cell Association | 1.1% | 3.4% | |
| Direct Antiglobulin Test (DAT) | ˂0.01 | ||
| DAT negative | 25.8% | 44.9% | |
| DAT positive | 21.4% | 7.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Hammou, H.; Elhidar, N.; Ouhammou, M.; Sansar, W.; Fazzani, S.; El Dhimni, T.; Sif Essalam, M. Immuno-Hematological Complications of Transfusion in Thalassemia Patients: First Report in the Marrakech Region (Morocco). Hemato 2025, 6, 35. https://doi.org/10.3390/hemato6040035
Ait Hammou H, Elhidar N, Ouhammou M, Sansar W, Fazzani S, El Dhimni T, Sif Essalam M. Immuno-Hematological Complications of Transfusion in Thalassemia Patients: First Report in the Marrakech Region (Morocco). Hemato. 2025; 6(4):35. https://doi.org/10.3390/hemato6040035
Chicago/Turabian StyleAit Hammou, Hanane, Najwa Elhidar, Mourad Ouhammou, Wafa Sansar, Samira Fazzani, Touria El Dhimni, and Mohamed Sif Essalam. 2025. "Immuno-Hematological Complications of Transfusion in Thalassemia Patients: First Report in the Marrakech Region (Morocco)" Hemato 6, no. 4: 35. https://doi.org/10.3390/hemato6040035
APA StyleAit Hammou, H., Elhidar, N., Ouhammou, M., Sansar, W., Fazzani, S., El Dhimni, T., & Sif Essalam, M. (2025). Immuno-Hematological Complications of Transfusion in Thalassemia Patients: First Report in the Marrakech Region (Morocco). Hemato, 6(4), 35. https://doi.org/10.3390/hemato6040035






