Multiple Myeloma and Precursor Plasma Cell Disorders: From Emerging Driver Mutations to Current and Future Therapeutic Strategies
Abstract
1. Introduction
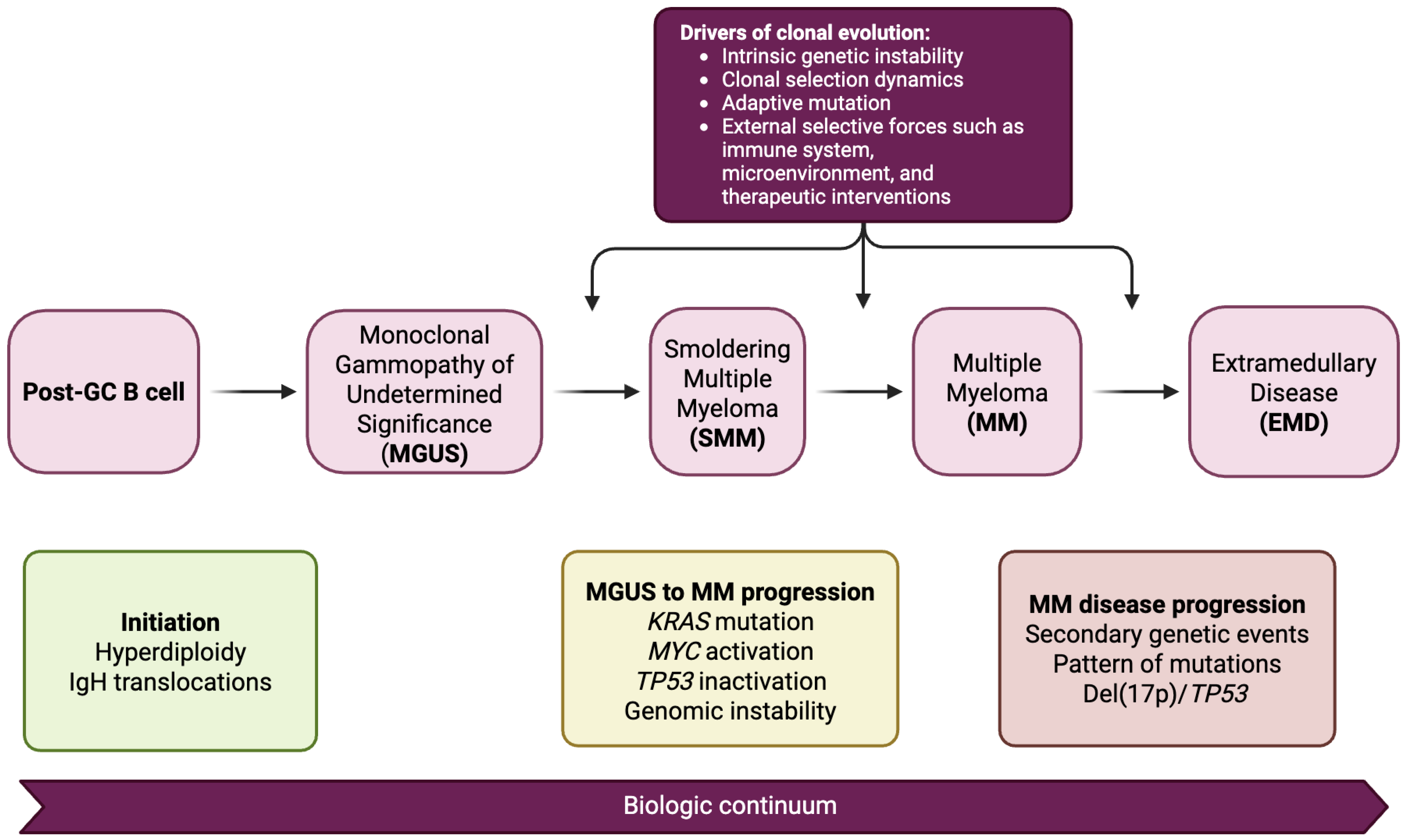
2. Pathophysiology and Disease Continuum
2.1. Clonal Evolution from MGUS to SMM to MM
2.2. Microenvironmental Influences
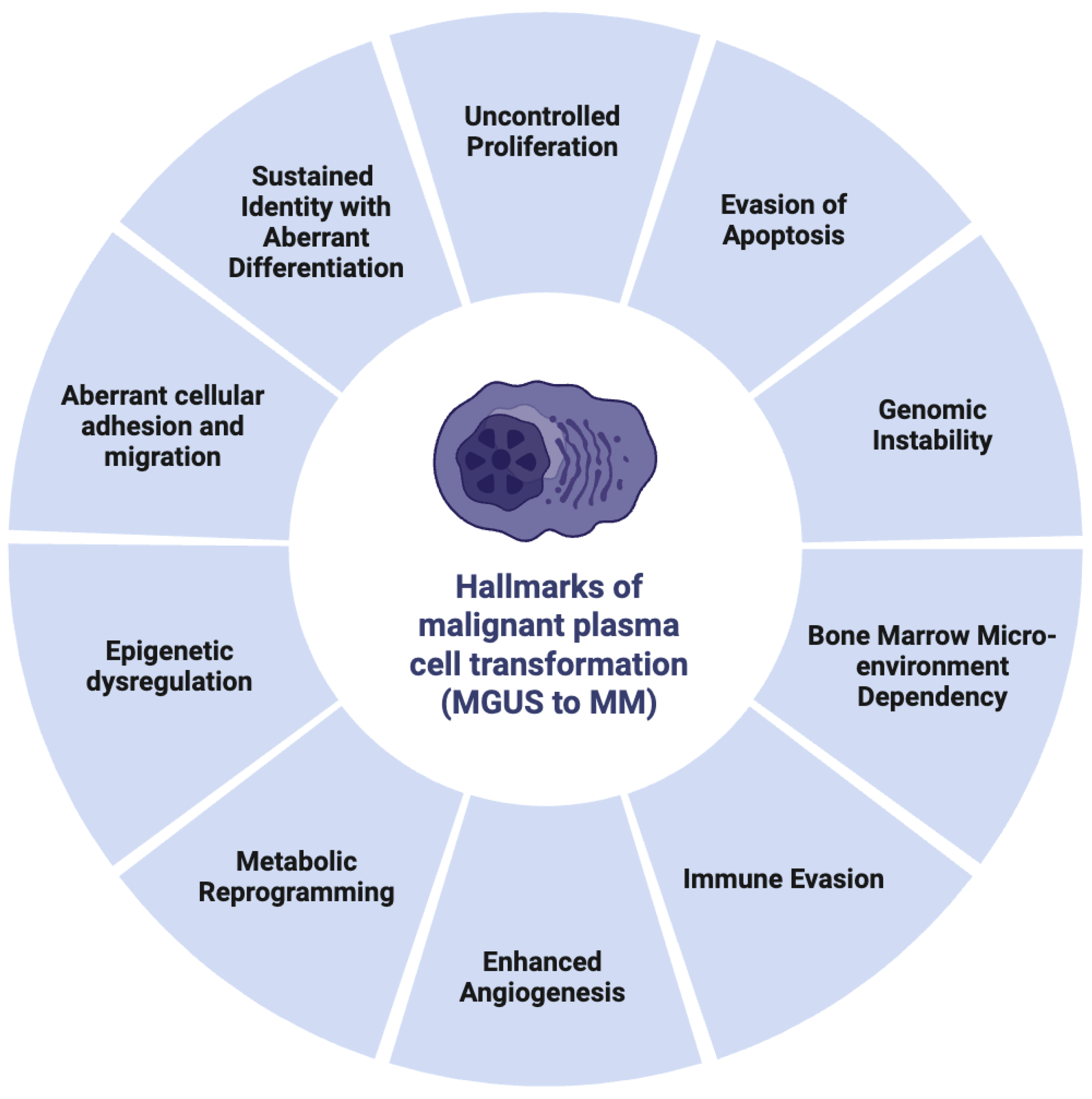
2.3. Hallmarks of Malignant Plasma Cell Transformation
3. Genomic and Epigenetic Landscape
3.1. Common Cytogenetic Abnormalities
3.1.1. Hyperdiploidy
3.1.2. IgH Translocations
3.1.3. Chromosome 13q and 17p Deletions
3.2. Emerging Driver Mutations
3.2.1. RAS Pathway Mutations
3.2.2. TP53 and DNA Repair Genes
3.2.3. MYC Deregulation
3.2.4. Mutational Signatures and Timing in Progression
3.3. Epigenetic Dysregulation
4. Disease Detection and Risk Stratification
4.1. Diagnostic Criteria
4.2. Molecular and Genomic Risk Markers
4.3. Imaging Modalities
5. Current Therapeutic Strategies
5.1. Frontline Therapy
5.2. Maintenance and Consolidation Therapy
5.3. Management of High-Risk Disease
5.4. Management of Bone Loss in Multiple Myeloma
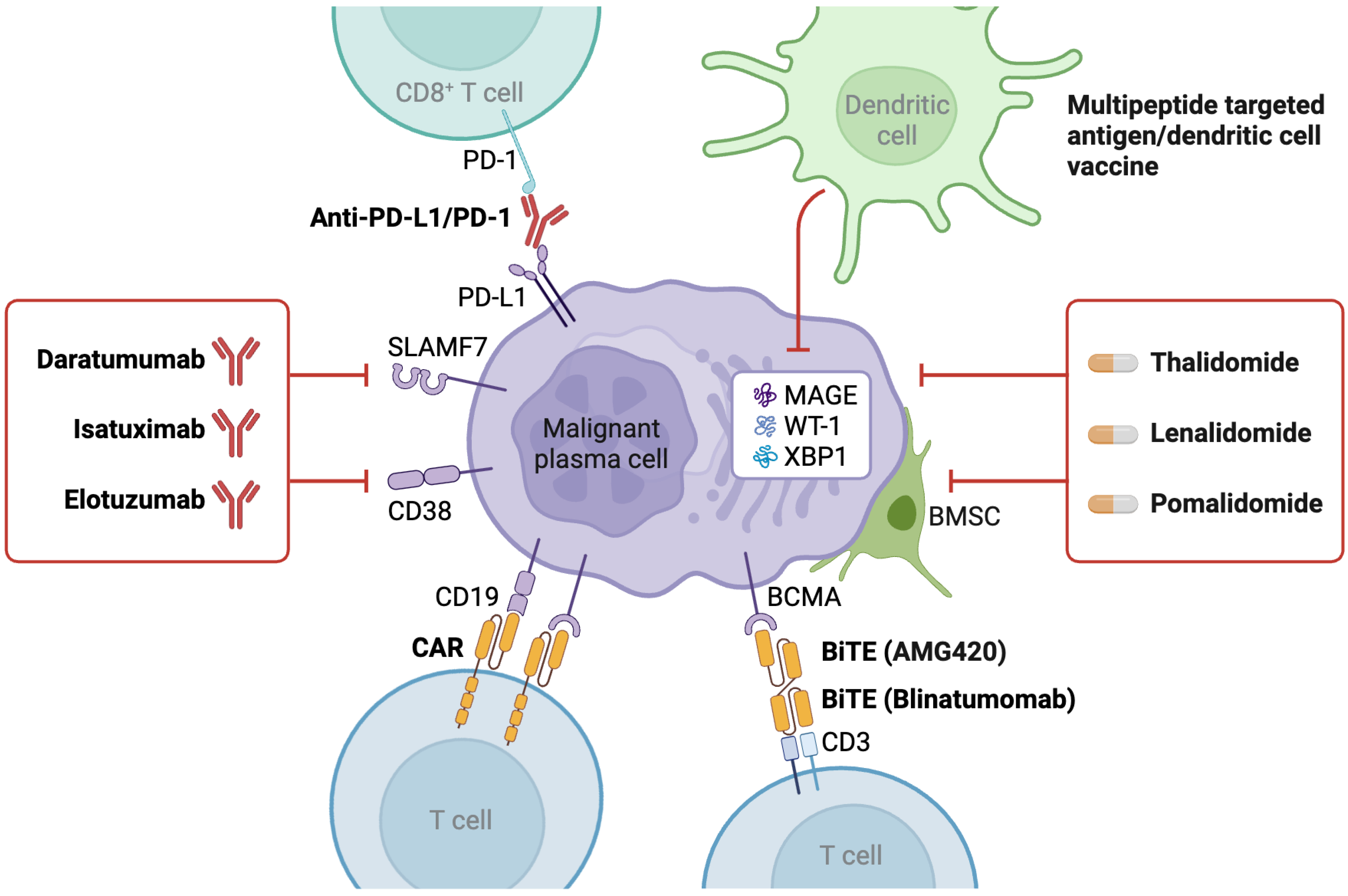
6. Immunotherapy and Targeted Agents
6.1. Monoclonal Antibodies
6.2. Antibody–Drug Conjugates
6.3. CAR-T-Cell Therapy
6.4. Bispecific T-Cell Engagers (BiTEs)
7. Future Directions in Therapeutics
7.1. Targeting Other Mutational Pathways
7.2. Epigenetic Modulators
7.3. Vaccine Strategies and Tumor Neoantigens
7.4. Microenvironment Modulation
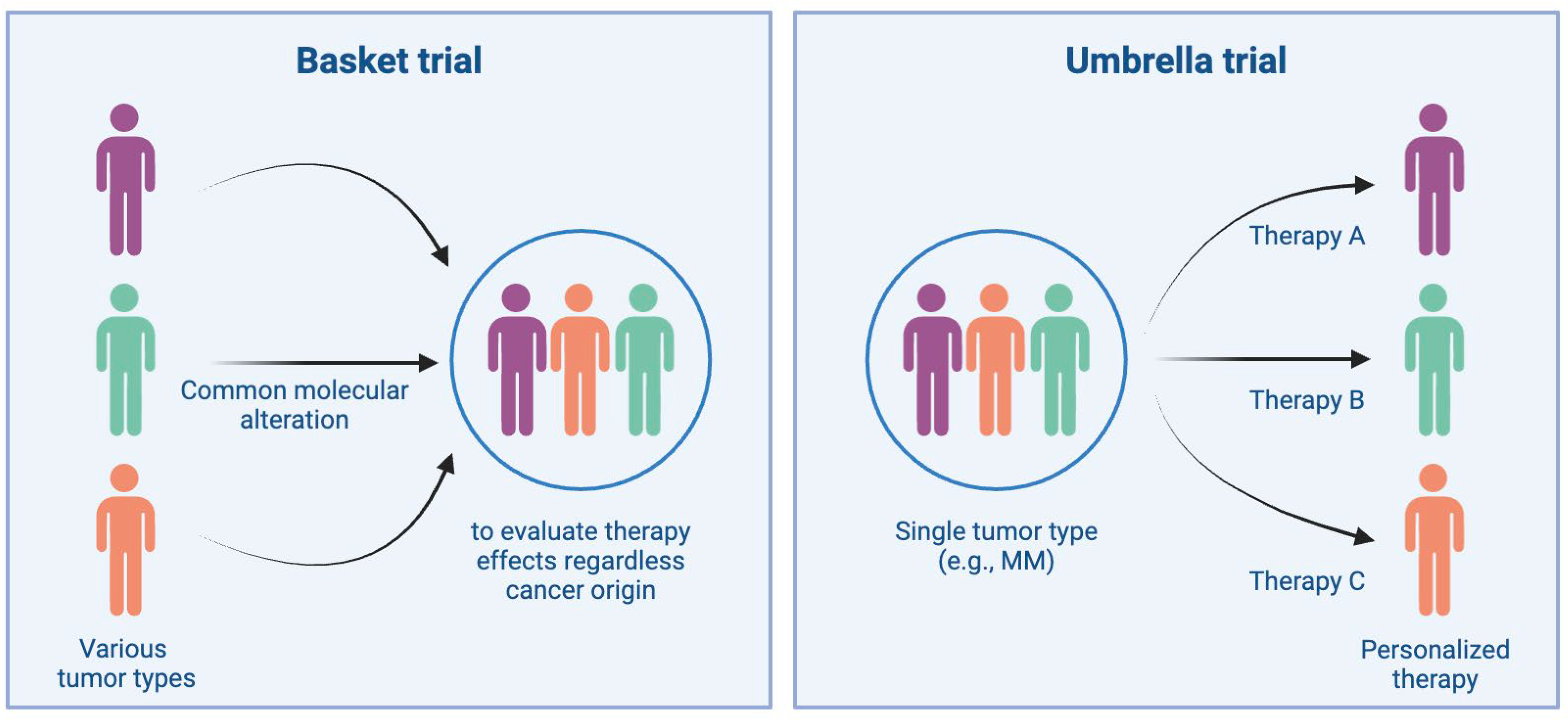
7.5. Precision Medicine and Biomarker-Driven Trials
8. Special Considerations
8.1. Management in Elderly and Frail Patients
8.2. Racial and Ethnic Disparities
8.3. Relapsed and Refractory MM
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mateos, M.-V.; Landgren, O. MGUS and Smoldering Multiple Myeloma: Diagnosis and Epidemiology. In Plasma Cell Dyscrasias; Roccaro, A.M., Ghobrial, I.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–12. ISBN 978-3-319-40320-5. [Google Scholar]
- Sutanto, H.; Sandra, D.Y.; Safira, A.; Adytia, G.J.; Waitupu, A.; Romadhon, P.Z. Genetic, Epigenetic, and Molecular Determinants of Multiple Myeloma and Precursor Plasma Cell Disorders: A Pathophysiological Overview. Med. Oncol. 2025, 42, 234. [Google Scholar] [CrossRef] [PubMed]
- Van De Donk, N.W.C.J.; Mutis, T.; Poddighe, P.J.; Lokhorst, H.M.; Zweegman, S. Diagnosis, Risk Stratification and Management of Monoclonal Gammopathy of Undetermined Significance and Smoldering Multiple Myeloma. Int. J. Lab. Hematol. 2016, 38, 110–122. [Google Scholar] [CrossRef] [PubMed]
- González-Calle, V.; Mateos, M.V. Monoclonal Gammopathies of Unknown Significance and Smoldering Myeloma: Assessment and Management of the Elderly Patients. Eur. J. Intern. Med. 2018, 58, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Patel, A.; Goh, C.Y.; Moscvin, M.; Zhang, L.; Bianchi, G. Changing Paradigms in Diagnosis and Treatment of Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering Multiple Myeloma (SMM). Leukemia 2020, 34, 3111–3125. [Google Scholar] [CrossRef]
- Korde, N.; Kristinsson, S.Y.; Landgren, O. Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering Multiple Myeloma (SMM): Novel Biological Insights and Development of Early Treatment Strategies. Blood 2011, 117, 5573–5581. [Google Scholar] [CrossRef]
- Blum, A.; Bazou, D.; O’Gorman, P. Smoldering Multiple Myeloma: Prevalence and Current Evidence Guiding Treatment Decisions. BLCTT 2018, 8, 21–31. [Google Scholar] [CrossRef]
- López-Corral, L.; Gutiérrez, N.C.; Vidriales, M.B.; Mateos, M.V.; Rasillo, A.; García-Sanz, R.; Paiva, B.; San Miguel, J.F. The Progression from MGUS to Smoldering Myeloma and Eventually to Multiple Myeloma Involves a Clonal Expansion of Genetically Abnormal Plasma Cells. Clin. Cancer Res. 2011, 17, 1692–1700. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kikuchi, J. Molecular Basis of Clonal Evolution in Multiple Myeloma. Int. J. Hematol. 2020, 111, 496–511. [Google Scholar] [CrossRef]
- Salomon-Perzyński, A.; Jamroziak, K.; Głodkowska-Mrówka, E. Clonal Evolution of Multiple Myeloma—Clinical and Diagnostic Implications. Diagnostics 2021, 11, 1534. [Google Scholar] [CrossRef]
- Qin, X.; An, G.; Hu, L.; Qin, Y.; Xu, Y.; Feng, X.; Zang, M.; Deng, S.; Sui, W.; Yi, S.; et al. Clonal Evolution Paths Significantly Impact the Outcome of Myeloma. Blood 2015, 126, 1784. [Google Scholar] [CrossRef]
- Yan, Y.; Qin, X.; Liu, J.; Fan, H.; Yu, Z.; Liu, W.; Hao, M.; Qiu, L.; An, G. OAB-011: Clonal Phylogeny and Evolution of Critical Cytogenetic Aberrations in Multiple Myeloma at Single Cell Level. Clin. Lymphoma Myeloma Leuk. 2021, 21, S7–S8. [Google Scholar] [CrossRef]
- Farswan, A.; Jena, L.; Kaur, G.; Gupta, A.; Gupta, R.; Rani, L.; Sharma, A.; Kumar, L. Branching Clonal Evolution Patterns Predominate Mutational Landscape in Multiple Myeloma. Am. J. Cancer Res. 2021, 11, 5659–5679. [Google Scholar] [PubMed]
- Melchor, L.; Brioli, A.; Wardell, C.P.; Murison, A.; Potter, N.E.; Kaiser, M.F.; Fryer, R.A.; Johnson, D.C.; Begum, D.B.; Hulkki Wilson, S.; et al. Single-Cell Genetic Analysis Reveals the Composition of Initiating Clones and Phylogenetic Patterns of Branching and Parallel Evolution in Myeloma. Leukemia 2014, 28, 1705–1715. [Google Scholar] [CrossRef]
- Suzuki, K.; Nishiwaki, K.; Yano, S. Treatment Strategies Considering Micro-Environment and Clonal Evolution in Multiple Myeloma. Cancers 2021, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Fink, J.L.; Grady, J.P.; Morgan, G.J.; Mullighan, C.G.; To, L.B.; Hewett, D.R.; Zannettino, A.C.W. Subclonal Evolution in Disease Progression from MGUS/SMM to Multiple Myeloma Is Characterised by Clonal Stability. Leukemia 2019, 33, 457–468. [Google Scholar] [CrossRef]
- Van Nieuwenhuijzen, N.; Spaan, I.; Raymakers, R.; Peperzak, V. From MGUS to Multiple Myeloma, a Paradigm for Clonal Evolution of Premalignant Cells. Cancer Res. 2018, 78, 2449–2456. [Google Scholar] [CrossRef]
- Bianchi, G.; Ghobrial, I. Biological and Clinical Implications of Clonal Heterogeneity and Clonal Evolution in Multiple Myeloma. CCTR 2014, 10, 70–79. [Google Scholar] [CrossRef]
- Corsale, A.M.; Di Simone, M.; Shekarkar Azgomi, M.; Speciale, M.; Gigliotta, E.; Garofano, F.; Aquilina, C.; Vullo, C.; Caccamo, N.; Dieli, F.; et al. Immune Cells, Cytokines, and Gut Microbiota Landscape Along Monoclonal Gammopathy of Undetermined Significance (MGUS) to Multiple Myeloma (MM) Evolution. Blood 2023, 142, 3292. [Google Scholar] [CrossRef]
- Manier, S.; Sacco, A.; Leleu, X.; Ghobrial, I.M.; Roccaro, A.M. Bone Marrow Microenvironment in Multiple Myeloma Progression. J. Biomed. Biotechnol. 2012, 2012, 157496. [Google Scholar] [CrossRef]
