Abstract
Complete or partial deletions of chromosome 7 (-7/del7q) represent the most frequent chromosomal abnormalities observed in myeloid neoplasms (MNs) and are associated with a poor prognosis. -7/del7q is observed in 10–15% of adult patients with myelodysplasia (MDS) or with acute myeloid leukemia (AML). The occurrence of -7/del7q is particularly frequent in pediatric MDS, often associated with germline mutations of GATA2 or SAMD9/SAMD9L genes. The disease biology of -7/del7q and the genes driving leukemic development have not been completely elucidated, but the haploinsufficiency of tumor suppressor genes located in chromosome 7 deleted regions seems to play a relevant role. The response to standard treatments based either on chemotherapy or hypomethylating agents plus Venetoclax is limited. No approved targeted therapies exist for patients with -7/del7q; however, some recent studies have discovered some vulnerabilities of these myeloid neoplasms than can be efficiently targeted.
1. Introduction
Genetic alterations are responsible for the development of myeloid neoplasia (MN). Both recurrent gene mutation events and structural chromosomal alterations are observed in MN. The development of sensitive cytogenetic techniques allows us to detect frequent chromosomal aberrations in various types of MN, such as myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Conventional karyotyping showed cytogenetic abnormalities in about 50% of MDS cases and in 50–60% of adult AML cases [1].
The chromosomal alterations observed in MN involve insertion, deletion, duplication, translocation and inversion events; deletion events may involve whole chromosomes or chromosomal subregions. Aberrations of chromosome 7 [abn(7)] are recurrently observed in adult AML patients (about 10% of cases) and in adult MDS patients (about 13%) and particularly in pediatric MDS (30–40%) [2,3]. The most common abn(7) includes complete loss of chromosome 7 (monosomy 7, -7) and deletions of the long arm of chromosome 7 [del(7q)]. Both these alterations may occur both in MDS and AML in the context of a complex karyotype (CK), defined as a condition in which at three least different chromosomal abnormalities coexist in the same leukemic cells.
The monosomy of chromosome 7 was initially reported in 1964 in the context of a study proposing the existence of a new clinical syndrome consisting of a refractory anemia lacking some chromosomes, including chromosome 7, in bone marrow cells [4]. This syndrome corresponds to what today is defined as myelodysplastic syndrome (MDS) carrying monosomy 7. Subsequent studies have shown that monosomy 7 is frequently observed in pediatric patients with preleukemic conditions, such as MDS and juvenile myelomonocytic leukemia (JMML) [5].
2. Techniques for Detecting Abnormalities of Chromosome 7 in MDS and AML
Various abnormalities of chromosome 7 have been detected in MDS and AML and their characterization requires multiple techniques. Human chromosome 7 encompasses nearly 158 million nucleotides of DNA and 1917 gene structures; more than 360 disease-associated genes and loci on chromosome 7 have been discovered [6].
Different types of chromosome 7 abnormalities have been reported in myeloid neoplasms: (i) full monosomy 7, corresponding to the complete loss of one copy of chromosome 7; (ii) deletions of the long arm of chromosome 7, del(7q), with some minimal commonly deleted regions (CDRs), defined as 7q22 (CDR1), 7q34 (CDR2) and 7q35–36 (CDR3); (iii) der(1;7)(q10;p10), an imbalanced translocation leading to +1q and -7q; and (iv) uniparental disomy (UPD) 7q, also called copy-neutral loss of heterozygosity, an event of somatic loss of 7q with duplication of the homologous 7q (Figure 1).
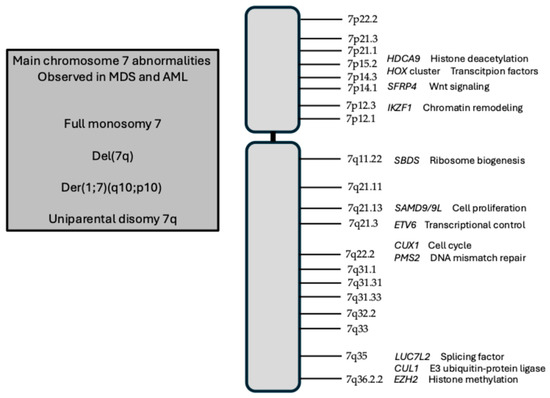
Figure 1.
Schematic representation of human chromosome 2, with major chromosome bands and with the localization of genes relevant in pathological alterations of this chromosome. In the box at the left, the main structural abnormalities of chromosome 7 in MDS and AML are shown.
Various cytogenetic and molecular techniques are currently used for the characterization of chromosome abnormalities.
Metaphase cytogenetics is based on Giemsa staining of metaphase chromosome alterations (such as trisomies or monosomies) unbalanced chromosomal defects, such as translocations and inversions, deletions and duplications. However, this technique cannot identify UDP and CN loss of heterozygosity (LOH) because the chromosome banding patterns remain unmodified.
Fluorescence in situ hybridization (FISH) in another important technique for the molecular analysis of chromosomal alterations in MDS and AML and is based on the use of fluorescently labeled DNA probes to detect chromosomal sequences of interest and to detect even small and hidden chromosomal alterations, such as gene fusions, deletions, or aneuploidies, with high sensitivity. The FISH technique is very useful for the detection of hidden cases of monosomy 7 or del(7q).
The single-nucleotide polymorphism array (SNP-A) technology is based on the hybridization of fluorescently labeled fragmented single-stranded DNA to a microarray chip containing hundreds of thousands of unique nucleotide probes. This technique allows a high-resolution scanning of the whole genome, detecting both copy number variation (CNV) and UDP.
Microarray-based comparative genome hybridization (Array-CGH) is an important technique allowing the detection and characterization of CNV and UDP together in a single analysis; this technique is based on comparing the hybridization of differentially labeled fragmented sample DNA and control DNA to the genome on the microarray platform for the detection of chromosomal aberrations. The fluorescence ratio of sample vs. control DNA hybridization signals is detected at the level of different positions of the genome and provides information about the relative DNA CN in the assayed genome in comparison with the normal diploid genome. The main advantage of Array-CGH over standard cytogenetic methods is that it can detect CNV simultaneously at the level of multiple genomic loci and can analyze a wide spectrum of genes on microarrays in a single experiment.
Next-generation sequencing (NGS) can provide valuable information for the detection of chromosomal aberrations; NGS can be used to detect CNV and structural variants in myeloid malignant genomes. NGS technologies display some relevant advantages compared to standard cytogenetic methods, related to a higher capacity of detection of genome-wide chromosomal alterations; higher sensitivity in that alterations, even present in 1% of cells, can be detected; and higher potential to longitudinally monitor alterations during treatment.
This technological armamentarium allows us to detect and characterize chromosome 7 alterations occurring in MDS and AML with high specificity and sensitivity [7]
In a screening on 1131 myeloid neoplasms, Hosono et al., using metaphase cytogenetics and SNP array-based karyotype analysis, detected LOH lesions affecting 7q in 14% of low-risk MDS, 34% of high-risk MDS, 8% of primary AML, 18% of secondary AML and 13% of MDS/myeloproliferative neoplasms [8].
Among the various chromosome 7 abnormalities, der(1;7)(q10;p10) displays some peculiarities. In fact, this unbalanced translocation is much more frequent in Asian than in European patients with myeloid neoplasms (5.8% vs. 0.4%, respectively) [9]. Der(1;7)(q10;p10) was more enriched in MDS (72.3% of total cases) than in AML (23.55) and MDS/myeloproliferative neoplasms (3.4%) [9]. In MDS, der(1;7)(q10;p10) and 7/del(7q) have a significantly lower overall survival compared to MDS without these abnormalities [9]. The co-mutational profile of der(1;7)(q10;p10) is significantly different to that observed in -7/del(7q) cases in that the frequency of RUNX1, EZH2, ETNK1, ETV6 and MYB genes is markedly higher in the former than in the latter, while the opposite is true for TP53 mutations [9]. Furthermore, del(5q) is absent in der(1;7)(q10;p10), while it is frequent in -7/del(7q) cases [9].
The types of chromosome 7 abnormalities observed in various types of myeloid neoplasms are similar. Thus, Haferlach and coworkers reported the characterization of 81 cases with myeloid malignancies and del((7q); Array-CGH showed interstitial del(7q) in 67 cases and terminal ones in 14 cases [10]. FISH analysis showed unbalanced translocations in 10 of the 14 cases with terminal deletion; partner chromosomes of these translocations were heterogeneous and the breakpoints on chromosome 7 were diverse, ranging from 7q11 to 7q32 [10]. In the 67 cases with interstitial del(7q), the size of del(7q) varied between 1.8 and 158.9 Mb [10]. Sizes and localizations of del(7q) largely overlapped between MDS and AML [10]. In total, 92% of all patients with del(7q) harbored at least one molecular mutation; TET2 and ASXL1 were the most frequently mutated genes and were present at comparable frequencies in MDS and AML; and AML with del(7q) was closely associated with RUNX1 mutations [10].
3. Chromosome 7 Abnormalities in MDS
3.1. Germline Genetic Factors Predisposing Individuals to Pediatric MDS with Monosomy 7 or del(7q)
The genomic sequencing of cohorts of pediatric MDS patients has supported the existence of monogenic disorders known as MDS predisposition syndromes. Particularly, gene sequencing studies have shown that a proportion, ranging from 7% to 31% of pediatric MDS cases, harbors germline variants, the two most frequent being GATA2 deficiency and SAMD9/SAMD9L syndromes, which together account for about 15% of primary pediatric MDS [11]. Less frequently, germline predispositions are associated with germline variants of RUNX1 and ETV6 genes [11].
3.2. Germline GATA-2 Deficiency
Germline GATA-2 deficiency is an autosomal dominant disorder predisposing individuals to myeloid malignancy and immunodeficiency [12] (Figure 2). Germline GATA-2 mutations occur in about 15% of advanced and 7% of all primary pediatrics MDS but are absent in pediatric patients developing MDS secondary to therapy or acquired aplastic anemia [12]. Monosomy 7 is the most frequent genetic alteration observed in GATA-2 germline-mutant patients: in 449 MDS pediatric patients, monosomy 7 was observed in 22.2% of cases and in 68% of patients with germline GATA-2 mutations, and within the group of pediatric MDS with monosomy 7, 37% displayed GATA-2 mutations and the median age at diagnosis was significantly higher for the GATA-2-mutant than for GATA-2-WT patients (12.5 vs. 4.5 years, respectively) [12].
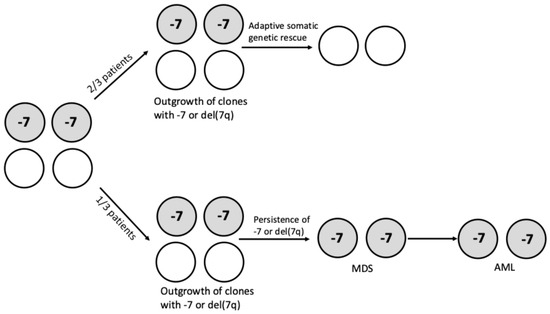
Figure 2.
Clinical and clonal evolution of patients with germline SAMD9/SAMD9L gain-of-function mutations. Genetic instability mechanisms and microenvironmental conditions, such as inflammatory stress, favor the generation and outgrowth of clones bearing chromosome 7 abnormalities (-7 and del(7q)). About two-thirds of these patients undergo somatic genetic rescue (SGR), eliminating/inactivating the toxic mutant allele in the hematopoietic compartment through the acquisition of somatic SAMD9/SAMD9L mutations or UDP7q (the duplication of the WT SAMD9/SAMD9L allele); the remaining one-third of patients display persistence of the clone with chromosome 7 abnormalities, with the acquisition of somatic mutations over time, driving, together with chromosome 7 abnormalities, the development of MDS and AML.
Importantly, in addition to monosomy 7, 7% of germline GATA-2-mutant patients display an unbalanced aberration der(1;7), resulting in the loss of the q-arm of chromosome 7 [13]. Der(1;7) was significantly enriched in GATA-2mut compared to GATA-2WT patients (9.2% vs. 0.4%, respectively) [13]. In total, 95% of these patients display the co-occurrence of additional secondary mutations in leukemia driver genes, thus indicating a malignant phenotype [13]. The presence of additional secondary mutations in leukemia driver genes observed in 95% of these patients supports the need for bone marrow transplantation [13].
