Survival and Prognostic Factors in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia Receiving Supportive Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Ethical Considerations
3. Results
3.1. Treatment
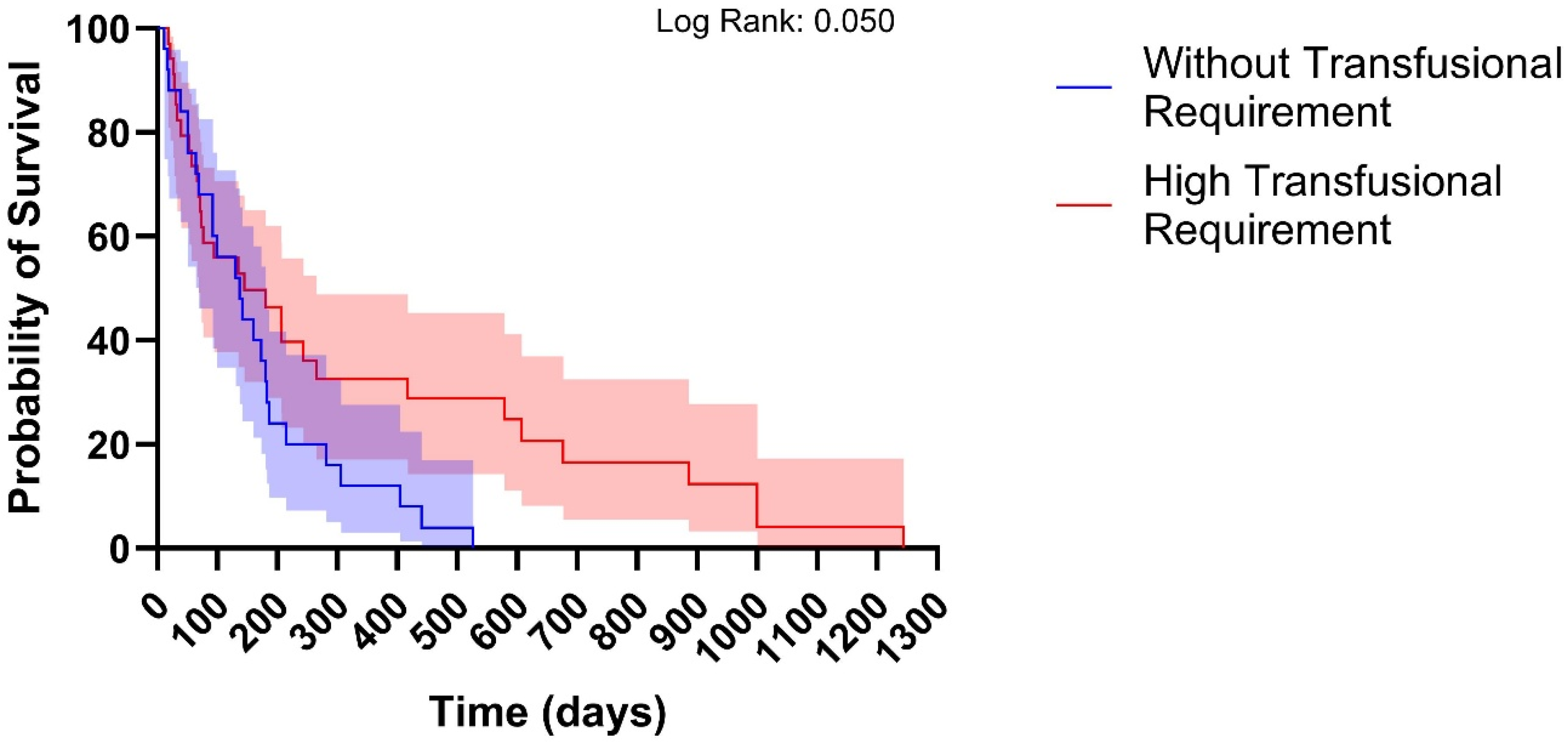
3.2. Supportive Care and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | Acute Lymphoblastic Leukemia |
| TKIs | Tyrosine kinase inhibitors |
| WBC | White Blood Cell Count |
| PPI | Palliative Prognostic Index |
| EASE | Emotion and Symptom-focused Engagement |
References
- Pagliaro, L.; Chen, S.; Herranz, D.; Mecucci, C.; Harrison, C.J.; Mullighan, C.G.; Zhang, M.; Chen, Z.; Boissel, N.; Winter, S.S.; et al. Acute lymphoblastic leukaemia. Nat. Rev. Dis. Primers 2024, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Sucre, O.; Pamulapati, S.; Muzammil, Z.; Bitran, J.D. Advances in Therapy of Adult Patients with Acute Lymphoblastic Leukemia. Cells 2025, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.A.; Hannon, B.; Zimmermann, C. Integrating Palliative Care into Oncology Care Worldwide: The Right Care in the Right Place at the Right Time. Curr. Treat. Options Oncol. 2023, 24, 353. [Google Scholar] [CrossRef]
- Gavralidis, A.; Brunner, A.M. Novel Therapies in the Treatment of Adult Acute Lymphoblastic Leukemia. Curr. Hematol. Malig. Rep. 2020, 15, 294–304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khanal, N.; Upadhyay Banskota, S.; Bhatt, V.R. Novel Treatment Paradigms in Acute Myeloid Leukemia. Clin. Pharmacol. Ther. 2020, 108, 506–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calderon, A.; Han, C.; Karma, S.; Wang, E. Non-genetic mechanisms of drug resistance in acute leukemias. Trends Cancer 2024, 10, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Gurnari, C.; Pagliuca, S.; Visconte, V. Deciphering the Therapeutic Resistance in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2020, 21, 8505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nachmias, B.; Aumann, S.; Haran, A.; Schimmer, A.D. Venetoclax resistance in acute myeloid leukaemia—Clinical and biological insights. Br. J. Haematol. 2024, 204, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.T.; Lasry, A.; Carroll, W.L.; Aifantis, I. Immune-Based Therapies in Acute Leukemia. Trends Cancer 2019, 5, 604–618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geramita, E.; Hou, J.-Z.; Shlomchik, W.D.; Ito, S. Maintenance strategies for relapse prevention and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2024, 2024, 635–643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ueno, H.; Yoshida, K.; Shiozawa, Y.; Nannya, Y.; Iijima-Yamashita, Y.; Kiyokawa, N.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; Isobe, T.; et al. Landscape of driver mutations and their clinical impacts in pediatric B-cell precursor acute lymphoblastic leukemia. Blood Adv. 2020, 4, 5165–5173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, Y.; Wu, L.; Zhang, M.; Ouyang, W.; Huang, J.; Chen, S. Emerging molecular subtypes and therapeutic targets in B-cell precursor acute lymphoblastic leukemia. Front. Med. 2021, 15, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Artioli, F.; Razzini, G.; Barbieri, D.; Secchi, C. Relationship Dynamics Underlying Cancer Overtreatment in Advanced Cancer Patients from an Oncologist Point of View. Front. Psychol. 