- Cenzano, I.; Cócera, M.; Larrayoz, M.; Campos-Dopazo, L.; Sanz, S.; Bantan, A.; Vilas-Zornoza, A.; San-Martin, P.; Aguirre-Ruiz, P.; Alignani, D.; et al. Stromal and Endothelial Transcriptional Changes during Progression from MGUS to Myeloma and after Treatment Response. bioRxiv 2024. [Google Scholar] [CrossRef]
- Capp, J.-P.; Bataille, R. The Ins and Outs of Endosteal Niche Disruption in the Bone Marrow: Relevance for Myeloma Oncogenesis. Biology 2023, 12, 990. [Google Scholar] [CrossRef]
- Lopes, R.; Caetano, J.; Ferreira, B.; Barahona, F.; Carneiro, E.A.; João, C. The Immune Microenvironment in Multiple Myeloma: Friend or Foe? Cancers 2021, 13, 625. [Google Scholar] [CrossRef]
- Rees, E.; Sodi, I.; Foster, K.; Ainley, L.; Lyon, E.; Galas-Filipowicz, D.; Rahman, J.; Allen, R.; Kimber, J.; Prabu, A.; et al. Single-Cell Profiling of Human Bone Marrow Reveals Multiple Myeloma Progression Is Accompanied by an Increase in CD56brightBone Marrow Resident NK Cells. bioRxiv 2025. [Google Scholar] [CrossRef]
- Janker, L.; Mayer, R.L.; Bileck, A.; Kreutz, D.; Mader, J.C.; Utpatel, K.; Heudobler, D.; Agis, H.; Gerner, C.; Slany, A. Metabolic, Anti-Apoptotic and Immune Evasion Strategies of Primary Human Myeloma Cells Indicate Adaptations to Hypoxia*. Mol. Cell. Proteom. 2019, 18, 936a–953. [Google Scholar] [CrossRef]
- Alagpulinsa, D.A.; Szalat, R.E.; Poznansky, M.C.; Shmookler Reis, R.J. Genomic Instability in Multiple Myeloma. Trends Cancer 2020, 6, 858–873. [Google Scholar] [CrossRef]
- Mahtouk, K.; Hose, D.; Rème, T.; De Vos, J.; Jourdan, M.; Moreaux, J.; Fiol, G.; Raab, M.; Jourdan, E.; Grau, V.; et al. Expression of EGF-Family Receptors and Amphiregulin in Multiple Myeloma. Amphiregulin Is a Growth Factor for Myeloma Cells. Oncogene 2005, 24, 3512–3524. [Google Scholar] [CrossRef] [PubMed]
- Saltarella, I.; Apollonio, B.; Lamanuzzi, A.; Desantis, V.; Mariggiò, M.A.; Desaphy, J.-F.; Vacca, A.; Frassanito, M.A. The Landscape of lncRNAs in Multiple Myeloma: Implications in the “Hallmarks of Cancer”, Clinical Perspectives and Therapeutic Opportunities. Cancers 2022, 14, 1963. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, M.; Chiu, M.; Accardi, F.; Vescovini, R.; Airoldi, I.; Storti, P.; Todoerti, K.; Agnelli, L.; Missale, G.; Andreoli, R.; et al. Dependence on Glutamine Uptake and Glutamine Addiction Characterize Myeloma Cells: A New Attractive Target. Blood 2016, 128, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, R.; Matulis, S.M.; Wei, C.; Nooka, A.K.; Von Hollen, H.E.; Lonial, S.; Boise, L.H.; Shanmugam, M. Targeting Glutamine Metabolism in Multiple Myeloma Enhances BIM Binding to BCL-2 Eliciting Synthetic Lethality to Venetoclax. Oncogene 2016, 35, 3955–3964. [Google Scholar] [CrossRef]
- Soncini, D.; Minetto, P.; Martinuzzi, C.; Becherini, P.; Fenu, V.; Guolo, F.; Todoerti, K.; Calice, G.; Contini, P.; Miglino, M.; et al. Amino Acid Depletion Triggered by l-Asparaginase Sensitizes MM Cells to Carfilzomib by Inducing Mitochondria ROS-Mediated Cell Death. Blood Adv. 2020, 4, 4312–4326. [Google Scholar] [CrossRef]
- Effenberger, M.; Bommert, K.S.; Kunz, V.; Kruk, J.; Leich, E.; Rudelius, M.; Bargou, R.; Bommert, K. Glutaminase Inhibition in Multiple Myeloma Induces Apoptosis via MYC Degradation. Oncotarget 2017, 8, 85858–85867. [Google Scholar] [CrossRef] [PubMed]
- Nadav, L.; Katz, B.-Z.; Baron, S.; Cohen, N.; Naparstek, E.; Geiger, B. The Generation and Regulation of Functional Diversity of Malignant Plasma Cells. Cancer Res. 2006, 66, 8608–8616. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, H.A.; Li, C.; Magrangeas, F.; Guerin, C.; Gouraud, W.; Harousseau, J.-L.; Attal, M.; Marit, G.; Mathiot, C.; Facon, T.; et al. In Myeloma, the Prognostic Impact of Hyperdiploidy Is Mainly Related to the Gain of Chromosome 5. Blood 2008, 112, 632. [Google Scholar] [CrossRef]
- Kaiser, M.F.; Boyle, E.M.; Walker, B.A.; Begum, D.B.; Proszek, P.; Johnson, D.C.; Pawlyn, C.; Jones, J.R.; Savola, S.; Owen, R.G.; et al. Molecular Subgroups of Hyperdiploidy and Their Prognostic Relevance—An Analysis of 1036 Myeloma Trial Patients. Blood 2015, 126, 2983. [Google Scholar] [CrossRef]
- Chng, W.J.; Kumar, S.; VanWier, S.; Ahmann, G.; Price-Troska, T.; Henderson, K.; Chung, T.-H.; Kim, S.; Mulligan, G.; Bryant, B.; et al. Molecular Dissection of Hyperdiploid Multiple Myeloma by Gene Expression Profiling. Cancer Res. 2007, 67, 2982–2989. [Google Scholar] [CrossRef]
- AlHashmi, H.; Al-Dayel, A.; Soliman, D.; Al-Sayegh, M.; Abduljalil, O.; Anezi, K.A.; Matrok, A.A.; Kaloyannidis, P.; Saber, A.A.; Kamel, M.M.; et al. Hyperdiploidy Is a Positive Prognostic Factor for Progression-Free Survival in Multiple Myeloma with High and Intermediate Risk Cytogenetics. Health Sci. J. 2018, 12, 590. [Google Scholar] [CrossRef]
- Pawlyn, C.; Melchor, L.; Murison, A.; Wardell, C.P.; Brioli, A.; Boyle, E.M.; Kaiser, M.F.; Walker, B.A.; Begum, D.B.; Dahir, N.B.; et al. Coexistent Hyperdiploidy Does Not Abrogate Poor Prognosis in Myeloma with Adverse Cytogenetics and May Precede IGH Translocations. Blood 2015, 125, 831–840. [Google Scholar] [CrossRef]
- Agnelli, L.; Fabris, S.; Bicciato, S.; Basso, D.; Baldini, L.; Morabito, F.; Verdelli, D.; Todoerti, K.; Lambertenghi-Deliliers, G.; Lombardi, L.; et al. Upregulation of Translational Machinery and Distinct Genetic Subgroups Characterise Hyperdiploidy in Multiple Myeloma. Br. J. Haematol. 2007, 136, 565–573. [Google Scholar] [CrossRef]
- Weinhold, N.; DeVos, J.; Hose, D.; Rossi, J.-F.; Axel, B.; Mahtouk, K.; Raab, M.S.; Rème, T.X.; Jauch, A.; Moreaux, J.; et al. Ribosomal Proteins Are Overexpressed in Hyperdiploid Multiple Myeloma. Blood 2007, 110, 2495. [Google Scholar] [CrossRef]
- Neri, A.; Todoerti, K.; Agnelli, L.; Fabris, S.; Bicciato, S.; Mosca, L.; Nobili, L.; Verdelli, D.; Ronchetti, D.; Intini, D.; et al. Identification of Specific Transcriptional Patterns Associated with Hyperdiploidy in Multiple Myeloma. Blood 2006, 108, 3412. [Google Scholar] [CrossRef]
- Rio-Machin, A.; Ferreira, B.I.; Henry, T.; Gómez-López, G.; Agirre, X.; Alvarez, S.; Rodriguez-Perales, S.; Prosper, F.; Calasanz, M.J.; Martínez, J.; et al. Downregulation of Specific miRNAs in Hyperdiploid Multiple Myeloma Mimics the Oncogenic Effect of IgH Translocations Occurring in the Non-Hyperdiploid Subtype. Leukemia 2013, 27, 925–931. [Google Scholar] [CrossRef]
- Samur, M.K.; Aktas Samur, A.; Shah, P.; Park, J.S.; Fulciniti, M.; Shammas, M.; Corre, J.; Anderson, K.C.; Parmigiani, G.; Avet-Loiseau, H.; et al. Development of Hyperdiploidy Starts at an Early Age and Takes a Decade to Complete. Blood 2025, 145, 520–525. [Google Scholar] [CrossRef]
- Miura, D.; Narita, K.; Kuzume, A.; Tabata, R.; Terao, T.; Tsushima, T.; Kobayashi, H.; Abe, Y.; Kitadate, A.; Takeuchi, M.; et al. Clinical and Prognostic Impact of (11;14)(Q13;Q32) Translocation on Patients with Multiple Myeloma. Blood 2019, 134, 5507. [Google Scholar] [CrossRef]