Recent studies have explored the role of somatic gene mutations and monosomy 7 in the progression of germline GATA-2 mutations to malignancy. Thus, Largeaud et al., in the context of a study of a French GATA-2 study group, reported clinical, biological and molecular features of 78 GATA-2 patients [14]. A comparison of the mutational profile of these patients with respect to AML patients showed (i) a higher occurrence of monosomy 7 (29% vs. 8%, respectively) and of der(1;7) (9% vs. 1%, respectively); (ii) a higher frequency of the mutations of STAG2 (33% vs. 4%), ASXL1 (22% vs. 8%) and EZH2 (8% vs. 3%) compared to AML; and (iii) a markedly lower frequency of NPM1 (0% vs. 37%), FLT3 (0% vs. 37%), IDH2 (0% vs. 10%), IDH1 (0% vs. 9%) or SRSF2 (0% vs. 6%) mutations [14]. According to their bone marrow morphology features, germline GATA-2mut patients were subdivided into three different groups: spectrum 0 with no pathological features; spectrum 1 with hyperplastic and/or low-grade MDS morphological features; and spectrum 2 with full MDS morphological features, with multilineage dysplasia [14]. In spectrum 0 patients, no somatic mutations and no monosomy 7 cases were observed; in spectrum 1, there was a median of one mutation/patient, with the enrichment of STAG2 mutations and monosomy 7 in 28% of cases; and in spectrum 2, there was a median of three mutations/patient, with the enrichment of SEPBP1, RAS pathway, ASXL1 and RUNX1 mutations and monosomy 7 in 47% of cases [14]. These observations suggest that SETBP1, RUNX1 and RAS pathway mutations and monosomy 7 and other chromosome alterations drive the leukemic transformation of germline GATA-2mut patients [14].
Another study reported that mutations in chromatin and cohesin-related genes, such as STAF2, ASXL1 and DNMT3A, are frequent in germline GATA-2 deficiency [15]. Mutations of many genes involved in myeloid malignancies, such as IDH1, IDH2, NPM1 and TET2, are absent in germline GATA-2 deficiency [15]. The presence of ASXL1 and STAG2 conferred a lower survival probability to these patients [15].
A recent study reported the extensive characterization of 218 patients (90% pediatric and 10% adult) with germline heterozygous GATA-2-deficient variants [16]. The study explored the age-dependent phenotypic and molecular evolution of these patients: a pronounced age-dependent incidence in GATA-2-related MDS was observed, with MDS being absent in infants, rare before the age of 6 years, and markedly increased in older children [16]. Among the various types of GATA-2 mutations, null mutations are those mostly associated with the risk of developing MDS. Monosomy 7 was the chromosomal abnormality most frequently observed in pediatric patients (about 55% of cases), while it was detected in a markedly lower proportion of adult patients (about 10% of cases). Somatic mutations were observed in 60% of symptomatic pediatric patients and in 77% of adult patients, with SETBP1 (26%), ASXL1 (22%), STAG2 (15%), RUNX1 (8.4%) and EZH2 (8%) being the most recurrent mutations [16]. All recurrent mutations, except for STAG2, co-occurred with monosomy 7; monosomy 7 was present in 74% of mutation-positive patients; and patients with monosomy 7 had the highest mutation frequency (76.5%) compared to 31.6% in karyotype-normal patients [16]. Virtually all patients with SETBP1 mutations had a monosomy 7 clone [16]. All patients with EZH2 mutations and almost all patients with ASXL1 mutations harbored monosomy 7 [16].
The mechanisms through which GATA2 germline mutations promote the development of myeloid neoplasia remain still unclear. GATA2 is a master regulator of hematopoiesis, required for the development and function of the hematopoietic system. Mice with a homozygous GATA2 deletion die early during embryogenesis due to the absent formation of the hematopoietic system, while the conditional loss of GATA2 in bone marrow causes bone marrow failure [17].
As above discussed, individuals born with monoallelic mutations in the GATA2 gene or GATA2 gene enhancers regulating GATA2 expression develop GATA2 deficiency syndrome, with a complex hematologic phenotype, encompassing bone marrow failure, cytopenias, MDS and AML. The mechanisms by which germline GATA2 mutations promote leukemia development remain largely unresolved. A few observations suggest the complexity of these mechanisms.
A study of some families bearing germline GATA2 variants showed variable penetrance of mutant GATA2 alleles, as supported by the observation that family members who carry an identical germline mutation display variable clinical manifestations; thus, reduced penetrance is a feature among certain GATA2-mutant MDS/AML families [18]. The analysis of five MDS/AML families harboring p.Thr354Met GATA2 mutations, showed significant intrafamilial and interfamilial variations in disease latency and phenotype [19]. These observations suggest that additional cooperating events, in addition to germline GATA2 mutations, are required for the development of hematological malignancy within the context of a shared germline mutation [19]. An additional element of complexity is given by the existence of epigenetic mechanisms regulating the level of expression of the mutant allele [19]. Similarly, variability in GATA2 mutant allele penetrance was impressive in a family in which the father, bearing GATA2c.1021_1031del and remaining asymptomatic in his sixth decade, transmitted the germline GATA2 mutant allele to three sibling who displayed, over an 18-year period, MDS or AML, with all three cases associated with monosomy 7 and one case also with trisomy 8 [20]. Extra-hematological manifestations were heterogeneous among these three siblings [20].
The need for additional mutational events for the development of MDS and AML is also suggested by a longitudinal analysis of individuals with germline GATA2 variants [14,15,16]. More than 400 different GATA2 germline mutations were identified, but a clear genotype–phenotype correlation could not be established due to high patient variability.
Studies in animal models have attempted to reproduce the main features associated with GATA2 haploinsufficiency in humans. One model was based on the study of GATA2 heterozygous mice (GATA2+/−): aging in these mice was associated with loss and functional defects at the level of the HSC compartment; after the transplantation of aged GATA2+/− bone marrow, B-lymphopenia and monocytopenia were generated; and the transplantation of a low number of GATA2 haplo-insufficient bone marrow in irradiated WT recipients resulted in a predisposition to develop BMF, preceding leukemia development [21].
Other studies have shown that germline GATA2 variants alter the proliferation and differentiation of HSCs/HPCs. A recent study showed that pathogenic GATA2 variants have both unique and GATA-like attributes and retain the capacity to activate an enhancer-dependent mechanism, which generates a fragmented, abnormal differentiation program [22]. This mechanism determines an elevation of C/EBPε levels, which in part counterbalances hematopoietic defects of GATA2-deficient HPCs [22].
The elevated expression of the hematopoietic transcription factor Interferon Regulatory Factor 8 (IRF8) observed in granulocyte–macrophage HPCs from GATA2-mutant mice causes macrophage-biased differentiation, and the genetic ablation of IRF8 in these mutant mice reverses the defective hematopoietic phenotype [23].
3.3. SAMD9 and SAMD9L Syndromes
Sterile alpha motif domain-containing protein 9 (SAMD9) and its paralog SAMD9-like (SADMD9L) are two interferon response genes located on chromosome 7q21, encoding antiviral proteins exerting a negative regulation of cell proliferation. Heterozygous germline gain-of-function SAMD9-SAMD9L variants determine the development of multisystem syndromes with variable clinical features. Some features related to hematologic dysfunction unify all these syndromes, including pancytopenia, bone marrow failure, immunodeficiency, infections, monosomy 7 and an increased risk of MDS [24]. Affected individuals display a variable clinical course, ranging from mild and transient alterations in bone marrow to a rapid progression of MDS or AML with monosomy 7.
Schwartz reported the characterization of 46 pediatric primary MDS and showed the presence of germline variants in SAMD9 and SAMD9L in 17% of cases; 42% of these patients bore monosomy 7 [25,26]. It is important to note that these SAMD9/SAMD9L variants were lost in the tumor cells by chromosomal deletions (monosomy 7) or copy number neutral loss of heterozygosity (CN-LOH) [20,21]. Pastor et al. described a familial syndrome in seven patients from unrelated pedigrees presenting with MDS and loss of chromosome 7/7q; genome studies showed constitutional mutations in the SAMD9L gene. The non-random loss of the mutated allele was attained with monosomy 7, deletion 7q, UDP7q, or acquired truncating variants [27]. Long-term outcomes showed either progression to leukemia and/or the accumulation of driver mutations, persistent monosomy 7 and transient monosomy 7 [27].
In a large cohort of 669 pediatric MDS patients, germline SAMD9/SAMD9L mutations were observed in 8% of cases and were mutually exclusive, with germline GATA-2 mutations observed in 7% of cases [28]. Acquired monosomy 7 was observed in 32% of these patients [28]. In pediatric MDS with monosomy 7, corresponding to 21% of total MDS, 33% of refractory cytopenias of childhood and 6% of MDS with excess blast subtypes are related to SAMD9/SAMD9L syndromes [28].
In a cohort of French patients with inherited bone marrow failure, Bluteau et al. reported the occurrence of SAMD9 and SAMD9L mutations in 18.9% of cases. These patients often experienced transient aplasia and monosomy7/del(7q). Monosomy 7 was observed in 73% of these patients [29].
The spectrum of most recurrent genetic abnormalities of SAMD9/SAMD9L pediatric MDS is predominantly defined by monosomy 7. In these patients, the genetic selection of chromosome 7 deleted is nonrandom since the allele harboring the germline SAMD9/SAMD9L variant is lost, while the resulting monosomy 7 retains the WT SAMD9/SAMD9L allele [30]. Thus, gain-of-function mutations in SAMD9/SAMD9L predispose individuals to malignancy through a mechanism of adaptation by aneuploidy; through this mechanism, hematopoietic stem cells that eliminate SAMD9 or SAMD9L gain a competitive advantage but simultaneously predispose individuals to the development of a malignant disease (MDS) [30].
The expression of gain-of-function SAMD9/SAMD9L mutations reduces cell cycle progression and favors the outgrowth of clones that have either lost the mutant clone or have acquired revertant mutations [31].
The compensation of SAMD9 or SAMD9L germline mutation by the loss of chromosome 7 bearing the mutant allele must be considered as a maladaptive compensation in that it eliminates the SAMD9 or SAMD9L allele with gain-of-function mutations but predisposes individuals to the risk of developing MDS (Figure 2). A study by Tesi et al. showed that patients with germline gain-of-function SAMD9/SAMD9L mutations undergo primary somatic genetic reversion in vivo through different molecular mechanisms, such as the uniparental disomy (UDP) of chromosome 7q, loss-of-function mutations in cis or adaptation by aneuploidy by monosomy 7 order(1;7) [30].
A study of seven patients with familial MDS associated with germline SAMD9L mutations confirmed the existence of clonal escape mechanisms, leading to the loss of the mutant allele through monosomy 7, deletion 7q, UPD7q, or acquired truncating SAMD9L variants [27]. Long-term observations of these patients showed divergent outcomes, represented by the persistence of monosomy 7 (most frequent), the evolution to a leukemic condition or transient monosomy followed by spontaneous recovery with SAMD9L-WT UPD7q [27]. It is of interest to note that, using single-cell DNA sequencing, it became evident that multiple adaptive clones and monosomy 7 arise independently and coexist within individual patients [27].
In total, 61% of SAMD9/SAMD9L patients underwent somatic genetic rescue, which resulted in clonal hematopoiesis, of which 95% had a maladaptive nature (monosomy 7) and 51% had an adaptive nature (revertant UDP7q and somatic SAMD9/SAMD9L mutations); single-cell studies showed the existence of multiple competing somatic genetic rescue mechanisms in the same individuals [28] (Figure 2).
Longitudinal studies on young children with SAMD9/SAMD9L syndrome have shown that monosomy 7 can be transient, often associated with hematological remission [32]. In fact, a longitudinal study of seven SAMD9/SAMD9L patients showed that three of these patients exhibited spontaneous hematologic remissions within a median of 0.6 years (range 0.4–2.9), associated with the disappearance of monosomy 7 and expansion of somatic SAMD9/SAMD9L mutations [32].
Somatic leukemia driver gene mutations such as SETBP1, ASXL1, STAG2, RUNX1, EZH2, ETV6 and RAS pathway members were observed in about 30% of germline SAMD9/SAMD9L-mutant patients, and most of these occurred in patients with monosomy 7 [28].
Germline loss-of-function SAMD9/SAMD9L mutations were observed in 3% of adult MDS patients; recurrent somatic alterations observed in these patients were del(5q) and TET2 mutations, while monosomy 7 and del(7q) were rare [33]. Genetic reversions via monosomy 7 or del(7q) or additional cis mutations are rare in adult MDS patients [33].