2021, 12, 754432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hauge, A.M.; Lydiksen, N.; Bech, M. Organizing to address overtreatment in cancer care near the end of life: Evidence from Denmark. J. Health Serv. Res. Policy 2025, 30, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pelcovits, A.; Olszewski, A.J.; Decker, D.; Guyer, D.; Leblanc, T.W.; Egan, P. Impact of Early Palliative Care on End-of-Life Outcomes in Hematologic Malignancies. J. Palliat. Med. 2022, 25, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.B.; Belanger, E.; Egan, P.C.; LeBlanc, T.W.; Olszewski, A.J. Early Palliative Care Services and End-of-Life Care in Medicare Beneficiaries with Hematologic Malignancies: A Population-Based Retrospective Cohort Study. J. Palliat. Med. 2021, 24, 63–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pergolizzi, J., Jr.; LeQuang, J.A.K.; Wagner, M.; Varrassi, G. Challenges in Palliative Care in Latin America: A Narrative Review. Cureus 2024, 16, e60698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwartz, M.S.; Muffly, L.S. Predicting relapse in acute lymphoblastic leukemia. Leuk. Lymphoma 2024, 65, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.R.; Ghoshal, A.; Udupa, K.; Pai, A.; Mailankody, S.; Damani, A.; Rao, K.S.; Prabhu, S.; Nayak, M.G.; Salins, N. Assessing palliative care needs in adult patients with hematological malignancies and their caregivers: Implications for referral practice. BMC Palliat. Care 2025, 24, 201. [Google Scholar] [CrossRef]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Aldoss, I.; DeAngelo, D.J.; Konopleva, M.; Leonard, J.; Logan, A.C.; Park, J.; Shah, B.; Stock, W.; Jabbour, E.; et al. Clinical use of measurable residual disease in adult acute lymphoblastic leukemia. Blood Adv. 2025, 9, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Colunga-Pedraza, P.R.; Colunga-Pedraza, J.E.; Peña-Lozano, S.P.; León, A.G.-D.; Ruiz-Delgado, G.J.; Ribeiro, R.C. Diagnosis and treatment of acute lymphoblastic leukemia in Latin America. Hematology 2022, 27, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Beyer, F.; Sistermanns, J.; Kuon, J.; Kahl, C.; Alt-Epping, B.; Stevens, S.; Ahlborn, M.; George, C.; Heider, A.; et al. Symptom Burden and Palliative Care Needs of Patients with Incurable Cancer at Diagnosis and During the Disease Course. Oncologist 2021, 26, e1058–e1065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuriakose, J.; Deodhar, J.K.; Jain, H.; Sengar, M.; Shetty, A.P.P.; Jayaseelan, P. Palliative Care Needs in Adult Patients with Acute Leukemia: A Prospective Observational Study. J. Palliat. Med. 2025, 28, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Rodin, G.; Malfitano, C.; Rydall, A.; Schimmer, A.; Marmar, C.M.; Mah, K.; Lo, C.; Nissim, R.; Zimmermann, C. Emotion and Symptom-focused Engagement (EASE): A randomized phase II trial of an integrated psychological and palliative care intervention for patients with acute leukemia. Support Care Cancer 2020, 28, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P.; Shostrom, V.; Al-Kadhimi, Z.S.; Maness, L.J.; Gundabolu, K.; Bhatt, V.R. Usefulness of Charlson Comorbidity Index to Predict Early Mortality and Overall Survival in Older Patients with Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2020, 20, 804–812.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoong, S.Q.; Bhowmik, P.; Kapparath, S.; Porock, D. Palliative prognostic scores for survival prediction of cancer patients: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2024, 116, 829–857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoong, S.Q.; Zhang, H.; Whitty, D.; Tam, W.W.S.; Wang, W.; Porock, D. Prognostic utility of Palliative Prognostic Index in advanced cancer: A systematic review and meta-analysis. Palliat. Support Care 2025, 23, e80. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, T.W.; Egan, P.C.; Olszewski, A.J. Transfusion dependence, use of hospice services, and quality of end-of-life care in leukemia. Blood 2018, 132, 717–726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Egan, P.C.; LeBlanc, T.W.; Olszewski, A.J. End-of-life care quality outcomes among Medicare beneficiaries with hematologic malignancies. Blood Adv. 2020, 4, 3606–3614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Odejide, O.O.; Cronin, A.M.; Earle, C.C.; Tulsky, J.A.; Abel, G.A. Why are patients with blood cancers more likely to die without hospice? Cancer 2017, 123, 3377–3384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vidal, M.; Hui, D.; Bruera, E. Palliative Care in Patients with Leukemia: When and How? Curr. Oncol. Rep. 2018, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Odejide, O.O.; Cronin, A.M.; Condron, N.B.; Fletcher, S.A.; Earle, C.C.; Tulsky, J.A.; Abel, G.A. Barriers to Quality End-of-Life Care for Patients with Blood Cancers. J. Clin. Oncol. 2016, 34, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Odejide, O.O.; Steensma, D.P. Patients with haematological malignancies should not have to choose between transfusions and hospice care. Lancet Haematol. 2020, 7, e418–e424. [Google Scholar] [CrossRef] [PubMed]
| Variables | Values |
|---|---|
| Age (years) | 31.0 (18.0–74.0) |
| WBC (×103/μL) | 14.0 (0.0–490.0) |
| Survivorship (days) | 137.0 (11.0–1244.0) |
| Gender Male Female | 29 (49.2%) 30 (50.8%) |
| Lineage at diagnosis B cell T cell | 57 (96.6%) 02 (03.4%) |
| Risk at diagnosis Standard High | 12 (20.3%) 47 (79.7%) |
| Relapse Absence Presence | 38 (64.4%) 21 (35.6%) |
| Transfusion requirement Without High | 25 (42.4%) 34 (57.6%) |
| Hyperleukocytosis Absence Presence | 39 (66.1%) 20 (33.9%) |
| Age Risk <35 years >35 years | 32 (54.7%) 27 (45.8%) |
| Overall survival Live Death | 04 (06.8%) 55 (93.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Peñafiel, C.; Cabrera García, Á.; Martínez Tovar, A.; Pérez Sámano, D.; Mendez Lomeli, I.; Villagrán Carpintero, E.; Olarte Carrillo, I.; Vargas Peña, S.M.; Gallardo Rodríguez, A.G. Survival and Prognostic Factors in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia Receiving Supportive Care. Hemato 2025, 6, 32. https://doi.org/10.3390/hemato6030032
Ramos Peñafiel C, Cabrera García Á, Martínez Tovar A, Pérez Sámano D, Mendez Lomeli I, Villagrán Carpintero E, Olarte Carrillo I, Vargas Peña SM, Gallardo Rodríguez AG. Survival and Prognostic Factors in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia Receiving Supportive Care. Hemato. 2025; 6(3):32. https://doi.org/10.3390/hemato6030032
Chicago/Turabian StyleRamos Peñafiel, Christian, Álvaro Cabrera García, Adolfo Martínez Tovar, Daniela Pérez Sámano, Isle Mendez Lomeli, Ernesto Villagrán Carpintero, Irma Olarte Carrillo, Sayuri Midori Vargas Peña, and Adán Germán Gallardo Rodríguez. 2025. "Survival and Prognostic Factors in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia Receiving Supportive Care" Hemato 6, no. 3: 32. https://doi.org/10.3390/hemato6030032
APA StyleRamos Peñafiel, C., Cabrera García, Á., Martínez Tovar, A., Pérez Sámano, D., Mendez Lomeli, I., Villagrán Carpintero, E., Olarte Carrillo, I., Vargas Peña, S. M., & Gallardo Rodríguez, A. G. (2025). Survival and Prognostic Factors in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia Receiving Supportive Care. Hemato, 6(3), 32. https://doi.org/10.3390/hemato6030032