- Chakraborty, R.; Lentzsch, S. Prognostic Impact of t(11;14) in Multiple Myeloma: Black and White or Shades of Gray? Cancer 2021, 127, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Sloan, S.; Li, D.; Zhuang, L.; Yi, Q.; Chen, C.I.; Reece, D.; Chun, K.; Keith Stewart, A. The t(4;14) Is Associated with Poor Prognosis in Myeloma Patients Undergoing Autologous Stem Cell Transplant. Br. J. Haematol. 2004, 125, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kamata, W.; Okada, S.; Tamai, Y. Clinical and Prognostic Significance of t(4;14) Translocation in Multiple Myeloma in the Era of Novel Agents. Int. J. Hematol. 2021, 113, 207–213. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Murison, A.; Boyle, E.M.; Melchor, L.; Pawlyn, C.; Kaiser, M.F.; Begum, D.; Dahir, N.; Proszek, P.; et al. Apobec Family Mutational Signatures Are Associated with Poor Prognosis Translocations in Multiple Myeloma. Blood 2014, 124, 723. [Google Scholar] [CrossRef]
- Marzocchi, G.; Ameli, G.; Pezzi, A.; Zamagni, E.; Cavallo, F.; Petrucci, M.T.; Waage, A.; Di Raimondo, F.; Patriarca, F.; Rambaldi, A.; et al. Rare Igh Translocations in Newly Diagnosed Multiple Myeloma (MM) Patients: Cytogenetic Characterization and Relevance on Prognosis. Blood 2014, 124, 2042. [Google Scholar] [CrossRef]
- Mao, X.; Zhuang, J.; Zhao, D.; Li, X.; Du, X.; Hao, M.; Xu, Y.; Yan, Y.; Liu, J.; Fan, H.; et al. IgH Translocation with Undefined Partners Is Associated with Superior Outcome in Multiple Myeloma Patients. Eur. J. Haematol. 2020, 105, 326–334. [Google Scholar] [CrossRef]
- Fonseca, R.; Oken, M.; Harrington, D.; Bailey, R.; Van Wier, S.; Henderson, K.; Kay, N.; Van Ness, B.; Greipp, P.; Dewald, G. Deletions of Chromosome 13 in Multiple Myeloma Identified by Interphase FISH Usually Denote Large Deletions of the q Arm or Monosomy. Leukemia 2001, 15, 981–986. [Google Scholar] [CrossRef]
- Kaufmann, H.; Krömer, E.; Nösslinger, T.; Weltermann, A.; Ackermann, J.; Reisner, R.; Bernhart, M.; Drach, J. Both Chromosome 13 Abnormalities by Metaphase Cytogenetics and Deletion of 13q by Interphase FISH Only Are Prognostically Relevant in Multiple Myeloma. Eur. J. Haematol. 2003, 71, 179–183. [Google Scholar] [CrossRef]
- Fassas, A.B.-T.; Spencer, T.; Sawyer, J.; Zangari, M.; Lee, C.; Anaissie, E.; Muwalla, F.; Morris, C.; Barlogie, B.; Tricot, G. Both Hypodiploidy and Deletion of Chromosome 13 Independently Confer Poor Prognosis in Multiple Myeloma. Br. J. Haematol. 2002, 118, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; An, G.; Li, C.; Xu, Y.; Deng, S.; Liu, X.; Qi, J.; Wang, Y.; Zou, D.; Zhao, Y.; et al. The significances of 13q14 deletion for development and prognosis of multiple myeloma. Zhonghua Xue Ye Xue Za Zhi 2011, 32, 217–220. [Google Scholar] [PubMed]
- Avet-Loiseau, H.; Leleu, X.; Roussel, M.; Mathiot, C.; Caillot, D.; Hulin, C.; Marit, G.; Facon, T.; Attal, M.; Harousseau, J.-L.; et al. Deletion of the 17p Chromosomal Region Is Associated with a Very Poor Outcome in Multiple Myeloma Independently of the Type of Treatment. Blood 2009, 114, 1817. [Google Scholar] [CrossRef]
- Lode, L.; Eveillard, M.; Trichet, V.; Soussi, T.; Wuilleme, S.; Richebourg, S.; Magrangeas, F.; Ifrah, N.; Campion, L.; Traulle, C.; et al. Mutations in TP53 Are Exclusively Associated with Del(17p) in Multiple Myeloma. Haematologica 2010, 95, 1973–1976. [Google Scholar] [CrossRef]
- Han, E.; Kwon, A.; Kim, Y.; Han, K.; Park, S.; Min, C.; Kim, M. Prevalence of Ras/Mapk Pathway Mutation (KRAS, NRAS, and BRAF) in Plasma Cell Myeloma at a Single Institute in Korea. Cytotherapy 2019, 21, S25–S26. [Google Scholar] [CrossRef]
- Janku, F.; Li, Q.; Huang, H.J.; Wang, Y.; Cao, Z.; Karlin-Neumann, G.; Liu, Z. Abstract 573: BRAF, KRAS, and NRAS Mutations in Archival Tumor Samples and Samples of Cell-Free DNA from Serum and Bone Marrow Aspirates from Patients with Multiple Myeloma. Cancer Res. 2018, 78, 573. [Google Scholar] [CrossRef]
- Mulligan, G.; Lichter, D.I.; Di Bacco, A.; Blakemore, S.J.; Berger, A.; Koenig, E.; Bernard, H.; Trepicchio, W.; Li, B.; Neuwirth, R.; et al. Mutation of NRAS but Not KRAS Significantly Reduces Myeloma Sensitivity to Single-Agent Bortezomib Therapy. Blood 2014, 123, 632–639. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.-S.; Min, C.-K.; Lee, G.D.; Son, J.; Jo, S.J.; Han, E.; Han, K.; Kim, M. KRAS, NRAS, and BRAF Mutations in Plasma Cell Myeloma at a Single Korean Institute. Blood Res. 2020, 55, 159–168. [Google Scholar] [CrossRef]
- Shirazi, F.; Jones, R.J.; Singh, R.K.; Zou, J.; Kuiatse, I.; Berkova, Z.; Wang, H.; Lee, H.C.; Hong, S.; Dick, L.; et al. Activating KRAS, NRAS, and BRAF Mutants Enhance Proteasome Capacity and Reduce Endoplasmic Reticulum Stress in Multiple Myeloma. Proc. Natl. Acad. Sci. USA 2020, 117, 20004–20014. [Google Scholar] [CrossRef]
- Lionetti, M.; Barbieri, M.; Todoerti, K.; Agnelli, L.; Marzorati, S.; Fabris, S.; Ciceri, G.; Galletti, S.; Milesi, G.; Manzoni, M.; et al. Molecular Spectrum of BRAF, NRAS and KRAS Gene Mutations in Plasma Cell Dyscrasias: Implication for MEK-ERK Pathway Activation. Oncotarget 2015, 6, 24205–24217. [Google Scholar] [CrossRef]
- Xu, J.; Pfarr, N.; Endris, V.; Mai, E.K.; Md Hanafiah, N.H.; Lehners, N.; Penzel, R.; Weichert, W.; Ho, A.D.; Schirmacher, P.; et al. Molecular Signaling in Multiple Myeloma: Association of RAS/RAF Mutations and MEK/ERK Pathway Activation. Oncogenesis 2017, 6, e337. [Google Scholar] [CrossRef] [PubMed]
- Mey, U.J.M.; Renner, C.; von Moos, R. Vemurafenib in Combination with Cobimetinib in Relapsed and Refractory Extramedullary Multiple Myeloma Harboring the BRAF V600E Mutation. Hematol. Oncol. 2017, 35, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Hofste Op Bruinink, D.; Hoogenboezem, R.; Bindels, E.; Sanders, M.; Erpelinck—Verschueren, C.; Van Strien, P.; Koenders, J.; Misund, K.; Beverloo, B.; Van Der Holt, B.; et al. Abstract 5023: Multiple Myeloma with a Clonal Del17p Aberration Is Characterized by Somatic TP53 Mutations, Which Negatively Affect Prognosis in This Cytogenetic Subgroup. Cancer Res. 2016, 76, 5023. [Google Scholar] [CrossRef]
- Xiong, W.; Wu, X.; Starnes, S.; Johnson, S.K.; Haessler, J.; Wang, S.; Chen, L.; Barlogie, B.; Shaughnessy, J.D.; Zhan, F. An Analysis of the Clinical and Biologic Significance of TP53 Loss and the Identification of Potential Novel Transcriptional Targets of TP53 in Multiple Myeloma. Blood 2008, 112, 4235–4246. [Google Scholar] [CrossRef]
- Petrilla, C.; Galloway, J.; Kudalkar, R.; Ismael, A.; Cottini, F. Understanding DNA Damage Response and DNA Repair in Multiple Myeloma. Cancers 2023, 15, 4155. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Hocking, J.; Ramachandran, M.; Choi, K.; Klarica, D.; Khong, T.; Reynolds, J.; Spencer, A. DNA-Repair Gene Mutations Are Highly Prevalent in Circulating Tumour DNA from Multiple Myeloma Patients. Cancers 2019, 11, 917. [Google Scholar] [CrossRef]