Few studies have explored the physiological role of SAMD9 and SAMD9L proteins. Initial studies have shown an antiproliferative effect in non-hematopoietic cells. The effect of WT and mutant SAMD9/SAMD9L overexpression in human CD34+ HSCs/HPCs was evaluated, showing an effect on cell proliferation and differentiation with a decrease in erythroid (BFU-E) and multipotential colonies (CFU-GEMM) and with the inhibition of cell proliferation and accumulation of cells in the G2/M phase [34]. The effect of mutant SAMD9 and SAMD9L was more pronounced than the effect of the respective WT forms [29]. Interactome analysis showed a role of SAMD9/SAMD9L in ribosome assembly and in protein synthesis [34]. SAMD9/SAMD9L overexpression in human CD34+ cells also activates DNA damage responses and apoptosis [34]. According to these observations, it was proposed that SAMD9 and SAMD9L regulate proteins involved in the cell cycle, protein synthesis, DNA damage response and apoptosis, these responses being amplified by mutant SAMD9 and SAMD9L. In the eventuality that these events remain unchecked by cell response mechanisms, it is necessary to determine at the level of bone marrow cells a condition of hypocellularity. Alternatively, cells lacking the mutant SAMD9/SAMD9L proteins due to monosomy 7 or somatic reversion may acquire secondary cooperating mutations and develop a leukemic process [34].
3.4. Monosomy 7 and del(7q) in Pediatric Myelodysplastic Syndromes
The WHO classification (fifth edition) subdivides pediatric mDS into two subgroups: pediatric MDS with low blasts (MDS-LB, <5% blasts in BM and <2% blasts in PB) and pediatric MDS with high blasts (MDS-HB, 5–19% blasts in BM and 2–19% blasts in PB) [35]. The International Consensus Classification (ICC) provides a more complete classification of pediatric MDS, with a Refractory Cytopenia of Childhood (RCC) including persistent cytopenia, BM dysplasia without blast cell increase; (ii) MDS not otherwise specified, including cases without morphological RCC features but with monosomy 7; and (iii) MDS with excess blasts including patients with 5–19% BM blasts and 2–19% PB blasts [36,37]. Most patients have as predominant presentation of RCC/MDS-LB (Figure 3).
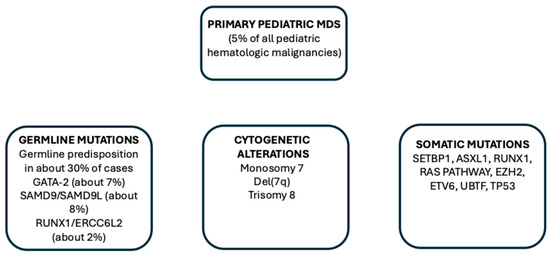
Figure 3.
Main genetic alterations observed in pediatric MDS, including germline mutations predisposing individuals to MDS development, cytogenetic alterations and somatic mutations.
It was estimated that about 20–60% of pediatric MDS at diagnosis exhibit a cytogenetic abnormality. The most frequent chromosome abnormalities are represented by the complete loss of chromosome 7 (monosomy 7) or partial [del(7q)] loss of chromosome 7 observed in 6–12% of RCC/MDS-LB and 27–32% of MDS-HB [11].
Somatic mutations are observed in some patients with pediatric primary MDS and their frequency is higher in MDS-HB than in RCC/MDS-LB. These studies have shown that the mutational landscape of pediatric MDS is significantly different to that observed in adult MDS: (i) DNMT3A, TET2, spliceosome gene SF3B1, U2AF35 and SRSF2 mutations, frequent in adult MDS, are virtually absent in pediatric MDS, and (ii) SETBP1, ASXL1 and RAS pathway gene (PTPN11, NRAS and CBL) mutations are frequent in pediatric MDS [19,38].
Monosomy 7 is strongly associated with EZH2 (100%), SETBP1 (90%), RUNX1 (79%) and ASXL1 (74%) mutations in children with MDS [39].
The studies carried out in pediatric MDS strongly support the major role of monosomy 7 as a major driver of the malignant evolution of pediatric MDS; in fact, various somatic mutations such as SETBP1, ASXL1, RUNX1 and RAS pathway mutations are observed in the context of a monosomy 7 background. According to these findings, it can be suggested that monosomy 7 arises as an ancestral clone, which rapidly and progressively acquires driver mutations, resulting in disease progression. In line with this hypothesis, a study by Kardos et al. showed that monosomy 7 is the main contributor to the progression of RCC to advanced MDS or AML, as supported by the finding that the median time to progression among 20 children with RCC and monosomy 7 is 1.7 years, with a cumulative incidence of progression higher compared to patients with other chromosomal abnormalities or with a normal karyotype [40].
3.5. Monosomy 7 and del(7q) in Adult MDS Patients
Monosomy 7 or del(7q) are observed in about 13% of adult MDS patients; chromosome 7 abnormalities often occur in the context of complex abnormalities, designed as complex karyotypes (CKs) [3].
Chromosome 7 abnormalities in adult MDS are frequently observed in the context of CKs; thus, in CK-MDS, with TP53 mutations, monosomy 7 was observed in 39% of cases and del(7q) in 14% of cases; in CK-MDS, without TP53 mutations, monosomy 7 was observed in 29% of cases and del(7q) in 7% of cases [41]. In these patients, the presence of TP53 mutations and monosomy 7 was associated with the greatest risk survival [28].
Crisà and coworkers reported the extensive characterization of 280 adult MDS patients with -7/del(7q) as isolated cytogenetic abnormalities [42]. Somatic mutations were observed in 82% in -7 patients and 73% in del(7q) patients; mutation frequency was similar in these two groups of patients [42]. In total, 54% had mutations in genes involved in epigenetic and chromatin modification, 33% in transcription factors, 30% in splicing factors and 20% in cell signaling; ASXL1, DNMT3A, U2AF1, RUNX1, EZH2, NRAS and TET2 were the most frequently mutated genes [42]. Untreated del(7q) patients had better OS compared to -7 patients (34 vs. 17 months, respectively) [42].
Bernard et al., in their extensive molecular characterization of 3233 adult MDS patients, reported that monosomy 7 cases were associated with TP53 mutations, while del(7q) cases were associated with EZH2 mutations [43]. Patients with cnLOH at 7/7q without CK display a markedly higher frequency of EZH2, ASXL1, TET2, RUNX1 and STAG2 co-mutations compared with LOH at 7/7q; on the contrary, patients with LOH at 7/7q without CK display a higher frequency of U2AF1 and DNMT3A co-mutations than patients with cnLOH at 7/7q [43].
4. Monosomy 7 and del(7q) in AML Patients
Genetic alterations are responsible for the development of myeloid neoplasia (MN). Both recurrent gene mutation events and structural chromosomal alterations are observed in MN. The development of sensitive cytogenetic techniques has allowed us to detect frequent chromosome aberrations in various types of MN, such as myelodysplastic syndromes and AML. Conventional karyotyping showed cytogenetic abnormalities in about 50% of MDS cases and in 50–60% of adult AML cases [44].
The chromosomal alterations observed in MN involve insertion, deletion, duplication, translocation and inversion events; the deletion events may involve whole chromosomes or chromosome subregions. Aberrations of chromosome 7 [abn(7)] are recurrently observed in adult AML patients (about 10% of cases) [45]. The most common abn(7) includes the complete loss of chromosome 7 (monosomy 7, -7) and deletions of the long arm of chromosome 7 [del(7q)]. Both these alterations may occur both in MDS and AML in the context of a complex karyotype (CK), defined as a condition in which at least three different chromosomal abnormalities coexist in the same leukemic cells.
4.1. Monosomy 7 and del(7q) in Adult AML Patients
Mori reported a large screening of chromosome 7 abnormalities in a large cohort (8142 patients) of patients with myeloid neoplasia; 645 of these patients displayed chromosome 7 abnormalities: 501 with -7 and 144 with del(7q) [46]. -7/Del(7q) abnormalities were observed with different frequencies in the various MNs: 7% in primary AML, 12% in MDS/s-AML, 7% in MDS/MPN, and 5% in MPN [46]. In patients with isolated -7 or del(7q), the survival was lower than in patients without chromosome 7 abnormalities and in -7 compared to del(7q) patients; in patients with chromosome 7 abnormalities associated with a CK, the survival was equally lower in -7 and del(7q) patients than in CK patients without chromosome 7 abnormalities [46].
Initial studies on AMLs bearing chromosome 7 abnormalities were based on limited cohorts of patients and provided initial evidence about the molecular heterogeneity of these leukemias related to the presence of different chromosome abnormalities [-7 or del(7q)], the association or not with CKs or the association with somatic mutations [47,48,49,50].
Only recent studies have reported the extensive characterization of large numbers of AML patients bearing chromosome 7 abnormalities. Halik and coworkers reported the detailed characterization of 519 AML patients with aberrations of chromosome 7 in the context of a multinational study [51]. According to the distribution of chromosome 7 abnormalities, various groups of AML patients were identified: (i) two large groups, one composed of 294 patients with chromosome 7 abnormalities, without CKs, and the other composed of 225 patients with chromosome 7 abnormalities, with CKs; (ii) the CK group was subdivided into two subgroups, -7/CK (136 patients) and del(7q)/CK (70 patients); (iii) the non-CK group was subdivided into two subgroups, -7/non-CK (192 patients) and del(7q)/non-CK (92 patients); (iv) the -7/non-CK group was subdivided into a subgroup without additional alterations (-7sole, 125 patients) and a subgroup with additional alterations (-7/non-CKns, 67 patients); and (v) similarly, del(7q)/non-CK group was subdivided into two subgroups according to the absence (del(7q) sole, 69 patients) or presence of additional abnormalities (del(7q)/non-CKns, 23 patients) [51]. The analysis of the mutational profile showed that the most frequently mutated genes were TP53 (33%), DNMT3A (18%), RUNX1 (16.7%), KMT2C (16.7%) ASXL1 (16.3&), NRAS (14.2%), TET2 (12.8%), PTPN11 (11%), EZH2 (10.3%) and IDH2 (9.4%); TP53 was mutated mostly in the CK group, while clonal hematopoiesis-associated mutations were mostly observed in the non-CK group [51]. An analysis of variant allele frequency (VAF) and reconstruction of mutation acquisition suggested that TP53 mutations are disease-initiating events, while -7 and del(7q) are subclonal events in one-third of patients [51]. The groups without CKs had a better survival than those with CKs; the group with the poorest survival was the -7/CK group [51], Mutations in TP53 and PTPN11 showed the strongest association with worse overall survival; in contrast, patients with mutated IDH2 exhibited prolonged OS and durable responses [51].
Mrozek and coworkers reported an extensive analysis of 160 CK-AML patients and proposed a classification of these AMLs into two groups: typical CK (corresponding to 70.5% of the total) and atypical CK (corresponding to 29.5% of the total) [52]. Typical CKs were characterized by the presence of abnormalities resulting in loss 5q, 7q and/or 17p; 65.5% of CK-AMLs displayed chromosome 7q loss [38]. Patients with atypical CKs differed from those with typical CKs for the lower frequency of TP53 mutations and increased frequency of PHF6, FLT3-TKD, MED12 and NPM1 mutations; furthermore, atypical CKs involve younger patients, have higher bone marrow and peripheral blood blast counts and exhibit higher complete remission rates and longer OS [52]. Importantly, typical CKs with abnormalities of 7q, but not of 5q and 17p, differ from typical CKs with other combinations of 5q, 7q and 17p abnormalities in that they have more frequently FLT3-ITD, BCOR, WT1, DNMT3A, NPM1 and RUNX1 mutations and less frequently TP53 mutations; furthermore, the OS of patients with only 7q abnormalities is longer than the OS of the remaining patients with typical CK-AML [52]. TP53 mutations are particularly frequent in AMLs bearing 5q, 7q and 17p abnormalities, or 5q, 17p without 7q abnormalities or 5q and 7q without 17p abnormalities or 7q and 17p without 5q abnormalities [52].
Kugler et al. reported the mutation dynamics of a group of 115 AML patients with chromosomal 7 deletions who achieved remission after induction treatments [38]. This group of patients were heterozygous with some showing -7/del(7q) as an isolated event or in association with other monosomies [53]. Importantly, in some of these patients, -7/del(7q) alone may represent a founder clone or a late subclonal event. A significant proportion of these patients have TP53 mutations, mostly associated with CKs, and display lower OS compared to those without TP53 mutations (8.6 vs. 13.04 months) [53]. Patients with TP53 mutations, with co-mutations such as NF1, GATA2 or RUNX1, have lower OS than those with TP53 mutations without these co-mutations [53].
Many studies suggest the existence of a link between abn(7) and TP53 mutations. Most patients with TP53 mutations have de novo AML, associated with chromosomal aneuploidies, including monosomy 7, monosomy 5/del(5q) or a complex karyotype/monosomal karyotype. Rucher et al., in a group of 234 CK AML, observed TP53 alterations (mutations or deletions) in 70% of cases; among the patients with TP53 alterations, 59% displayed -7/del(7q), and among those with TP53-WT, 37% showed -7/del(7q) [54].