- Shyamsunder, P.; Sridharan, S.P.; Madan, V.; Dakle, P.; Zeya, C.; Kanojia, D.; Chng, W.-J.; Ong, S.T.; Koeffler, H.P. THZ531 Induces a State of BRCAness in Multiple Myeloma Cells: Synthetic Lethality with Combination Treatment of THZ 531 with DNA Repair Inhibitors. IJMS 2022, 23, 1207. [Google Scholar] [CrossRef]
- Rahmat, M.; Clement, K.; Sklavenitis-Pistofidis, R.; Kodgule, R.; Fulco, C.; Alberge, J.-B.; Boehner, C.J.; Agius, M.P.; Kitzenberg, E.M.; Dorfman, D.; et al. Identification of a Novel Epigenetic Mechanism of MYC Deregulation in Smoldering and Newly Diagnosed Multiple Myeloma Patients. Blood 2021, 138, 504. [Google Scholar] [CrossRef]
- Mikulasova, A.; Ashby, C.; Tytarenko, R.G.; Qu, P.; Rosenthal, A.; Dent, J.A.; Ryan, K.R.; Bauer, M.A.; Wardell, C.P.; Hoering, A.; et al. Microhomology-Mediated End Joining Drives Complex Rearrangements and Overexpression of MYC and PVT1 in Multiple Myeloma. Haematologica 2020, 105, 1055–1066. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Begum, D.; Dahir, N.; Johnson, D.C.; Davies, F.E.; Morgan, G.J. MYC Translocations In Multiple Myeloma Involve Recruitment Of Enhancer Elements Resulting In Over-Expression and Decreased Overall Survival. Blood 2013, 122, 274. [Google Scholar] [CrossRef]
- Rahmat, M.; Clement, K.; Alberge, J.-B.; Sklavenitis-Pistofidis, R.; Kodgule, R.; Fulco, C.P.; Heilpern-Mallory, D.; Nilsson, K.; Dorfman, D.; Engreitz, J.M.; et al. Novel Mechanism of MYCD eregulation in Multiple Myeloma. bioRxiv 2023. [Google Scholar] [CrossRef]
- Handa, H.; Honma, K.; Oda, T.; Kobayashi, N.; Kuroda, Y.; Kimura-Masuda, K.; Watanabe, S.; Ishihara, R.; Murakami, Y.; Masuda, Y.; et al. Long Noncoding RNA PVT1 Is Regulated by Bromodomain Protein BRD4 in Multiple Myeloma and Is Associated with Disease Progression. IJMS 2020, 21, 7121. [Google Scholar] [CrossRef]
- Anguiano, A.; Acharya, C.; Salter, K.; McCluskey, D.; Gasperetto, C.; Zhan, F.; Dhodapkar, M.; Nevins, J.; Barlogie, B.; Shaughnessy, J.D.; et al. Gene Expression Profiles for Prognosis in MGUS, Coupled with Signatures of Oncogenic Pathway Deregulation Provide a Novel Approach for Selection of Molecular Targets in Multiple Myeloma. Blood 2007, 110, 655. [Google Scholar] [CrossRef]
- Alderton, G.K. Targeting MYC? You BET. Nat. Rev. Drug Discov. 2011, 10, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Rustad, E.H.; Yellapantula, V.; Leongamornlert, D.; Bolli, N.; Ledergor, G.; Nadeu, F.; Angelopoulos, N.; Dawson, K.J.; Mitchell, T.J.; Osborne, R.J.; et al. Timing the Initiation of Multiple Myeloma. Nat. Commun. 2020, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Bolli, N.; Minvielle, S.; Gloznik, D.; Szalat, R.; Fullam, A.; Martincorena, I.; Samur, M.K.; Tarpey, P.; Davies, H.; et al. Analysis of Mutational Signatures Suggest That Aid Has an Early and Driver Role in Multiple Myeloma. Blood 2016, 128, 116. [Google Scholar] [CrossRef]
- Farswan, A.; Gupta, A.; Jena, L.; Ruhela, V.; Kaur, G.; Gupta, R. Characterizing the Mutational Landscape of MM and Its Precursor MGUS. Am. J. Cancer Res. 2022, 12, 1919–1933. [Google Scholar]
- Aktas Samur, A.; Fulciniti, M.; Szalat, R.; Corre, J.; Bolli, N.; Moreau, P.; Attal, M.; Anderson, K.C.; Parmigiani, G.; Avet-Loiseau, H.; et al. Lack of Significant Differences in Somatic Alterations between MGUS, SMM and Symptomatic Multiple Myeloma: A Result from Comprehensive Genomic Profiling Study. Blood 2019, 134, 3089. [Google Scholar] [CrossRef]
- Ohguchi, H.; Hideshima, T.; Anderson, K.C. The Biological Significance of Histone Modifiers in Multiple Myeloma: Clinical Applications. Blood Cancer J. 2018, 8, 83. [Google Scholar] [CrossRef]
- Walker, B.A.; Leone, P.E.; Dickens, N.J.; Boyd, K.D.; Gonzalez, D.; Davies, F.E.; Morgan, G.J. UTX, a Histone Demethylase, Is Inactivated through Homozygous Deletion, Mutation, and DNA Methylation in Multiple Myeloma. Blood 2009, 114, 1798. [Google Scholar] [CrossRef]
- Deligezer, U.; Akisik, E.E.; Erten, N.; Dalay, N. Sequence-Specific Histone Methylation Is Detectable on Circulating Nucleosomes in Plasma. Clin. Chem. 2008, 54, 1125–1131. [Google Scholar] [CrossRef]
- Muers, M. Mapping Histone Modifications and DNA Methylation Together. Nat. Rev. Genet. 2012, 13, 299. [Google Scholar] [CrossRef]
- Fuks, F. DNA Methylation and Histone Modifications: Teaming up to Silence Genes. Curr. Opin. Genet. Dev. 2005, 15, 490–495. [Google Scholar] [CrossRef]
- Pawlyn, C.; Bright, M.D.; Buros, A.F.; Stein, C.K.; Walters, Z.; Aronson, L.I.; Mirabella, F.; Jones, J.R.; Kaiser, M.F.; Walker, B.A.; et al. Overexpression of EZH2 in Multiple Myeloma Is Associated with Poor Prognosis and Dysregulation of Cell Cycle Control. Blood Cancer J. 2017, 7, e549. [Google Scholar] [CrossRef] [PubMed]
- Harding, T.; Swanson, J.; Van Ness, B. EZH2 Inhibitors Sensitize Myeloma Cell Lines to Panobinostat Resulting in Unique Combinatorial Transcriptomic Changes. Oncotarget 2018, 9, 21930–21942. [Google Scholar] [CrossRef]
- Adamik, J.; Jin, S.; Sun, Q.; Zhang, P.; Weiss, K.R.; Anderson, J.L.; Silbermann, R.; Roodman, G.D.; Galson, D.L. EZH2 or HDAC1 Inhibition Reverses Multiple Myeloma–Induced Epigenetic Suppression of Osteoblast Differentiation. Mol. Cancer Res. 2017, 15, 405–417. [Google Scholar] [CrossRef]
- Carew, J.S.; Espitia, C.M.; Zhao, W.; Visconte, V.; Anwer, F.; Kelly, K.R.; Nawrocki, S.T. Rational Cotargeting of HDAC6 and BET Proteins Yields Synergistic Antimyeloma Activity. Blood Adv. 2019, 3, 1318–1329. [Google Scholar] [CrossRef]
- Alzrigat, M.; Párraga, A.A.; Majumder, M.M.; Ma, A.; Jin, J.; Österborg, A.; Nahi, H.; Nilsson, K.; Heckman, C.A.; Öberg, F.; et al. The Polycomb Group Protein BMI-1 Inhibitor PTC-209 Is a Potent Anti-Myeloma Agent Alone or in Combination with Epigenetic Inhibitors Targeting EZH2 and the BET Bromodomains. Oncotarget 2017, 8, 103731–103743. [Google Scholar] [CrossRef]
- Puła, A.; Robak, P.; Robak, T. MicroRNA in Multiple Myeloma—A Role in Pathogenesis and Prognostic Significance. CMC 2021, 28, 6753–6772. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, D.; Luo, Y.; Sun, Y.; Duan, C.; Yang, J.; Wei, J.; Li, X.; Lu, Y.; Lai, X. miR-34a Promotes the Immunosuppressive Function of Multiple Myeloma-Associated Macrophages by Dampening the TLR-9 Signaling. Cancer Med. 2024, 13, e7387. [Google Scholar] [CrossRef]
- Szudy-Szczyrek, A.; Ahern, S.; Krawczyk, J.; Szczyrek, M.; Hus, M. MiRNA as a Potential Target for Multiple Myeloma Therapy–Current Knowledge and Perspectives. J. Pers. Med. 2022, 12, 1428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.; Wang, H.; Leng, Y.; Li, Z.-L.; Yang, Y.-F.; Xiao, F.-J.; Li, Q.-F.; Chen, X.-Q.; Wang, L.-S. Overexpression of microRNA-29b Induces Apoptosis of Multiple Myeloma Cells through down Regulating Mcl-1. Biochem. Biophys. Res. Commun. 2011, 414, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Long, S.; He, H.; Chen, G. microRNA-765 Is Pregulated in Multiple Myeloma and Serves an Oncogenic Role by Directly Targeting SOX6. Exp. Ther. Med. 2019, 17, 4741–4747. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kuroda, Y.; Masuda, Y.; Yamane, A.; Hattori, H.; Tahara, K.; Kaneko, A.; Suda, I.; Takahashi, N.; Gotoh, N.; et al. Loop Regulation Between microRNAs and Epigenetics Underlie microRNA Dysregulation in Multiple Myeloma and Is Associated with the Disease Progression. Blood 2015, 126, 3013. [Google Scholar] [CrossRef]
- Gregorova, J.; Vlachova, M.; Vychytilova-Faltejskova, P.; Dostalova, A.; Ruzickova, T.; Vecera, M.; Radova, L.; Pospichalova, V.; Sladecek, S.; Hyzdalova, M.; et al. MicroRNA Profiling of Bone Marrow Plasma Extracellular Vesicles in Multiple Myeloma, Extramedullary Disease, and Plasma Cell Leukemia. Hematol. Oncol. 2025, 43, e70036. [Google Scholar] [CrossRef]
- Rossi, M.; Tagliaferri, P.; Tassone, P. MicroRNAs in Multiple Myeloma and Related Bone Disease. Ann. Transl. Med. 2015, 3, 334. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Leone, E.; Amodio, N.; Foresta, U.; Lionetti, M.; Pitari, M.R.; Gallo Cantafio, M.E.; Gullà, A.; Conforti, F.; Morelli, E.; et al. Synthetic miR-34a Mimics as a Novel Therapeutic Agent for Multiple Myeloma: In Vitro and in Vivo Evidence. Clin. Cancer Res. 2012, 18, 6260–6270. [Google Scholar] [CrossRef]
- Zarone, M.R.; Misso, G.; Grimaldi, A.; Zappavigna, S.; Russo, M.; Amler, E.; Di Martino, M.T.; Amodio, N.; Tagliaferri, P.; Tassone, P.; et al. Evidence of Novel miR-34a-Based Therapeutic Approaches for Multiple Myeloma Treatment. Sci. Rep. 2017, 7, 17949. [Google Scholar] [CrossRef]
- Kastritis, E.; Malandrakis, P.; Solia, I.; Ntanasis-Stathopoulos, I.; Theodorakakou, F.; Spiliopoulou, V.; Fotiou, D.; Migkou, M.; Kanellias, N.; Roussou, M.; et al. Risk and Patterns of Progression Among Smoldering Myeloma Patients after the Implementation of New Criteria and Modern Imaging. Blood 2024, 144, 4675. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Evolving Diagnostic Criteria for Multiple Myeloma. Hematology 2015, 2015, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wang, M.; Shih, Y.-H.; Huber, J.; Fiala, M.A.; Wang, R.; Sanfilippo, K.M.; Thomas, T.S.; Wang, S.-Y.; Schoen, M.W.; et al. From Criteria to Clinic: How Updated SLiM CRAB Criteria Influence Multiple Myeloma Diagnostic Activity. JCO 2024, 42, 7556. [Google Scholar] [CrossRef]
- Ludwig, H.; Kainz, S.; Schreder, M.; Zojer, N.; Hinke, A. SLiM CRAB Criteria Revisited: Temporal Trends in Prognosis of Patients with Smoldering Multiple Myeloma Who Meet the Definition of ‘Biomarker-Defined Early Multiple Myeloma’—A Systematic Review with Meta-Analysis. EClinicalMedicine 2023, 58, 101910. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Uno, H.; Jacobus, S.J.; Wier, S.A.V.; Ahmann, G.J.; Henderson, K.J.; Callander, N.S.; Haug, J.L.; Siegel, D.S.; Greipp, P.R.; et al. Impact of Gene Expression Profiling-Based Risk Stratification in Patients with Myeloma Receiving Initial Therapy with Lenalidomide and Dexamethasone. Blood 2011, 118, 4359–4362. [Google Scholar] [CrossRef]
- Fonseca, R.; Braggio, E.; Keats, J.J.; Ahmann, G.J.; Mantei, J.; Stewart, A.K.; Bergsagel, P.L. Generation of an Automated Tool for Querying Myeloma Transcriptomics for Multiple Gene Expression Signatures Used in Risk-Stratification. JCO 2011, 29, 8024. [Google Scholar] [CrossRef]
- Meißner, T.; Seckinger, A.; Rème, T.; Hielscher, T.; Möhler, T.; Neben, K.; Goldschmidt, H.; Klein, B.; Hose, D. Metascoring and Gene Expression Profiling in Clinical Routine in Multiple Myeloma. Blood 2011, 118, 3940. [Google Scholar] [CrossRef]
- Heuck, C.J.; Johnson, S.K.; Zhang, Q.; Shaughnessy, J.D. Gene Expression Signature in MGUS and Multiple Myeloma. In Genetic and Molecular Epidemiology of Multiple Myeloma; Lentzsch, S., Ed.; Springer: New York, NY, USA, 2013; pp. 17–41. ISBN 978-1-4614-4660-6. [Google Scholar]
- Cerchione, C.; Usmani, S.Z.; Stewart, A.K.; Kaiser, M.; Rasche, L.; Kortüm, M.; Mateos, M.-V.; Spencer, A.; Sonneveld, P.; Anderson, K.C. Gene Expression Profiling in Multiple Myeloma: Redefining the Paradigm of Risk-Adapted Treatment. Front. Oncol. 2022, 12, 820768. [Google Scholar] [CrossRef]
- D’Agostino, M.; Cairns, D.A.; Lahuerta, J.J.; Wester, R.; Bertsch, U.; Waage, A.; Zamagni, E.; Mateos, M.-V.; Dall’Olio, D.; van de Donk, N.W.C.J.; et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. JCO 2022, 40, 3406–3418. [Google Scholar] [CrossRef]
- D’Agostino, M.; Zaccaria, G.M.; Ziccheddu, B.; Rustad, E.H.; Genuardi, E.; Capra, A.; Oliva, S.; Auclair, D.; Yesil, J.; Colucci, P.; et al. Early Relapse Risk in Patients with Newly Diagnosed Multiple Myeloma Characterized by Next-Generation Sequencing. Clin. Cancer Res. 2020, 26, 4832–4841. [Google Scholar] [CrossRef]
- Fiala, M.A.; Dukeman, J.; Stockerl-Goldstein, K.; Tomasson, M.H.; Wildes, T.M.; Auclair, D.; Vij, R. Next Generation Sequencing Based Revised International Staging System (R-ISS) for Multiple Myeloma. Blood 2016, 128, 2349. [Google Scholar] [CrossRef]
- Szalat, R.; Munshi, N.C. Next-Generation Sequencing Informing Therapeutic Decisions and Personalized Approaches. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e442–e448. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, L.; Mashudi, F.H.; Ningtyas, M.C.; Sutanto, H.; Romadhon, P.Z. Genetic Profiling of Acute and Chronic Leukemia via Next-Generation Sequencing: Current Insights and Future Perspectives. Hematol. Rep. 2025, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Pratiwi, L.; Romadhon, P.Z.; Bintoro, S.U.Y. Advancing Chronic Myeloid Leukemia Research with Next-Generation Sequencing: Potential Benefits, Limitations, and Future Clinical Integration. Hum. Genet. 2025, 144, 481–503. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.; Perumal, D.; Melnekoff, D.; Readhead, B.; Kidd, B.; Leshchenko, V.V.; Kuo, P.-Y.; Keats, J.J.; Derome, M.; Yesil, J.; et al. Integrative Network Analysis of Newly Diagnosed Multiple Myeloma Identifies a Novel RNA-Seq Based High Riskgene Signature. Blood 2016, 128, 3285. [Google Scholar] [CrossRef]
- Bustoros, M.; Park, J.; Salem, K.Z.; Liu, C.-J.; Capelletti, M.; Huynh, D.; Tai, Y.-T.; Mouhieddine, T.H.; Freeman, S.; Ha, G.; et al. Next Generation Sequencing Identifies Smoldering Multiple Myeloma Patients with a High Risk of Disease Progression. Blood 2017, 130, 392. [Google Scholar]
- Gozzetti, A.; Bocchia, M. Minimal Residual Disease in Multiple Myeloma: An Important Tool inClinical Trials. RRCT 2022, 17, 9–10. [Google Scholar] [CrossRef]