Abbas et al. reported a retrospective analysis of 243 treatment-naïve AML patients with chromosome 7 or 7q deletions; 69% of these patients had -7 and 31% del(7q) [55]. The composite CR + CRi in the whole population of leukemic patients was 49% and similar in the two groups of patients; RFS was significantly longer among AML patients with del(7q) compared to those with del(7) (6.0 months vs. 2.7 months), but OS was similar (7.4 months vs. 8.4 months) [55]. The frequency of TP53 mutations was similar among del(7) and del(7q) (55% vs. 54%, respectively); patients harboring TP53 alterations had significantly lower OS compared to those without TP53 alterations (5.5 months vs. 10.5 months) [55]. The presence of co-occurring del5/5q conferred worse outcomes in del(7) and del(7q) [55]. The profile of various somatic mutations was similar in del(7) and del(7q) AMLs [55].
Fleming et al. reported a study of 256 patients with TP53-mutant AML; among de novo AMLs, monosomy AML was observed in 36% and in secondary AMLs in 49.9% of cases [56]. The presence of TP53 mutations concomitantly with monosomy 7 was associated with a significantly lower OS compared to monosomy alone [56].
Single-cell transcriptomic studies have shown in -7 HSC/HPCs a downregulation of genes involved in the maintenance of DNA, repair, cell cycle, apoptosis, immune response and hematopoietic differentiation [57]. According to these findings, it was hypothesized that the downregulation of genes required for the maintenance of DNA stability and apoptosis may result in genomic instability, thus favoring the acquisition of a series of genetic alterations and ultimately inducing the development of a leukemic process [57].
Kaur et al. reported the results of a real-world study on a cohort of patients with TP53-mutated myeloid neoplasms (mostly AML, 82.4% of cases) mostly treated using HMA + VEN (74% of cases) [58]. Some pre-therapy factors predicted an inferior response, including the presence of ≥2 autosomal monosomies, multihit TP53 allelic state and CUX1 co-alterations (either deletions or mutations) [58].
A monosomal karyotype (MK) is defined as the presence of one autosomal and at least one structural aberration or two or more autosomal monosomies in the absence of recurrent AML genetic abnormalities including t(8;21), inv(16), and t(15;17) [59]. Kayser et al. explored 1058 adult AML patients and observed that 11.9% of these patients display an MK; among MK+ patients, 6% display -7 and 7% del(7q) [46]. Among AML patients with chromosome 7 abnormalities, MK+ patients with -7 and del(7q) display an MK with a frequency of 52.8% and 18.4%, respectively, while MK- patients with -7 and del(7q) exhibit an MK with a frequency of 14.4% and 18.4%, respectively [59]. An MK may be observed alone or in association with CKs: MK alone (7.5% of total AMLs, 74% with -7 and 9%with del(7q)), CK alone (9.2% of total AMLs, 1% with -7 and 14% with del(7q)) and MK/CK (24% of total AMLs, 35% with -7 and 19% with del(7q)) [60].
A recent study reported the analysis of 156 adult AML patients with an MK; in these patients, the most common monosomies were -17 (41%) and -7 (37%), with 88% having them and the most frequent mutations were TP53 (69%), DNMT3A (19%), TET2 (13%) and IDH1 (7%) [61]. The OS of these patients was affected by the presence of TP53 mutations (reduced in patients with TP53 mutations compared to those without TP53 mutations) but was not affected by the presence or absence of CKs [46]. Among the various monosomies, only monosomy 17 affected outcomes [61].
An MK is associated with a dismal prognosis in AML patients. In a South Korean Registry, an MK was observed in 5.7% of adult AM patients [62]. In these patients, the presence of single monosomy and absence of abn(17p) were associated with a better prognosis following aloo-HSCT [62].
4.2. Monosomy 7 and del(7q) in Pediatric AML Patients
Chromosome 7 abnormalities are observed in a minority of pediatric AML patients, with 2–3% of monosomy 7 and of del(7q). A retrospective analysis on 100 [63] and 258 [47] children with AML or refractory anemia with an excess of blasts in transformation (RAEB-T) allowed a characterization of these patients [47,63]. A part of these patients exhibited -7 or del(7q) as isolated abnormalities or in association with other cytogenetic abnormalities. Overall survival was superior for patients with del(7q) compared to those with monosomy 7 [47,63]. Cytogenetic aberrations associated with favorable prognosis, such as t(8;21), inv(16), t(15;17), and t(9;11), were strongly associated with del(7q) and displayed a higher 5-year survival rate compared with del(7q) without favorable cytogenetics [47]. On the contrary, patients with -7 in association with inv(3), 5del/del(5q) or +21 had a markedly reduced survival rate [47].
Ries et al. have reported a genomic and transcriptomic analysis of 45 pediatric AML patients with monosomy 7: 22 of these patients had additional karyotypic alterations, such as CNAs or translocations [64]. The patients were subdivided into two groups according to MECOM expression: MECOM high with a poor prognosis and MECOM low, with a better prognosis [64]. In some of these patients, MECOM high expression was associated with 3q26 variants [64].
Westover et al. explored a group of 108 pediatric and young patients with myeloid neoplasms, including 48% of AML patients, showing, in these patients, frequent mutations involving the Ras/MAPK pathway [65]. Clonal analysis showed that chromosome 7 alterations are early-initiating events in these pediatric patients; it was hypothesized that chromosome 7 deletions could cooperate with secondary mutations and inflammatory stress in the development of a leukemic process [65].
Poor-risk cytogenetic abnormalities at diagnosis predict survival after allo-HSCT in pediatric AML patients; an analysis carried out in 845 patients with these features (36% with 11q23 abnormality, 24% with monosomy7/del(7q) or monosomy5/del(5q), 24% with a MK or CK and 16% with other poor-risk cytogenetic abnormalities) showed that patients with monosomy 5/del(5q) and monosomy7/del(7q) had a higher risk of disease relapse compared to 11q23 and other poor-risk cytogenetic abnormalities [66].
4.3. Chromosome 7 Abnormalities in Therapy-Related Myeloid Neoplasms
Therapy-related myeloid neoplasms (t-MNs) include a group of myeloid neoplasms comprising AML, MDS and MDS/myeloproliferative neoplasms (MDS/MPNs). They occur as a late complication of cytotoxic therapy–chemotherapy and/or radiation therapy used for the treatment of malignant disorders or immunosuppressive therapy for autoimmune diseases [53,54,55]. It was estimated that the risk of developing an MN is about 4.7-fold higher among cancer survivors compared to the general population of an equivalent age [67]. The risk of developing a t-MN is influenced both by the type of primary cancer, by the type of primary cancer, by the type and dose of cytotoxic factors, by individual, patient specific-factors, genetic factors, and comorbidities and by the age of the patient. Currently, it is estimated that t-MNs correspond to 5–15% of newly diagnosed MDS, AML and MDS/MPN [67].
T_MNs are characterized by the presence of genetic alterations similar to those observed in the primary tumors, with an accentuation of the frequency of high-risk genetic alterations. Particularly, chromosomal alterations are observed in > 90% of t-MNs; the most frequent karyotype alterations observed in tMNs are represented by a decreased prevalence of normal karyotype and by the predominance of complex and unbalanced karyotypes with frequent chromosomal deletions compared to de novo MDS and AML [68]. Complete or partial deletions of chromosomes 5 and 7 and CKs have been observed in a large majority of t-MNs occurring after CT with alkylating agents or with radiotherapy [67,68,69]. Smith et al. reported the analysis of 306 patients with t-MDS or t-AML: 40% of these patients had undergone CT alone (alkylating agents, 78%, and topoisomerase 2 inhibitors, 39%), 14% RT alone and 45% both CT and RT [70]. At diagnosis, 92% of these patients had karyotypic clonal abnormalities involving chromosome 5 (20.5%), chromosome 7 (27.7%), both chromosomes 5 and 7 (21.5%), balanced rearrangements (10%) and other clonal abnormalities (12.7%) or a normal karyotype (7.8%). Importantly, abnormalities of chromosomes 5 or 7 or both accounted for 76% of all cases with an abnormal karyotype [70].
A recent analysis on most of the available studies on the characterization of t-MN in comparison with p-MN showed a marked increase in del7/7q (26% vs. 8%, respectively), del5/5q (24% vs. 5%, respectively), MK (26% vs. 12%, respectively) and CK (36% vs. 10%, respectively) [67].
TP53 mutations are more frequent in t-MN than in p-MN (30.9% vs. 7.9%, respectively), t-MDS vs. p-MDS (35.1% vs. 11.3%, respectively) and t-AML vs. p-AML (26.2% vs. 11.3%) [67].
Chromosome 7 abnormalities, as well as chromosome 5 abnormalities, are enriched in TP53-mutated t-MDS and t-AML, in association with CKs [57,58]. The association between del(7q), CK and TP53 mutations was particularly evident for t-MN with TP53 mutations with a variant allele frequency of ≥10% [71]. Chromosome 7 abnormalities were significantly more frequent in TP53-mutated t-MN compared to TP53-WT t-MN [71,72].
5. Pathogenesis of Myeloid Neoplasia with Chromosome 7 Abnormalities
Chromosome 7 alterations in hematologic malignancies are almost always deletion events or copy neutral losses of heterozygosity (LOH), in contrast to solid tumors where amplifications are frequently observed [73]. The loss of all or part of chromosome 7 del(7) or del(7q) is one of the most common chromosomal abnormalities observed in myeloid neoplasms [74]. The high frequency of chromosome 7 loss observed in high-risk myeloid neoplasia supports the hypothesis that chromosome 7 harbors tumor suppressor genes (TSGs), whose loss could contribute to the development of MN. This tumor suppressor seems to act in part in a haploinsufficiency manner since the other allele present on the other intact chromosome 7 is seemingly functional. The study of the regions deleted at the level of 7q has led us to identify CUX1 as a potential TSG. CUX1 is a gene encoding a homeodomain-containing transcription factor. CUX1 is the most significantly differentially expressed gene within the commonly deleted region of 7q and is expressed at the haploinsufficiency level in -7/del(7q) MNs [75]. Importantly, the haploinsufficiency of CUX1 confers to human HSCs a significant engraftment advantage on transplantation into immunodeficient mice [75].
Several experimental studies support a functional role of CUX1 haploinsufficiency in MN development. Thus, Aly et al. showed that the introduction of CUX1 mutations or the experimental decrease in CUX1 expression in bone marrow cells induces a defective base repair, thus favoring the accumulation of genetic alterations [76]. Furthermore, these authors also showed that CUX1 is mutated in 2.4% of MNs [76].
CUX1 knockdown in human CD34+ HSCs/HPCs induced a gene signature similar to that observed in leukemic cells of patients with -7/del (7q) [77]. CUX1−/+ mice develop mild anemia and bone marrow dysplasia; furthermore, CUX1 haploinsufficiency induces apoptosis evasion and favors leukemia development [78].
KMT2C, KMT2E and EZH2 genes are collectively lost together with CUX1 in 70% of patients with del(7q). EZH2 deficiency synergizes with CUX1 deficiency in promoting hematopoietic expansion after exposure to alkylating agents [65]. Multiple 7q gene knockout experiments in HSCs provided evidence that combined CUX1 and EZH2 deficiency promotes cell expansion after chemotherapy exposure and thus drives chemoresistance [79]. Furthermore, combined CUX1 and EZH2 deficiency induces the abrogation of DNA damage response after genotoxic stress [79].
CUX1 mutations are frequently associated with RAS pathway mutations; the association of activating NRAS mutation with CUX1 deficiency causes AML generation in mice, not seen with either mutation alone [80].
The generation of mice with heterozygous deletions of different chromosome bands systemic to commonly deleted segments of human 7q22 d determines different phenotypes and cooperation when associated with the expression of genes commonly mutated in -7/del(7q) leukemias [81,82].
As above mentioned, CUX1 mutations are observed in 4% of AML patients; interestingly, the most frequent cytogenetic abnormality observed in CUX1mut AMLs is monosomy 7 (20% of cases) [83]. The study of clonal hierarchies in MDS and AML suggests that chromosome 7 abnormalities are early events. This conclusion is also supported by the studies of clonal hematopoiesis, defined as a clonal hematopoietic population carrying somatic mutations in one of the leukemia-associated genes. In CH, healthy individuals harbor alterations of genes involved in myeloid malignancy (such as DNMT3A, ASXL1 or TET2) at low frequency; these individuals have an increased risk of developing a hematologic malignancy. A study of large cohorts of normal individuals showed the existence in CH not only of somatic mutational variants but also of copy number alterations [84,85]. These studies also identified, among these copy number alterations, chromosome 7 deletions and LOH [84,85]. Importantly, one of these studies showed that individuals with del(7q) and 7qLOH display a significantly increased risk of developing a hematologic malignancy, particularly pronounced for the development of myeloid malignancies [85]. It is important to note that, according to the study, the risk of developing a hematologic malignancy is equally pronounced for CH bearing chromosome 7 abnormalities and 17p deletions (the chromosome arm encoding TP53) [86]. In addition to CNAs involving chromosome 7, CUX1 gene mutations have also been identified in some individuals with CH [86,87,88].