- Ferla, V.; Antonini, E.; Perini, T.; Farina, F.; Masottini, S.; Malato, S.; Marktel, S.; Lupo Stanghellini, M.T.; Tresoldi, C.; Ciceri, F.; et al. Minimal Residual Disease Detection by Next-Generation Sequencing in Multiple Myeloma: Promise and Challenges for Response-Adapted Therapy. Front. Oncol. 2022, 12, 932852. [Google Scholar] [CrossRef]
- Mina, R.; Bonello, F.; Oliva, S. Minimal Residual Disease in Multiple Myeloma: Ready for Prime Time? Cancer J. 2021, 27, 247–255. [Google Scholar] [CrossRef]
- Aljama, M.A.; Sidiqi, H.M.; Gertz, M.A. Are We Maintaining Minimal Residual Disease in Myeloma? Leuk. Lymphoma 2025, 66, 1001–1009. [Google Scholar] [CrossRef]
- Yao, Q.; Bai, Y.; Orfao, A.; Kumar, S.; Chim, C.S. Upgraded Standardized Minimal Residual Disease Detection by Next-Generation Sequencing in Multiple Myeloma. J. Mol. Diagn. 2020, 22, 679–684. [Google Scholar] [CrossRef]
- Mina, R.; Oliva, S.; Boccadoro, M. Minimal Residual Disease in Multiple Myeloma: State of the Art and Future Perspectives. JCM 2020, 9, 2142. [Google Scholar] [CrossRef]
- Genovesi, L.; Schirm, M.; Dupuis, N.; Pottiez, G. Measuring Minimal Residual Disease in Plasma of Multiple Myeloma Patients By Intact Mass Spectrometry. Blood 2023, 142, 6652. [Google Scholar] [CrossRef]
- Medina-Herrera, A.; Sarasquete, M.E.; Jiménez, C.; Puig, N.; García-Sanz, R. Minimal Residual Disease in Multiple Myeloma: Past, Present, and Future. Cancers 2023, 15, 3687. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Dimopoulos, M.A.; Moulopoulos, L.A. The Role of Imaging in the Treatment of Patients with Multiple Myeloma in 2016. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e407–e417. [Google Scholar] [CrossRef] [PubMed]
- Withofs, N.; Nanni, C.; Simoni, P.; Fanti, S.; Beguin, Y.; Caers, J. Imaging Myeloma and Related Monoclonal Plasma Cell Disorders Using MRI, Low-Dose Whole-Body CT and FDG PET/CT. Clin. Transl. Imaging 2015, 3, 95–109. [Google Scholar] [CrossRef]
- Rama, S.; Suh, C.H.; Kim, K.W.; Durieux, J.C.; Ramaiya, N.H.; Tirumani, S.H. Comparative Performance of Whole-Body MRI and FDG PET/CT in Evaluation of Multiple Myeloma Treatment Response: Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2022, 218, 602–613. [Google Scholar] [CrossRef]
- Hillengass, J.; Moulopoulos, L.A.; Delorme, S.; Koutoulidis, V.; Mosebach, J.; Hielscher, T.; Drake, M.; Rajkumar, S.V.; Oestergaard, B.; Abildgaard, N.; et al. Whole-Body Computed Tomography versus Conventional Skeletal Survey in Patients with Multiple Myeloma: A Study of the International Myeloma Working Group. Blood Cancer J. 2017, 7, e599. [Google Scholar] [CrossRef]
- Pianko, M.J.; Terpos, E.; Roodman, G.D.; Divgi, C.R.; Zweegman, S.; Hillengass, J.; Lentzsch, S. Whole-Body Low-Dose Computed Tomography and Advanced Imaging Techniques for Multiple Myeloma Bone Disease. Clin. Cancer Res. 2014, 20, 5888–5897. [Google Scholar] [CrossRef]
- Prieto, E.; García-Velloso, M.J.; Aquerreta, J.D.; Rosales, J.J.; Bastidas, J.F.; Soriano, I.; Irazola, L.; Rodríguez-Otero, P.; Quincoces, G.; Martí-Climent, J.M. Ultra-Low Dose Whole-Body CT for Attenuation Correction in a Dual Tracer PET/CT Protocol for Multiple Myeloma. Phys. Medica 2021, 84, 1–9. [Google Scholar] [CrossRef]
- Wu, F.; Bernard, S.; Fayad, L.M.; Ilaslan, H.; Messiou, C.; Moulopoulos, L.A.; Mulligan, M.E. Updates and Ongoing Challenges in Imaging of Multiple Myeloma: AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2021, 217, 775–785. [Google Scholar] [CrossRef]
- Mosebach, J.; Thierjung, H.; Schlemmer, H.-P.; Delorme, S. Multiple Myeloma Guidelines and Their Recent Updates: Implications for Imaging. Fortschr. Röntgenstr. 2019, 191, 998–1009. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with Lenalidomide and Dexamethasone versus Lenalidomide and Dexamethasone Alone in Patients with Newly Diagnosed Myeloma without Intent for Immediate Autologous Stem-Cell Transplant (SWOG S0777): A Randomised, Open-Label, Phase 3 Trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Facon, T.; Hungria, V.; Bahlis, N.J.; Venner, C.P.; Braunstein, M.; Pour, L.; Martí, J.M.; Basu, S.; Cohen, Y.C.; et al. Daratumumab plus Bortezomib, Lenalidomide and Dexamethasone for Transplant-Ineligible or Transplant-Deferred Newly Diagnosed Multiple Myeloma: The Randomized Phase 3 CEPHEUS Trial. Nat. Med. 2025, 31, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; Gautam, S.; Lafeuille, M.; Rossi, C.; Moore, B.; Tardif-Samson, A.; Thompson-Leduc, P.; Fu, A.Z.; Cortoos, A.; Kaila, S.; et al. Comparison of Time to Next Treatment or Death Between Front-Line Daratumumab, Lenalidomide, and Dexamethasone (DRd) Versus Bortezomib, Lenalidomide, and Dexamethasone (VRd) Among Transplant-Ineligible Patients with Multiple Myeloma. Cancer Med. 2024, 13, e70308. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Park, S.-S.; Yoon, S.-S.; Ahn, A.; Kim, M.; Lee, J.Y.; Jeon, Y.-W.; Shin, S.-H.; Yahng, S.-A.; Koh, Y.; et al. Advantage of Achieving Deep Response Following Frontline Daratumumab-VTd Compared to VRd in Transplant-Eligible Multiple Myeloma: Multicenter Study. Blood Res. 2023, 58, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Bove, V.; Villano, F.; Riva, E. Survival Analysis of Newly Diagnosed Multiple Myeloma Patients after Frontline Autologous Stem Cell Transplantation in a Real-Life Setting. Acta Med. 2023, 66, 117–121. [Google Scholar] [CrossRef]
- Pasvolsky, O.; Wang, Z.; Milton, D.R.; Tanner, M.R.; Bashir, Q.; Srour, S.; Saini, N.; Lin, P.; Ramdial, J.; Nieto, Y.; et al. Multiple Myeloma Patients with a Long Remission after Autologous Hematopoietic Stem Cell Transplantation. Blood Cancer J. 2024, 14, 82. [Google Scholar] [CrossRef]
- Pawlyn, C.; Cairns, D.; Menzies, T.; Jones, J.; Jenner, M.; Cook, G.; Boyd, K.; Drayson, M.; Kaiser, M.; Owen, R.; et al. Autologous Stem Cell Transplantation Is Safe and Effective for Fit, Older Myeloma Patients: Exploratory Results from the Myeloma XI Trial. Haematologica 2020, 107, 231–242. [Google Scholar] [CrossRef]
- Rosenberg, A.S.; Brunson, A.; Jonas, B.A.; Keegan, T.H.M.; Wun, T. Association Between Autologous Stem Cell Transplant and Survival Among Californians with Multiple Myeloma. JNCI J. Natl. Cancer Inst. 2019, 111, 78–85. [Google Scholar] [CrossRef]
- Ricciuti, G.; Falcone, A.; Cascavilla, N.; Martinelli, G.; Cerchione, C. Autologous Stem Cell Transplantation in Multiple Myeloma. Panminerva Med. 2021, 62, 220–224. [Google Scholar] [CrossRef]
- Attal, M.; cances Lauwers, V.; Marit, G.; Caillot, D.; Facon, T.; Hulin, C.; Moreau, P.; Mathiot, C.; Roussel, M.; Payen, C.; et al. Maintenance Treatment with Lenalidomide After Transplantation for MYELOMA: Final Analysis of the IFM 2005-02. Blood 2010, 116, 310. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Jung, S.-H.; Richardson, P.G.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Updated Analysis of CALGB 100104 (Alliance): A Randomised Phase III Study Evaluating Lenalidomide vs. Placebo Maintenance after Single Autologous Stem Cell Transplant for Multiple Myeloma. Lancet Haematol. 2017, 4, e431–e442. [Google Scholar] [CrossRef]
- Syed, Y.Y. Lenalidomide: A Review in Newly Diagnosed Multiple Myeloma as Maintenance Therapy After ASCT. Drugs 2017, 77, 1473–1480. [Google Scholar] [CrossRef]
- Rifkin, R.M.; Jagannath, S.; Durie, B.G.M.; Narang, M.; Terebelo, H.R.; Gasparetto, C.J.; Toomey, K.; Hardin, J.W.; Wagner, L.; Parikh, K.; et al. Treatment Outcomes and Health Care Resource Utilization in Patients with Newly Diagnosed Multiple Myeloma Receiving Lenalidomide-Only Maintenance, Any Maintenance, or No Maintenance: Results from the Connect MM Registry. Clin. Ther. 2018, 40, 1193–1202.e1. [Google Scholar] [CrossRef]
- Alonso, R.; Cedena, M.-T.; Wong, S.; Shah, N.; Ríos-Tamayo, R.; Moraleda, J.M.; López-Jiménez, J.; García, C.; Bahri, N.; Valeri, A.; et al. Prolonged Lenalidomide Maintenance Therapy Improves the Depth of Response in Multiple Myeloma. Blood Adv. 2020, 4, 2163–2171. [Google Scholar] [CrossRef]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide Maintenance versus Observation for Patients with Newly Diagnosed Multiple Myeloma (Myeloma XI): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ohashi, K.; Kakihana, K.; Nakamura, Y.; Komeno, T.; Kojima, H.; Morita, S.; Sakamaki, H. A Multicenter Clinical Study to Determine the Feasible Initial Dose of Lenalidomide for Maintenance Therapy in Patients with Multiple Myeloma Following Autologous Peripheral Blood Stem-Cell Transplantation. Mol. Clin. Oncol. 2016, 4, 965–970. [Google Scholar] [CrossRef]
- Parrondo, R.D.; Reljic, T.; Iqbal, M.; Ayala, E.; Kharfan-Dabaja, M.A.; Kumar, A.; Murthy, H. Efficacy of Proteasome Inhibitor-Based Maintenance Post Autologous Hematopoietic Cell Transplant in Multiple Myeloma: A Systematic Review and Meta-Analysis. Biol. Blood Marrow Transplant. 2020, 26, S244–S245. [Google Scholar] [CrossRef]
- Karam, D.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Hayman, S.R.; Dingli, D.; Kapoor, P.; Kourelis, T.; Warsame, R.M.; Hogan, W.J.; et al. Use of Maintenance Therapy Post Autologous Stem Cell Transplantation Outside of Clinical Trial Setting for Multiple Myeloma: Single Institution Experience. Blood 2019, 134, 2013. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Owzar, K.; Hahn, T. Autologous Hematopoietic Stem Cell Transplantation and Maintenance Therapy for Multiple Myeloma. Int. J. Hematol. Oncol. 2013, 2, 71–83. [Google Scholar] [CrossRef]
- Sonneveld, P.; Schmidt-Wolf, I.; van der Holt, B.; el Jarari, L.; Bertsch, U.; Salwender, H.; Zweegman, S.; Vellenga, E.; Schubert, J.; Blau, I.W.; et al. HOVON-65/GMMG-HD4 Randomized Phase III Trial Comparing Bortezomib, Doxorubicin, Dexamethasone (PAD) Vs. VAD Followed by High-Dose Melphalan (HDM) and Maintenance with Bortezomib or Thalidomide In Patients with Newly Diagnosed Multiple Myeloma (MM). Blood 2010, 116, 40. [Google Scholar] [CrossRef]
- Sonneveld, P.; Schmidt-Wolf, I.G.H.; van der Holt, B.; el Jarari, L.; Bertsch, U.; Salwender, H.; Zweegman, S.; Vellenga, E.; Broyl, A.; Blau, I.W.; et al. Bortezomib Induction and Maintenance Treatment in Patients with Newly Diagnosed Multiple Myeloma: Results of the Randomized Phase III HOVON-65/GMMG-HD4 Trial. JCO 2012, 30, 2946–2955. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Lokhorst, H.M.; Mai, E.K.; van der Holt, B.; Blau, I.W.; Zweegman, S.; Weisel, K.C.; Vellenga, E.; Pfreundschuh, M.; Kersten, M.J.; et al. Bortezomib before and after High-Dose Therapy in Myeloma: Long-Term Results from the Phase III HOVON-65/GMMG-HD4 Trial. Leukemia 2018, 32, 383–390. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Špička, I.; Quach, H.; Oriol, A.; Hájek, R.; Garg, M.; Beksac, M.; Bringhen, S.; Katodritou, E.; Chng, W.-J.; et al. Ixazomib as Postinduction Maintenance for Patients with Newly Diagnosed Multiple Myeloma Not Undergoing Autologous Stem Cell Transplantation: The Phase III TOURMALINE-MM4 Trial. JCO 2020, 38, 4030–4041. [Google Scholar] [CrossRef]
- Martello, M.; Poletti, A.; Borsi, E.; Solli, V.; Dozza, L.; Barbato, S.; Zamagni, E.; Tacchetti, P.; Pantani, L.; Mancuso, K.; et al. Clonal and Subclonal TP53 Molecular Impairment Is Associated with Prognosis and Progression in Multiple Myeloma. Blood Cancer J. 2022, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Perrot, A.; Caillot, D.; Belhadj, K.; Hulin, C.; Leleu, X.; Mohty, M.; Facon, T.; Buisson, L.; Do Souto, L.; et al. Del(17p) without TP53 Mutation Confers a Poor Prognosis in Intensively Treated Newly Diagnosed Patients with Multiple Myeloma. Blood 2021, 137, 1192–1195. [Google Scholar] [CrossRef]
- Novoa Jáuregui, S.; Gabarros Subira, M.; Garrido, A.M.; Palomo, L.; Medina, I.; Beas, F.; Bosch, M.; Muzio, S.; Camarillas, S.; Hidalgo-Gomez, G.; et al. Ultra-Deep Sequencing of TP53 in Multiple Myeloma: Double-Hit Prevalence and Clinical Phenotype. Blood 2024, 144, 1935. [Google Scholar] [CrossRef]
- Munawar, U.; Barrio, S.; Roth, M.; Einsele, H.; Bargou, R.C.; Stuehmer, T.; Kortum, M. Implications of TP53 Alterations for Therapy Response in Multiple Myeloma. Blood 2018, 132, 3189. [Google Scholar] [CrossRef]
- Munawar, U.; Roth, M.; Barrio, S.; Wajant, H.; Siegmund, D.; Bargou, R.C.; Kortüm, K.M.; Stühmer, T. Assessment of TP53 Lesions for P53 System Functionality and Drug Resistance in Multiple Myeloma Using an Isogenic Cell Line Model. Sci. Rep. 2019, 9, 18062. [Google Scholar] [CrossRef]
- Singh, C.; Sreedharanunni, S.; Panakkal, V.; Jandial, A.; Jain, A.; Lad, D.; Prakash, G.; Khadwal, A.; Malhotra, P. Early Mortality and Aggressive Presentation Lead to Poor Outcomes in Patients with Newly Diagnosed Double Hit and Triple Hit Multiple Myeloma. Blood 2021, 138, 3762. [Google Scholar] [CrossRef]
- D’Agostino, M.; De Paoli, L.; Conticello, C.; Offidani, M.; Ria, R.; Petrucci, M.T.; Spada, S.; Marcatti, M.; Catalano, L.; Gilestro, M.; et al. Continuous Therapy in Standard- and High-Risk Newly-Diagnosed Multiple Myeloma: A Pooled Analysis of 2 Phase III Trials. Crit. Rev. Oncol. Hematol. 2018, 132, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Marneni, N.; Chakraborty, R. Current Approach to Managing Patients with Newly Diagnosed High-Risk Multiple Myeloma. Curr. Hematol. Malig. Rep. 2021, 16, 148–161. [Google Scholar] [CrossRef]
- Cazaubiel, T.; Mulas, O.; Montes, L.; Schavgoulidze, A.; Avet-Loiseau, H.; Corre, J.; Perrot, A. Risk and Response-Adapted Treatment in Multiple Myeloma. Cancers 2020, 12, 3497. [Google Scholar] [CrossRef]