In addition to CUX1 and EZH2, MLL3, SAMD9/SAMD9L and LUC7L2 represent additional TSGs present in chromosome 7 regions commonly deleted in del(7q). As such, myeloid cancers driven by a loss of chromosome 7 are seen to be consistent with contiguous gene syndrome in which the combined loss of multiple neighboring TSGs drives malignant transformation [89].
Chromosome 7 abnormalities often occur in the context of a CK, a condition characterized by complex chromosomal rearrangements, intratumor heterogeneity, therapy resistance and poor overall survival. The characterization of molecular abnormalities occurring in CK AML is complex and requires adequate molecular techniques. In fact, copy number analyses failed to capture the full karyotypic heterogeneity occurring in CK-AML because copy-balanced and complex rearrangement structures remain unresolved in these malignancies [84]. To obviate to this limitation, Leppa and coworkers have developed a single-cell multiomics analysis, based on structural variant discovery, nucleosome occupancy, and transcriptomic and immunophenotypic changes in single cells [76]. Individual leukemic cells of patients with CK-AML were characterized by linear and circular breakage–fusion–bridge cycles and chromotripsis. Three different patterns of clonal evolution were observed in these patients, monoclonal, linear and branched polyclonal, with 75% of cases displaying multiple clones, often exhibiting karyotype remodeling [90]. Patient-derived xenograft models provided evidence about the consistent heterogeneity of clonal evolution of leukemic stem cells, showing subclone-specific drug responses [90]. Finally, longitudinal studies in some patients further supported the heterogeneity of genetic evolution and showed the existence of a consistent cell-type plasticity as mechanisms of disease progression [90]. In conclusion, this study provided strong evidence about the dynamic genomic, phenotypic and functional heterogeneity of CK-AML.
Chromoangiogenesis (CAG) events, represented by a spectrum of catastrophic events such as chromotripsis, chromoanasynthesis and chromoplexy, are very frequent in CK-AML (92% of cases) [91]. Among patients with MK, those with CAG have a much higher frequency of chromosome 7 abnormalities compared to those without CAG (88% vs. 12%, respectively) [91]. Patients with CAG have a clearly shorter mOS compared to those without CAG [91].
As above discussed, a significant proportion (43%) of AML patients with -7/del(7q) exhibit a CK; the group with CKs was strongly associated with TP53 mutations, while those without CKs were rarely TP53-mutated [51]. These observations suggest two main pathogenetic pathways which involve AMLs with chromosome 7 alterations: one related to AMLs with CKd and TP53 mutations with chromosome 7 alterations as subclonal events and associated with high genomic instability, and another related to AMLs without CKs, where predominantly clonal chromosome 7 alterations cooperate with co-mutational events at the level of some genes to drive leukemic development. Chromosome alterations, such as chromosome 5 and 7 alterations significantly contribute to the malignant development of TP53-mutant AMLs, as supported by the observation that TP53-mutant AMLs with low TP53 VAF (variant allele frequency) without chromosome 5/7 alterations have a clearly better OS than those with low TP53 VAF associated with chromosome 5/7 abnormalities [92].
In addition to CNAs, structural variants can generate fusion proteins or remove or create new enhancer–promoter interactions. Examples of these events are given by 5% of AML cases harboring inv(3)(q21q26.2) or t(3;3)(q21q26.2), which reposition the GATA2 enhancer in close vicinity of MECOM, leading to aberrant MECOM expression and the haploinsufficiency of GATA2 [93]. Other examples of genes activated by enhancer hijacking in AML are given by BCL11B in AML with a mixed phenotype and MNX1 in pediatric AML with t(7;12)(q36;p13). The development of Pyjacker, a computational tool, showed the existence of an enhancer hijacking events in AML patient samples with CKs, including the aberrant expression of MNX1, which can result from del(7q)(q22p36) and is associated with the hijacking of a CDK6 enhancer [93]. MNX1 activation occurs in 1.4% of patients with AML (8.7% of AMLs with del(7q)) and co-occurrs with BCOR mutations [93]. Xenograft mouse models showed that MNX1 activation is required for leukemia cell fitness. Thus, the discovery of MNX1 overexpression showed that deletions on chromosome 7q can not only lead to haploinsufficiency but also the activation of oncogenes by enhancer hijacking.
6. Therapy of Myeloid Neoplasms with Chromosome 7 Abnormalities
Deletions in chromosome 7 are associated with poor outcomes and allogeneic hematopoietic stem cell transplantation (HSCT) is recommended for patients who achieve post-induction complete remission. Two induction therapies were available for these patients; one based on intensive chemotherapy (IC) and the other on a hypomethylating agent (HMA) plus venetoclax (VEN). It is unclear in these patients whether IC is superior to HMA + VEN. A recent retrospective study reported the results of the analysis of 228 AML patients treated with induction therapy based on IC (38% of patients) or HMA + VEN (62% of patients) [94]. Patients treated with HMA + VEN were older than those treated with IC (72 years vs. 61 years) [94]. TP53 mutations and CK were more frequent among patients treated with HMA + VEN than among patients treated with IC (for TP53 mutations 70% vs. 37% and for CK 84% vs. 63%, respectively) [94]. In the entire cohort of patients, also including 897 AML patients without chromosome 5 or 7 abnormalities, median OS was lower in patients with deletions in chromosomes 5 or 7 than in those without these alterations (7.4 months vs. 27 months) [94]. Overall survival for patients with chromosome 5 or 7 deletions was improved by allo-HSCT (24 months for those undergoing allo-HSCT compared to 5.5 months without allo-HSCT) [94]. There was no difference in induction with IC vs. HMA + VEN in patients with ages between 60 and 75 years and in patients who received allo-HSCT after induction therapy [94]. Patients with chromosome abnormalities treated with IC had longer OS compared to those treated with HMA + VEN (10 months vs. 6.1 months, respectively). As HMA + VEN-treated patients were older, an OS comparison was made for patients with ages between 60 and 65 years. There was no difference in mOS following induction with IC vs. HMA + VEN in patients with concomitant TP53 mutations [94] (Figure 4).
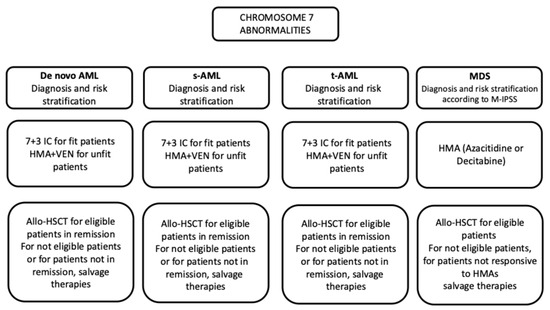
Figure 4.
Outline of treatment of MDS and AML patients with chromosome 7 abnormalities.
Retrospective studies on AML patients undergoing allo-HSCT in the first CR showed that monosomal kayotypes negatively affect outcomes of transplantation: the adverse prognostic impact of MK tended to be more prominent in the younger age group (< 40 years) than in the older age (>40 years) [95]. A recent study reported the results of a large retrospective analysis on a group of 1735 adult AML patients with adverse ELN2022 cytogenetics allografted in first remission, showing that monosomy 7 or monosomy 7 or del(5q) in the presence of CKs or monosomy 17 or 17p abnormalities are associated with poor leukemia-free survival and overall survival; patients with monosomy 7 or monosomy 5 or del(5q) without CK or monosomy 17 or 17p abnormalities had significantly better survival compared to those with CK and/or 17 abnormalities [96].
A second recent study confirmed these findings in a group of 240 patients with TP53-mutated MDS or AML with a median age of 72 years who underwent allo-HSCT. Variant allele frequency (VAF) of TP53 mutations and cytogenetics (5q deletions/7q deletions) were identified as the two most important prognostic factors [92]. Patients with TP53 mutants > 50% had a 2-year PFS of 3%; patients with TP53-mutant VAF < 50% and chromosome 5/7 abnormalities had a 2-year PFS of 22%; and patients with TP53-mutant VAF < 50% without chromosome 5/7 deletions had a 2-year PFS of 60% [92].
No specific targeted therapies for chromosome 7 abnormalities have been developed. Monosomy 7 and del(7q) determine the haploinsufficiency of many genes, some of which contribute to the development of myeloid malignancies, such as SAMD9, SAMD9L, KMT2C, EZH2 and CUX1. A recent study showed that the haploinsufficiency of NAMPT (nicotinamide phosphoribosyltransferase) determines a level of vulnerability that can be exploited at therapeutic level [97]. Thus, in vitro studies using primary AML cells showed that -7/del(7q) leukemic blasts are particularly sensitive to the cytotoxic effects of Daprinad and other NAMPT inhibitors [82]. Biochemical studies confirmed that AML blasts with -7/del(7q) have low NAMPT levels. The combination of Daprinad with Venetoclax (a BCL-2 inhibitor) efficiently eradicated AML blasts with -7/del(7q) [97].
Another recent study confirmed these findings, showing that -7/del(7q) AML cells are sensitive to the cytotoxicity induced by the NAMPT inhibitor KPT-9274; interestingly, both -7/del(7q) cells without or with TP53 abnormalities) are equally sensitive to KPT-9274 [83]. Interestingly, KPT-9274 synergistically interacts with PARP inhibitors to target NAMPT-deficient AML cells [98].
AML and MDS patients with inv(397t(3;3) have an aggressive myeloid neoplasia, associated with low survival. In total, 70% of these patients harbor additional monosomy 7. 3q-rearranged myeloid cancers drive leukemic transformation through the expression of EVI that acts as an oncoprotein with multiple activities. A recent study showed the existence of specific vulnerabilities that could be targeted in these leukemias. Thus, high throughput drug screens and functional assays have shown that EZH2 inhibitors induce apoptosis preferentially in MDS/AML cells with inv(3)/t(3;3) and monosomy 7 through the activation of the GADD45γ-p38-p53 axis [84]. EVI 1 activated in 3q-rearranged MDS/AML was responsible for GADD45γ silencing by directly binding to its consensus sequence present at the level of the GADD45γ promoter and recruitment of the PCR2 complex through its interaction with EXH2, which can be therapeutically targeted by EZH2 inhibition [99]. Thus, MDS/AML cells with inv(3)/t(3;3) and -7 display preferential sensitivity to EZH2 inhibition.
A comprehensive analysis of bone marrow including an evaluation of cytomorphology, cytogenetic analysis with chromosome banding, and gene sequencing is essential to establishing the diagnosis and classification of patients with MDS. The Revised International Prognostic Scoring System (IPSS-R) distinguishes MDS into two groups: low-risk MDS (including those classified as low, very-low and intermediate risk) and high risk (including those classified as high and very high risk) [100]. According to the MDS Cytogenetic Scoring System contained in the IPSS-R, del(7q) pertains to the intermediate-risk group, -7 and double chromosomal abnormalities including -7/del(7q) as poor risk and -7/del(7q) in the context of CKs as very poor risk [100]. The Molecular International Prognostic Scoring System (IPSS-M) risk stratification system introduced additional criteria based on molecular genetics and allowed a more accurate risk stratification [101]. The International Working Group for Prognosis in MDS defined disease subtypes according to genetic lesions; this analysis enabled the formulation of a molecular taxonomy comprising 18 distinct groups [43]. Monosomy 7 pertains to a group characterized by aggressive disease with poor survival and a high risk of leukemic transformation [43]. MDS patients with monosomy 7 are considered potential transplant candidates [102]. In these patients, allo-HSCT should be considered at the time of diagnosis in all eligible patients [102].
The outcome of MDS patients is mainly related to the biology and molecular genetic of the disease and by its general status prior to allo-HSCT, which remains the only curative option in suitable patients. Early in the management of MDS patients, it is fundamental to provide a general prognostication in terms of HSCT planning [103]. It was estimated that only 15% of all MDS patients are generally eligible for allo-HSCT [103]. HSCT in MDS patients is potentially associated with a high rate of complications and a significant risk of associated mortality [103].
No randomized clinical trial directly compared allo-HSCT and conventional therapy in MDS. However, a number of studies have indirectly compared these two therapeutic approaches and have shown significant improvement in long-term outcomes with HSCT, thus supporting the superiority of allo-HSCT over non-HSCT therapy [104]. Few studies have specifically analyzed the outcome of MDS with chromosome 7 abnormalities after allo-HSCT. Thus, van Gelder et al. analyzed 277 adult MDS patients with a chromosomal 7 abnormality undergoing allo-HSCT, present in the European Group for Blood and Marrow Transplantation (EGBMT) database [105]. These patients were classified according to the presence of monosomy 7 (-7) or other chromosome 7 abnormalities (no-7); particularly, among -7 MDS, 77% had an MK but not a CK, while no-7 MDS did not display these features; furthermore, among -7 MDS, 24% had an MK and CK, while only 7% of no-7 MDS exhibited these features [105]. In the whole MDS population, 5-year PFS and OS were 22% and 28%, respectively; in multivariate analysis, the presence of a CK or MK were predictors of a worse outcome [105].
In a study by Versluis et al., featuring 309 adult MDS patients characterized at the genomic level by targeted sequencing and undergoing allo-HSCT, it was observed that 22$% of these patients were classified as very-high-risk (VHR) patients according to the IPSS-M; 57% of these VHR MDS displayed a TP53 mutation and 43% were without TP53 mutations [106]. In total, 27% of VHR patients displayed chromosome 7 abnormalities: 38% in the TP53-mutated group and 13% in the no-TP53-mutated group [106]. In the TP53-mutated group, 94% of chromosome 7 alterations occurred in the context of a CK; in the TP53-WT group, most MDS cases with chromosome 7 alterations co-occurred with ASXL1 mutations [106]. Importantly, allo-HSCT improved OS compared with no transplantation in both subgroups of VHR patients, TP53-mutated and TP53-WT; VHR TP53-WT patients allo-transplanted displayed a better OS compared to VHR TP53-mutated patients [106].
The majority of MDS patients with chromosome 7 abnormalities are considered as high-risk patients and hypomethylating agents currently are the only approved non-transplant therapy for these patients and the standard of care for patients not eligible for allo-HSCT [107].
7. Conclusions
Myeloid neoplasms bearing chromosomal abnormalities of chromosome 7, complete or partial deletions, are characterized by high genomic instability and are associated with poor outcomes. Chromosome 7 abnormalities are frequent in MDS and AML and, only recently, these tumors have been extensively characterized at the molecular level, showing their heterogeneity and their association with other cytogenetic abnormalities and with some somatic mutations.
Chromosome 7 abnormalities are particularly frequent in pediatric MDS associated with germline mutations of GATA-2 and SAMD9/SAMD9L genes; these germline variants represent genes predisposing to MDS development and contribute to defining a peculiar and separate group of myeloid neoplasms.
The pathogenic mechanisms underlying the development of myeloid neoplasms with chromosome 7 abnormalities have been only in part elucidated, but it appears evident now that chromosome 7 deletions usually represent an early key event, and their leukemogenic potential is mediated through the deletion of tumor suppressor genes and they cooperate with other cytogenetic abnormalities and with some gene mutations.
Alterations of chromosome 7 in MDS and AML patients are frequently associated with other chromosomal abnormalities in the context of MK or CK and with TP53 mutations. MNs with deletions of whole chromosome 7 or del(7q) present great challenges due to inherent chemoresistance and poor outcomes, and only a part of these patients benefit from induction therapies and allo-HSCT (basically those without other concomitant chromosomal abnormalities and TP53 mutations).
To date, no targeted specific treatment for chromosome 7 deleted MDS and AML has been approved. However, recent studies have led to the identification of some vulnerabilities that could be specifically targeted by a pharmacologic approach.
Author Contributions
G.C. and E.P. were involved in researching, writing and editing the manuscript. U.T. was involved in conceptualization, organization, and researching and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haase, D. Cytogenetic features in myelodysplastic syndromes. Ann. Hematol. 2008, 87, 515–526. [Google Scholar] [CrossRef]
- Schiffer, C.A.; Lee, E.J.; Tomiyasu, T.; Wiernik, P.H.; Testa, J.R. Prognostic impact of cytogernetic abnormalities in patients with de novo acute nonlymphocytic leukemia. Blood 1989, 73, 263–270. [Google Scholar] [CrossRef]
- Ogawa, S. Genetics of MDS. Blood 2019, 133, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Freireich, E.J.; Whang, J.; Tjio, J.H.; Levin, R.H.; Brittin, G.M.; Frei, I.E. Refractory anemia, granulocytic hyperplasia of bone marrow, and a missing chromosome in marrow cells. A new clinical syndrome? Clin. Res. 1964, 12, 284. [Google Scholar]
- Gyger, M.; Bonny, Y.Y. Monosomy 7 syndrome. N. Engl. J. Med. 1981, 305, 1155–1156. [Google Scholar]
- Scherer, S.W.; Cheung, J.; MacDonald, J.R.; Osborne, L.R.; Nababayashi, K.; Herbrick, J.A.; Carson, A.R.; Parker-Katiraee, L.; Skang, J.; Khaja, R.; et al. Human chromosome 7: DNA sequence and biology. Science 2003, 300, 767–772. [Google Scholar] [CrossRef]
- Kendrick, T.S.; Buic, D.; Fuller, K.A.; Erber, W.N. Abnormalities in chromosomes 5 and 7 in myelodysplastic syndrome and acute myeloid leukemia. Ann. Lab. Med. 2025, 45, 133–145. [Google Scholar] [CrossRef]
- Hosono, N.; Makishima, H.; Jerez, A.; Yoshida, K.; Przychodzen, B.; McMahon, S.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; Miyano, S.; et al. Recurrent genetic defects on chromosome 7q in myeloid neoplasms. Leukemia 2014, 28, 1348–1351. [Google Scholar] [CrossRef]
- Okada, R.; Ochi, Y.; Saiki, R.; Yamanaka, T.; Terao, C.; Yoshizato, T.; Nakagawa, T.; Zhao, L.; Ohyashiki, K.; Hiramoto, N.; et al. Genetic analysis of myeloid neoplasms with der(1;7)(q10;p10). Leukemia 2025, 39, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, C.; Fasan, A.; Meggendorfer, M.; Zenger, M.; Schnittger, S.; Kern, W.; Haferlach, T. Myeloid malignancies with isolated 7q deletion can be further characterized by their accompanying molecular mutations. Blood 2015, 126 (Suppl. 1), 3811. [Google Scholar] [CrossRef]
- Kotmayer, L.; Kennedy, A.L.; Wlodarrski, M.W. Germline and somatic genetic landscape of pediatric myelodysplatic syndromes. Haematologica 2025, 110, 1974–1986. [Google Scholar]
- Wlodarski, M.W.; Hirabayashi, S.; Pator, V.; Stary, J.; Hasle, H.; Moretti, R.; Dworzak, M.; Schmugge, M.; Heuven-Eibrink, M.; Ussawicz, M.; et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood 2016, 127, 1387–1397. [Google Scholar] [CrossRef]
- Kozyra, E.J.; Gohring, G.; Hickstein, D.D.; Calvo, K.R.; DiNardo, C.D.; Dworzak, M.; de Haas, V.; Satry, J.; Hasle, H.; Shimamura, A.; et al. Association of unbalanced translocation der(1;7) with germline GATA2 mutations. Blood 2021, 138, 2441–2445. [Google Scholar] [CrossRef]
- Largeaud, L.; Collin, M.; Monselet, N.; Vergez, F.; Fregona, V.; Larcher, L.; Hirsch, P.; Duployez, N.; Bidet, A.; Luquey, I.; et al. Somatic genetic alterations predict hematological progression in GATA2 deficiency. Haematologica 2023, 108, 1515–1529. [Google Scholar] [CrossRef]
- West, R.R.; Calvo, K.R.; Embree, L.J.; Wang, W.; Tuschong, L.M.; Bauer, T.R.; Tillo, D.; Lack, J.; Droll, S.; Hsu, A.-P.; et al. ASXL1 and STAG2 are common mutations in GATA2 deficiency patients with bone marrow disease and myelodysplastic syndrome. Blood Adv. 2022, 6, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Kotmayer, L.; Kozyra, E.J.; Kong, G.; Strahm, B.; Yoshimi, A.; Sahoo, S.S.; Pastor, V.B.; Attardi, E.; Voss, R.; Vinci, L.; et al. Age-dependent phenotypic and molecular evolution of pediatric MDS arising from GATA2 deficiency. Blood Cancer J. 2025, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Gonzalez, J.B.; Vukovic, M.; Abdelfattah, A. GATA2 is a critical regulator of stem cells in adult hemeatopoiesis and acute myeloid leukemia. Stem Cell Rep. 2019, 13, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.N.; Chang, C.E.; Carmichael, C.L.; Wilkins, E.J.; Brautigan, P.J.; Li, X.C.; Bubic, M.; Lin, M.; Carmignac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2012, 43, 1012–1017. [Google Scholar] [CrossRef]
- Al Serahi, A.F.; Rio-Machin, A.; Tawana, K.; Bodor, C.; Wang, J.; Nagano, A.; Heward, J.A.; Iqbal, S.; Best, S.; Lea, N.; et al. GATA2 monoallelic expression underlies reduced penetrance in inherited GATA2-mutated MDS/AML. Leukemia 2018, 32, 2502–2507. [Google Scholar] [CrossRef]
- Ellingford, J.M.; Telford, N.; Uzqubart, J.; Will, A.M.; Bonney, D.; Adams, B.; Dixon, R.; Kerr, B.; Block, G.C.; Wynn, R.F.; et al. High penetrance of myeloid neoplasia with diverse clinical and cytogenetic features in three siblings with a familial GATA2 deficiency. Cancer Genet. 2021, 257, 77–80. [Google Scholar] [CrossRef]
- Fernandze-Orth, J.; Kotylar, C.; Weiss, J.M.; Gioacchino, E.; de Looper, H.; Endrieux, G.; Ter Borg, M.; Zink, J.; Gonzalez-Menendez, I.; Hoogenboezem, R.; et al. Hematological phenotypes in GATA2 deficiency syndrome arise from secondary injuries and maladaptation to proliferation. Blood Adv. 2025, 9, 2794–2807. [Google Scholar] [CrossRef]
- Katsumura, K.R.; Liu, P.; Kim, J.; Mehta, C.; Bresnick, E.H. Pathogenic GATA2 genetic variants utilize an obligate enhancer mechanism to distort a multilineage differentiation program. Proc. Natl. Acad. Sci. USA 2024, 121, e2317147121. [Google Scholar] [CrossRef]
- Johnson, K.D.; Soukup, A.A.; Bresnick, E.H. GATA2 deficiency elevates interferon regulatory factor-8 to subvert a progenitor cell differentiation program. Blood Adv. 2022, 6, 1464–1473. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Erlacher, M.; Wlosarski, M.W. Genetic and clinical spectrum of SAMD9 and SAMD9L syndromes: From variant interpretation to patients’ management. Blood 2025, 145, 475–485. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Wang, S.; Ma, J.; Lamprecht, T.; Song, G.; Raimondi, S.C.; Wu, G.; Walsh, M.F.; McGee, R.B.; Kesserwan, C.; et al. Germline SAMD9 mutation is sibling with monosomy 7 and myelodysplastic syndrome. Leukemia 2017, 31, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Ma, J.; Lamprecht, T.; Walsh, M.; Wang, S.; Brisnt, V.; Sarg, G.; Wu, G.; Eastan, J.; Kesserwan, C.; et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat. Commun. 2017, 8, 1557. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.B.; Sahoo, S.S.; Boklan, J.; Schwabe, J.C.; Saibryoglu, E.; Strahm, B.; Labrecht, D.; Voss, M.; Bryceson, Y.T.; Erlacher, M.; et al. Constitutional mutations cause familial myelodysplastic syndrome and transient monosomy 7. Haematologica 2018, 103, 427–437. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Pastor, V.B.; Goodings, C.; Voss, R.K.; Kozyra, E.J.; Szvetinik, A.; Noellke, P.; Dworzak, M.; Stary, J.; Locatelli, F.; et al. Clinical evolution, genetic landscape and trajectories of clonal hematopoiesis in SAMD9/SAMD9L syndromes. Nat. Med. 2021, 27, 1806–1817. [Google Scholar] [CrossRef]
- Bluteau, O.; Sebert, M.; Leblanc, T.; Peffault de la Tour, R.; Quentin, S.; Luney, E.; Hernandez, L.; Dalle, J.H.; Sicre de Fantbrune, F.; Itkynson, R.; et al. A landscape of germline mutations in a cohort of inherited bone marrow failure patients. Blood 2018, 131, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Tesi, B.; Davidson, J.; Voss, M.; Rahikkala, E.; Holmes, T.D.; Chiang, S.; Komulainen-Ebrahim, K.; Gorcenco, S.; Rundberg Nilsson, A.; Ripperberger, T.; et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immudeficiency, MDS, and neurological symptoms. Blood 2017, 129, 2266–2279. [Google Scholar] [CrossRef]
- Wong, J.C.; Bryant, V.; Lamporecht, T.; Ma, J.; Waish, M.; Schwartz, J.; Alzamora, M.; Mullighan, C.G.; Ribeiro, R.; Downing, J.R.; et al. Germline SAMD9 and SAMD9L mutations are associated with extensive genetic evolution and diverse hematologic outcomes. JCI Insight 2018, 3, e121086. [Google Scholar] [CrossRef]
- Erlacher, M.; Andresan, F.; Sukova, M.; Stary, J.; de Morelose, B.; van der Werff Ten Bosch, J.; Dworzak, M.; Seidel, M.G.; Polychronopoulou, G.; Beier, R.; et al. Spontaneous remission and loss of monosomy 7: A window of opportunity for young children with SAMD9L syndrome. Haematologica 2024, 109, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Nanumi, S.; Guan, Y.; Przychodzen, B.P.; Hirsch, C.M.; Makishiuma, H.; Shima, H.; Aly, M.; Pastor, V.; Kuzmanovic, T.; et al. Germline loss-of-function SAMD9 and SAMD9L alterations in adult myelodysplastic syndromes. Blood 2018, 132, 2309–2313. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.; Abdelhamed, S.; Hiltebrand, R.; Schwartz, J.R.; Sakurada, S.M.; Walsh, M.; Song, G.; Ma, J.; Pruett, M.; Klco, J.M. Pedaitric MDS and bone marrow failure-associated germline mutations in SAMD9 and SAMD9L impair multiple pathways in primary hemartopoietic cells. Leukemia 2021, 35, 3232–3244. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.; Bejar, R.; Berti, E.; Busquet, L.; Chau, J.; et al. The 5th edition of the World Health Organization Classification og hematolymphoid tumors: Myeloid and histiocytic/dendritic neoplasms. Leuekemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Rudelius, M.; Weinberg, O.K.; Niemeyer, C.M.; Shimamura, A.; Calvo, K.R. The International Consensus Classification (ICC) of hematologic neoplasms with germline predisposition, pediatric myelodysplastic syndrome and juvenile myelomonocytic leukemia. Virchows Arch. 2023, 482, 113–130. [Google Scholar] [CrossRef]
- Pastor, V.; Hirabayashi, S.; Karow, A.; Wehrle, J.; Kozyra, E.J.; Nienhold, R.; Ruzaike, G.; Labrecht, D.; Yoshimi, A.; Nievisch, M.; et al. Mutational landscape in children with myelodysplastic syndromes is distinct from adults: Specific somatic drivers and novel germline variants. Leukemia 2017, 31, 759–762. [Google Scholar] [CrossRef]
- Hasle, H.; Maseth, R.; Pastor Loyola, V.B.; Karow, A.; Catala, A.; DeMoerloose, B.; Dworbak, M.; Hasle, H.; Mosetti, R.; Schmugge, M.; et al. Clonal mutational landscape of childhood myelodysplastic syndromes. Blood 2015, 126 (Suppl. 1), 1162. [Google Scholar] [CrossRef]
- Kardos, G.; Baumann, I.; Panmore, S.J.; Locatelli, F.; Hasle, H.; Schultz, K.R.; Starý, J.; Schmitt-Graeff, A.; Fischer, A.; Harbott, J.; et al. Refractory anemias in childhood: A retrospective analysis of 67 patients with particular reference to monosomy 7. Blood 2003, 102, 1997–2003. [Google Scholar] [CrossRef]
- Haase, D.; Stevenson, K.E.; Neuberg, D.; Maciejewski, J.P.; Nazha, A.; Sekeres, M.A.; Ebert, B.L.; Garcia-Manero, G.; Haferlach, C.; Haferlach, T.; et al. TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups. Leukemia 2019, 33, 1747–1758. [Google Scholar] [CrossRef]
- Crisà, E.; Kulasekararaj, A.G.; Adema, V.; Such, E.; Shanz, J.; Haase, D.; Shirenshan, K.; Best, S.; Milan, S.A.; Kizilors, A.; et al. Impact of somatic mutations in myelodysplastic patients with isolated partial ot total loss of chromosome 7. Leukemia 2020, 34, 2441–2450. [Google Scholar] [CrossRef]
- Bernard, E.; Hasserjian, R.P.; Greenberg, P.L.; ArangoOssa, J.E.; Creigns, M.; Tuechler, H.; Gutierez-Abril, J.; Domenico, D.; Medina-Martinez, J.S.; Levine, M.; et al. Molecular taxonomy of myelodysplastic syndromes and its clinical implications. Blood 2024, 144, 1617–1632. [Google Scholar] [CrossRef]
- Cazzola, M. Myelodysplastic syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahapatra, M.; Saxena, R. Cytogenetics’ impact on the prognosis of acute myeloid leukemia. J. Lab. Physicians 2019, 11, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.M.; Kubota, Y.; Durmaz, A.; Goosdings, C.; Adema, V.; Ponwuilawars, B.; Bahaj, W.S.; Kawan, T.; La Framboise, T.; Meggendorfer, M.; et al. Genomics of deletion 7 and 7q in myeloid neoplasm: From pathogenic culprits to potential synthetic lethal therapeutic targets. Leukemia 2023, 37, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Hasle, H.; Alonzo, T.A.; Auivrignon, A.; Behar, C.; Chang, M.; Creutzig, U.; Fischer, A.; Forestier, E.; Fynn, A.; Haas, O.A.; et al. Monosomy 7 and deletion 7q in children and adolescents with acute myeloid leukemia: An international retrospective study. Blood 2007, 109, 4641–4647. [Google Scholar] [CrossRef]
- McNerney, N.E.; Brown, C.D.; Peterson, A.L.; Banejee, M.; Lçrson, R.A.; Anastasi, J. The spectrum of somatic mutations in high-risk acute myeloid leukemia with -7/del(7q). Br. J. Haematol. 2014, 166, 550–556. [Google Scholar] [CrossRef]
- Grob, T.; AlHinai, A.S.A.; Sanders, M.A.; Kavelaars, F.G.; Rijken, M.; Gradowska, P.L. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef]
- Eisfeld, A.K.; Kolschdmioth, J.; Mrózek, K.; Volinia, S.; Blachly, J.S.; Nicolet, D.; Oakes, C.; Krill, K.; Orwick, S.; Carrol, A.J.; et al. Mutational landscape and gene expression patterns in acute myeloid leukemias with monosomy 7 as a sole abnormality. Clin. Cancer Res. 2017, 77, 207–218. [Google Scholar] [CrossRef]
- Halik, A.; Tilgner, M.; Silva, P.; Estrada, N.; Attwasser, R.; Jahn, E.; Heuser, M.; Hou, H.A.; Protcorona, M.; Hills, R.K.; et al. Genomic characterization of AML with aberrations of chromosome 7: A multinational cohort of 519 patients. J. Hematol. Oncol. 2024, 17, 70. [Google Scholar] [CrossRef]
- Mrozek, K.; Eisfeld, A.K.; Kohlschmidt, J.; Carroll, A.J.; Walker, C.J.; Nicolet, D.; Blachly, J.S.; Bill, M.; Papaioannu, D.; Wang, E.S.; et al. Complex karyotype in de novo acute myeloid leukemia: Typical and atypical subtypes differ molecularly and clinically. Leukemia 2019, 33, 1620–1634. [Google Scholar] [CrossRef]
- Kugler, E.; Enes, D.; Bataller, A.; Wang, B.; DiNardo, C.; Daver, N.; Yilmaz, M.; Short, N.J.; Borthakur, G.; Kadia, T.M.; et al. Mutation dynamics from diagnosis to relapse in acute myeloid leukemia with chromosomal 7 deletions. Leuk. Lymphoma 2025, 66, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Rucker, F.G.; Schlenk, R.F.; Bullinger, L.; Kayser, S.; Teleanu, V.; Kett, H.; Habdank, M.; Kugler, C.M.; Holzmann, K.; Gaidzik, V.I.; et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 2012, 119, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Ayoub, E.; Sun, H.; Kasnagal-Shamanna, R.; Short, N.J.; Issa, G.; Yilmaz, M.; Pierce, S.; Rivera, D.; Chan, B.; et al. Clinical and molecular profiling of AML patients with chromosome 7 or 7q deletions in the context of TP53 alterations and venetoclax treatment. Leuk. Lymphoma 2022, 63, 3105–3116. [Google Scholar] [CrossRef]
- Fleming, S.; Tsai, X.C.H.; Morris, R.; Hou, H.A.; Wei, A.H. TP53 status and impact on AML prognosis within the ELN 2022 risk classification. Blood 2023, 142, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, S.; Wu, Z.K.; Ajigaya, S.; Feng, X.; Liu, C.; Townsley, D.M.; Cooper, J.; Chen, J.; Keyvanfer, K.; et al. Single-cell RNA-seq reveals a distinct transcriptome signature of aneuploid hematopoietic cells. Blood 2017, 130, 2762–2773. [Google Scholar] [CrossRef]
- Kaur, A.; Rajek, A.E.; Syymes, E.; Nawas, M.T.; Patel, A.A.; Patel, J.L.; Sejitra, P.; Aqil, B.; Sukhanova, M.; McNerney, M.E.; et al. Real-wold predictors of response and 24-month survival in high-grade TP53-mutated myeloid neoplasms. Blood Cancer J. 2024, 14, 99. [Google Scholar] [CrossRef]
- Breems, D.A.; van Patten, V.L.; DeGreef, G.E. Monosomal karyotype in acute myeloid leukemia: A better indicator of poor prognostication for a complex karyotype. J. Clin. Oncol. 2008, 26, 4791–4797. [Google Scholar] [CrossRef]
- Kayser, S.; Zucknick, M.; Dohner, K.; Krauter, J.; Kohne, C.H.; Harst, H.A.; Held, G.; von Lillenfeld-Toal, M.; Wihelm, S.; Rummel, Y.; et al. Monosomal karyotype in adult myeloid leukemia: Prognostic impact and outcome after treatment strategies. Blood 2021, 119, 551–558. [Google Scholar] [CrossRef]
- Wangulu, C.; Hezaveh, E.B.; Zarif, M.; Zhou, Q.; Lu, W.; Wei, C.; Sibai, H.; Chang, H. Genomic profile and clinical outcomes in acute myeloid leukemia with monosomal karyotype. Int. J. Mol. Sci. 2025, 29, 5845. [Google Scholar] [CrossRef]
- Jang, J.E.; Min, Y.H.; Kim, I.; Lee, J.H.; Shin, H.J.; Lee, W.S.; Hong, D.S.; Kim, H.J.; Kim, H.J.; Park, S.; et al. Single monosomy as a relatively better survival factor in acute myeloid leukemia patients with monosomal karyotype. Blood Cancer J. 2015, 5, e358. [Google Scholar] [CrossRef]
- Hasle, H.; Aricò, M.; Basso, G.; Biondi, A.; Rajnoldi, A.C.; Creutzing, U.; Fenu, S.; Fonatsch, C.; Haas, O.A.; Harbott, J.; et al. Myelodysplastic syndrome, juvenile myelomonocytic leukemia, and acute myeloid leukemia associated with complete or partial monosomy 7. European Working Group on MDS in Childhood (EWOG-MDS). Leukemia 1999, 13, 376–385. [Google Scholar] [CrossRef]
- Ries, R.E.; Triche, T.J.; Smith, J.L.; Leonti, A.R.; Alonzo, T.A.; Farrar, J.E.; Chen, X.; Liu, Y.; Shaw, T.; Huang, B.J.; et al. Genome and transcriptome profiling of monosomy 7 AML defines novel risk and therapeutic cohorts. Blood 2020, 136 (Suppl. 1), 20. [Google Scholar] [CrossRef]
- Westover, T.; Walsh, M.P.; Abdelhamed, S.; Xiong, E.; Ma, J.; Song, G.; Thomas, M.E., III; Umeda, M.; Maciaszec, J.L.; Wong, J.C.; et al. Genomic landscape and clonal architecture in pediatric myeloid neoplasms with chromosome 7 deletions. Blood Neoplasia 2025, 2, 1–5. [Google Scholar] [CrossRef]
- Sharma, A.; Gallimard, J.E.; Pryce, A.; Bhoopalan, S.V.; Dalissier, A.; Dalle, J.H.; Locatelli, F.; Jubert, C.; Micri-Danicor, O.; Kitra-Roussou, V.; et al. Cytogenetic abnormalities predict survival after allogeneic hematopoiertic stem cell transplantation for pediatric acute myeloid leukemia: A PDWP/EBMT study. Bone Marrow Transplant. 2024, 59, 451–458. [Google Scholar] [CrossRef]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.A. Therapy-related myeloid neoplasms: When genetic and environment collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef]
- Voso, M.T.; Falconi, G.; Fabiani, E. What’s new in the pathogenesis and treatment of therapy-related myeloid neoplasms. Blood 2021, 138, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Singhal, D.; Kutyna, M.M.; Hahn, C.N.; Shah, M.V.; Hiwase, D.K. Therapy-related myeloid neoplasms: Complex interactions among cytotoxic therapies, genetic factora, and aberrant microenvironment. Blood Cenecer Discov. 2024, 5, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; LeBeau, M.; Huo, D.; Karrison, T.; Sotecks, R.M.; Anastasi, J.; Vardiman, J.W.; Rowly, J.D.; Larson, R.A. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: The University of Chicago series. Blood 2003, 103, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.V.; Tran, E.; Shah, S.; Chhetri, R.; Barankal, A.; Ladon, D.; Shultz, C.; Al-kali, A.; Brown, A.L.; Chen, D.; et al. TP53 mutation variant allele frequency of ≥10% is associated with poor prognosis in therapy-related myeloid neoplasms. Blood Cancer J. 2023, 13, 51. [Google Scholar] [CrossRef]
- Buo, Z.; Li, B.; Qin, T.; Xu, Z.; Qu, S.; Jia, Y.; Li, C.; Pan, L.; Gao, Q.; Jiao, M.; et al. Molecular characetristics and clinical implications of TP53 mutations in therapy-related myelodysplastic syndromes. Blood Cancer J. 2025, 5, 58. [Google Scholar] [CrossRef]
- Davoli, T.; Xu, A.W.; Mengwasser, K.E.; Sack, L.M.; Yean, J.C.; Park, P.J.; Elledge, S.J. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell 2013, 155, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- McNerney, M.E.; Brown, C.D.; Wang, X.; Barton, E.T.; Karmakar, S.; Bandlamudi, C.; Yu, S.; Ko, J.; Sandall, B.P.; Stricker, T.; et al. CUX1 is a haploinsufficient tumor suppressor gene on chromosome 7 frequently inactivated in acute myeloid leukemia. Blood 2013, 121, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.; Ramdzan, Z.; Nagata, Y.; Balabrusamanina, S.K.; Honono, N.; Makishima, H.; Visconte, V.; Kuzmanovic, T.; Adema, V.; Nuzha, A.; et al. Distinct clinical and biological implications of CUX1 in meyloid neoplasms. Blood Adv. 2019, 3, 2164–2172. [Google Scholar] [CrossRef]
- An, N.; Khan, S.; Imgruet, M.K.; Gurbuxani, S.K.; Konecki, S.N.; Burgess, M.R.; McNerney, M.E. Gene dosage effect of CUX1 in a muirine model disruptrs HSC homeostasis and controls the severity and mortality of MDS. Blood 2018, 131, 2682–26976. [Google Scholar] [CrossRef] [PubMed]
- Supper, E.; Rudat, S.; Ieyr, V.; Droop, A.; Wong, K.; Spinella, J.F.; Thomas, P.M.; Savageau, G.; Aìdams, D.J.; Wong, C.C. Cut-like homeobox 1 (CUX1) tumor suppressor gene haploinsufficiency induces apoptosis evasion to sustain myeloid leukemia. Nat. Commun. 2021, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- Jotte, M.; Steddart, A.; Martinez, T.C.; Moten, R.; Xue, Y.; Imgruet, M.; Blayylock, H.; Nam, H.; Hu, B.; Austin, J.; et al. Multiplex gene editing models of del(7q) revea combined CUX1 and EZH2 loss drives clonal expansion and drug resistance. Blood Neoplasia 2025, 2, 1–17. [Google Scholar] [CrossRef]
- An, N.; Khan, S.; Imgruet, M.K.; Jueng, L.; Gurbuxani, S.; McNerney, M.E. Oncogenic RAS promotes leukemic transformation of CUX1-deficient cells. Oncogene 2023, 42, 881–893. [Google Scholar] [CrossRef]
- Wong, J.C.; Weinfurtner, K.M.; Alzamore Mdel, P.; Kogan, S.C.; Burgess, M.R.; Zhang, Y.; Nakitandwe, J.; Ma, J.; Cheng, J.; Chen, S.C.; et al. Functional evidence implicating chromosome 7q22 haploinsufficiency in myelodysplastic syndrome pathogenesis. eLife 2015, 4, e07839. [Google Scholar] [CrossRef]
- Wong, J.C.; Weinfurtner, K.M.; Westover, T.; Nim, J.; Lebish, E.J.; Alzamora, M.; Huang, B.J.; Walsh, M.; Abdelòhamed, S.; Ma, J.; et al. 5G3 mice model loss of a commonly deleted segment of chromosome 7q22 in myeloid malignancies. Leukemia 2024, 38, 1182–1186. [Google Scholar] [CrossRef]
- Boulignyy, I.; Murrayy, G.; Ho, T.; Gor, J.; Zacholski, K.; Wages, N.; Grant, S.; Maher, K. CUX1mut acute myeloid leukemia as a distinct biological entity: An analysis of clinical outcomes and implications. Blood 2023, 142 (Suppl. 1), 5978–5979. [Google Scholar] [CrossRef]
- Gao, T.; Ptashkin, R.; Bolton, K.L.; Sirenko, M.; Fong, C.; Spitzer, B.; Megheriani, K.; Arango Ossa, J.E.; Zhou, Y.; Bernard, E.; et al. Interplay between chromosomal alterations and gene mutations shapes the evolutionary trajectory of clonal hematopoiesis. Nat. Commun. 2021, 12, 338. [Google Scholar] [CrossRef]
- Saiki, R.; Momozawa, Y.; Nannya, Y.; Nakgawa, M.; Ochi, Y.; Ypshizato, T.; Terao, T.; Terao, C.; Kurda, Y.; Shiraishi, T.; et al. Combined landscape of single-nucleotide variants and copy number alterations in clonal hematopoiesis. Nat. Med. 2021, 27, 1239–1249. [Google Scholar] [CrossRef]
- Yoshizato, T.; Dumutriu, B.; Hosokawa, K.; Makishima, H.; Yoshida, K.; Townlers, D.; Sat-Otserbo, A.; Sato, Y.; Liu, D.; Suziki, H.; et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N. Engl. J. Med. 2015, 373, 35–47. [Google Scholar] [CrossRef]
- Zink, F.; Stacey, S.N.; Norddahl, G.L.; Frigge, M.L.; Magnusson, O.T.; Jonsdottir, I.; Thorgeirsson, T.E.; Sigurdsson, A.; Gudjonsson, S.A.; Gudmundsson, J.; et al. Clonal hematopoiesis with and without candidate driver mutations is common on the elderly. Blood 2017, 130, 742–752. [Google Scholar] [CrossRef]
- Robertson, N.A.; Latorre-Crespo, E.; Terradas-Terradas, M.; Lemos-Portela, J.; Purcell, A.C.; Livesey, B.J.; Hillary, R.F.; Murphy, L.; Fawkes, A.; MacGillivray, L.; et al. Longitudinal dynamics of clonal hematopoiesis identifies gene-specific fitness effects. Nat. Med. 2022, 28, 1439–1446. [Google Scholar] [CrossRef]
- Jotte, M.; McNerney, M. The significance of CUX1 and chromosome 7 in myeloid malignancies. Curr. Opin. Hematol. 2022, 29, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Leppa, A.M.; Grimes, K.; Jeong, H.; Huang, F.Y.; Andrades, A.; Waclawiczek, A.; Boch, T.; Janch, A.; Renders, S.; Stelmach, P.; et al. Single-cell multiomics analysis reveals dynamic clonal evolution and targetable phenotypes in acute myeloid leukemia with complex karyotype. Nat. Genet. 2024, 56, 2790–2803. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Hu, S.; Loghavi, S.; Toruner, G.A.; Ravandi-Kashani, F.; Tang, Z.; Li, S.; Xu, J.; Daver, N.; Madeiros, J.; et al. Chromoangiogenesis is frequently associated with highly complex karyotypes, extensive clonal heterogeneity, and treatment refractoriness in acute myeloid leukemia. Am. J. Hematol. 2025, 100, 417–426. [Google Scholar] [CrossRef]
- Lontos, K.; Saliba, R.M.; Kanagal-Shamanna, R.; Ozcan, G.; Ramdial, J.; Chen, G.; Kadia, T.; Short, N.J.; Daver, N.G.; Kantarjian, H.; et al. TP53-mutant allele frequency and cytogenetics determine prognostic groups in MDS/AML for transplantation. Blood Adv. 2025, 9, 2845–2854. [Google Scholar] [CrossRef]
- Sollier, E.; Riedel, A.; Toprak, U.; Wierbinska, J.; Weichenan, O.; Schmid, J.P.; Hakobyan, M.; Touzart, A.; Jahn, E.; Vick, B.; et al. Enhancer hijacking discovery in acute myeloid leukemia by Pyjacker identifies MNX1 activation via deletion 7q. Blood Cancer Discov. 2025, 6, 343–363. [Google Scholar] [CrossRef]
- Boussi, L.; Bewersdorf, P.; Liu, Y.; Shallis, R.M.; Aguirre, L.E.; Zucenka, A.; Garciaz, S.; Bystrom, R.P.; DeAngelo, D.; Stone, R.M.; et al. Outcomes with HMA plus venetoclax vs intensive chemotherapy in AML patients with chromosome 5 and 7 abnormalities. Blood 2024, 144 (Suppl. 1), 4281–4283. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.H.; Lee, J.; Park, H.S.; Choi, E.J.; Jo, J.C.; Lee, Y.J.; Lee, Y.S.; Kang, Y.A.; Lee, K.H. Monosomal karyotype affecting outcomes of allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia in first complete remission. EUR J. Hematol. 2020, 108, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Galimard, J.E.; Dalle, I.A.; Sociè, G.; Versluis, J.; Wu, D.; Eder, M.; Labussière-Wallet, H.; Yakub-Agha, I.; Maerten, J.; et al. Challenging the adverse label: Diverse outcomes of ELN2022 adverse cytogenetic subgroups in acute myeloid leukemia patients allografted in first remission: From EBMT ALWP. Am. J. Hematol. 2025, 100, 1374–1386. [Google Scholar] [CrossRef]
- Eldfors, S.; Saad, J.; Ikonen, N.; Malani, D.; Vaha-Hoskela, M.; Gjersten, B.T.; Kontro, M.; Porkka, K.; Heekman, C.A. Monosomy 7/del(7q) cause sensitivity to inhibitors of nicotinanimide phosphoribosyltransferase in acute myeloid leukemia. Blood Adv. 2024, 8, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Senagolage, M.D.; Blaylock, H.Z.; Khan, S.; Skuli, S.J.; Carroll, M.P.; McNerney, M.E. NAMPT Haploinsufficiency is a Collateral Lethat Therapeutic Vulnerability in High-Risk Myeloid Malignancies with TP53 Inactivation. Blood Neoplasia 2025, 2, 100119. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, H.; Muira, A.; Sagisaka, M.; Ito, M.; Menosono, R.; Yamauchi, H.; Nishimura, K.; Honma, D.; Tsutsumi, S.; Adachi, A.; et al. PRC2-mediated apoptosis evasion is a therapeutic target of MDS/AML harboring inv(3)/t(3;3) and monosomy 7. Hemasphere 2025, 9, e70149. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arongo Ossa, J.E.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid. 2022, 1, EVIDoa22000008. [Google Scholar] [CrossRef]
- Gurnari, C.; Robin, M.; Adès, L.; Aljuf, M.; Almeida, A.; Duarte, F.B.; Bernard, E.; Cutler, C.; Della Porta, M.G.; De Witta, T.; et al. Clinical-genomic profiling of MDS to inform allo-HCT: Recommendations from an international panel on behalf of the EBMT. Blood 2025, 145, 1987–2001. [Google Scholar] [CrossRef]
- Gurnari, C.; Gagelmann, N.; Badbaran, A.; Awada, H.; Dima, D.; Pagliuca, S.; D’Aveni-Piney, M.; Attardi, E.; Voso, M.T.; Cerretti, R.; et al. Outcome prediction in myelodysplastic neoplasm undergoing hematopoietic cell transplant in the molecular era of IPSS-M. Leukemia 2023, 37, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C. Revisiting timing and decision modeling for allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes. J. Clin. Oncol. 2024, 42, 2843–2848. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, M.; de Weede, L.C.; van Biezen, A.; Volin, L.; Maertens, J.; Robin, M.; Petersen, E.; de Witte, T.; Kroger, N.; on behalf of EBMT for Chronic Malignancies Woking Party. Mononomial karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia. Leukemia 2013, 27, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Versluis, J.; Saber, W.; Tsai, H.K.; Gibson, C.J.; Dillon, L.W.; Mishra, A.; McGiurk, J.; Maziarz, R.T.; Westervelt, P.; Hedge, P.; et al. Allogeneic hematopoietic cell transplantation improves outcome in myelodysplastic syndrome across high-risk genetic subgroups: Genetic analysis of the blood and bone marrow transplant clinical trials network 1102 study. J. Clin. Oncol. 2023, 41, 4497–4510. [Google Scholar] [CrossRef]
- Kroger, N. Treatment of high-risk myelodysplastic syndromes. Haematologica 2025, 110, 339–350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).