- Goel, U.; Usmani, S.; Kumar, S. Current Approaches to Management of Newly Diagnosed Multiple Myeloma. Am. J. Hematol. 2022, 97, S3–S25. [Google Scholar] [CrossRef]
- Chatziravdeli, V.; Katsaras, G.N.; Katsaras, D.; Doxani, C.; Stefanidis, I.; Zintzaras, E. A Systematic Review and Meta-Analysis of Interventional Studies of Bisphosphonates and Denosumab in Multiple Myeloma and Future Perspectives. J. Musculoskelet. Neuronal Interact. 2022, 22, 596–621. [Google Scholar]
- Adam, Z.; Straub, J.; Krejčí, M.; Pour, L.; Brančíková, D.; Ostřížková, L.; Sandecká, V.; Štork, M. Osteoprotective therapy with bisphosphonates or denosumab in patients with multiple myeloma: Benefit and risks. Vnitr. Lek. 2017, 63, 311–321. [Google Scholar] [CrossRef]
- Lei, M.M.; Tavares, E.; Buzgo, E.; Lou, U.; Raje, N.; Yee, A.J. Denosumab versus Intravenous Bisphosphonate Use for Hypercalcemia in Multiple Myeloma. Leuk. Lymphoma 2022, 63, 3249–3252. [Google Scholar] [CrossRef]
- John, P.; Khalil, A.; Gao, J.; Nishioka, J.; Kamangar, F.; Badros, A. Effects of Denosumab Therapy on Renal Function in Multiple Myeloma Patients: A Single Center Retrospective Analysis. Blood 2024, 144, 6972. [Google Scholar] [CrossRef]
- Leleu, X.; Martin, T.; Weisel, K.; Schjesvold, F.; Iida, S.; Malavasi, F.; Manier, S.; Min, C.-K.; Ocio, E.M.; Pawlyn, C.; et al. Anti-CD38 Antibody Therapy for Patients with Relapsed/Refractory Multiple Myeloma: Differential Mechanisms of Action and Recent Clinical Trial Outcomes. Ann. Hematol. 2022, 101, 2123–2137. [Google Scholar] [CrossRef]
- Moreno, L.; Perez, C.; Zabaleta, A.; Manrique, I.; Alignani, D.; Ajona, D.; Blanco, L.; Lasa, M.; Maiso, P.; Rodriguez, I.; et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3176–3187. [Google Scholar] [CrossRef]
- Yashar, D.; Regidor, B.S.S.; Jew, S.K.; Bujarski, S.; Goldwater, M.-S.; Swift, R.; Schwartz, G.E.; Eshaghian, S.; Vescio, R.; Berenson, J.R. Retrospective Analysis of Response Rates to Anti-CD38 Monoclonal Antibody Containing Regimens Among Multiple Myeloma Patients with Prior Exposure to Daratumumab or Isatuximab. Blood 2021, 138, 3785. [Google Scholar] [CrossRef]
- Diamond, B.; Baughn, L.; Poorebrahim, M.; Poos, A.M.; Lee, H.; Kaddoura, M.; Wiedmeier-Nutor, J.E.; Durante, M.; Otteson, G.; Jevremovic, D.; et al. CD38 Biallelic Loss Is a Recurrent Mechanism of Resistance to Anti-CD38 Antibodies in Multiple Myeloma. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, H.; Koob, T.; Fromm, J.R.; Gopal, A.; Carter, D.; Lieber, A. CD46 and CD59 Inhibitors Enhance Complement-Dependent Cytotoxicity of Anti-CD38 Monoclonal Antibodies Daratumumab and Isatuximab in Multiple Myeloma and Other B-Cell Malignancy Cells. Cancer Biol. Ther. 2024, 25, 2314322. [Google Scholar] [CrossRef] [PubMed]
- Einsele, H.; Schreder, M. Treatment of Multiple Myeloma with the Immunostimulatory SLAMF7 Antibody Elotuzumab. Ther. Adv. Hematol. 2016, 7, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Pazina, T.; James, A.M.; MacFarlane, A.W.; Bezman, N.A.; Henning, K.A.; Bee, C.; Graziano, R.F.; Robbins, M.D.; Cohen, A.D.; Campbell, K.S. The Anti-SLAMF7 Antibody Elotuzumab Mediates NK Cell Activation through Both CD16-Dependent and –Independent Mechanisms. OncoImmunology 2017, 6, e1339853. [Google Scholar] [CrossRef] [PubMed]
- Magen, H.; Muchtar, E. Elotuzumab: The First Approved Monoclonal Antibody for Multiple Myeloma Treatment. Ther. Adv. Hematol. 2016, 7, 187–195. [Google Scholar] [CrossRef]
- Taniwaki, M.; Yoshida, M.; Matsumoto, Y.; Shimura, K.; Kuroda, J.; Kaneko, H. Elotuzumab for the Treatment of Relapsed or Refractory Multiple Myeloma, with Special Reference to its Modes of Action and SLAMF7 Signaling. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018014. [Google Scholar] [CrossRef]
- Balasa, B.; Yun, R.; Belmar, N.A.; Fox, M.; Chao, D.T.; Robbins, M.D.; Starling, G.C.; Rice, A.G. Elotuzumab Enhances Natural Killer Cell Activation and Myeloma Cell Killing through Interleukin-2 and TNF-α Pathways. Cancer Immunol. Immunother. 2015, 64, 61–73. [Google Scholar] [CrossRef]
- Danhof, S.; Rasche, L.; Mottok, A.; Steinmüller, T.; Zhou, X.; Schreder, M.; Kilian, T.; Strifler, S.; Rosenwald, A.; Hudecek, M.; et al. Elotuzumab for the Treatment of Extramedullary Myeloma: A Retrospective Analysis of Clinical Efficacy and SLAMF7 Expression Patterns. Ann. Hematol. 2021, 100, 1537–1546. [Google Scholar] [CrossRef]
- Almodovar Diaz, A.; Alouch, S.; Chawla, Y.; Gonsalves, W. The Antibody Drug Conjugate, Belantamab-Mafodotin, in the Treatment of Multiple Myeloma: A Comprehensive Review. BLCTT 2024, 14, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Tzogani, K.; Penttilä, K.; Lähteenvuo, J.; Lapveteläinen, T.; Lopez Anglada, L.; Prieto, C.; Garcia-Ochoa, B.; Enzmann, H.; Gisselbrecht, C.; Delgado, J.; et al. EMA Review of Belantamab Mafodotin (Blenrep) for the Treatment of Adult Patients with Relapsed/Refractory Multiple Myeloma. Oncologist 2021, 26, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab Mafodotin for Relapsed or Refractory Multiple Myeloma (DREAMM-2): A Two-Arm, Randomised, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Nooka, A.K.; Cohen, A.D.; Lee, H.C.; Badros, A.; Suvannasankha, A.; Callander, N.; Abdallah, A.-O.; Trudel, S.; Chari, A.; Libby, E.N.; et al. Single-Agent Belantamab Mafodotin in Patients with Relapsed/Refractory Multiple Myeloma: Final Analysis of the DREAMM-2 Trial. Cancer 2023, 129, 3746–3760. [Google Scholar] [CrossRef]
- Abeykoon, J.P.; Vaxman, J.; Patel, S.V.; Kumar, S.; Malave, G.C.; Young, K.S.; Ailawadhi, S.; Larsen, J.T.; Dispenzieri, A.; Muchtar, E.; et al. Impact of Belantamab Mafodotin-induced Ocular Toxicity on Outcomes of Patients with Advanced Multiple Myeloma. Br. J. Haematol. 2022, 199, 95–99. [Google Scholar] [CrossRef]
- Terpos, E.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Fotiou, D.; Migkou, M.; Theodorakakou, F.; Spiliopoulou, V.; Syrigou, R.; Eleutherakis-Papaiakovou, E.; et al. P884: Belantamab mafodotin plus lenalidomide and dexamethasone in transplant ineligible patients with newly diagnosed multiple myeloma: Updated results from the phase 1/2 belard study. HemaSphere 2023, 7, e828385f. [Google Scholar] [CrossRef]
- Lasica, M.; Spencer, A.; Campbell, P.; Wallington-Gates, C.; Wong Doo, N.; Janowski, W.; McCaughan, G.; Puliyayil, A.; Yuen, F.; Le, K.; et al. P946: A PHASE I/II SINGLE ARM STUDY OF BELANTAMAB MAFODOTIN, CARFILZOMIB AND DEXAMETHASONE IN PATIENTS WITH RELAPSED MULTIPLE MYELOMA: AMARC 19-02 BELACARD STUDY. HemaSphere 2022, 6, 836–837. [Google Scholar] [CrossRef]
- Eastman, S.; Shelton, C.; Gupta, I.; Krueger, J.; Blackwell, C.; Bojczuk, P.M. Synergistic Activity of Belantamab Mafodotin (Anti-BCMA Immuno-Conjugate) with PF-03084014 (Gamma-Secretase Inhibitor) in Bcma-Expressing Cancer Cell Lines. Blood 2019, 134, 4401. [Google Scholar] [CrossRef]
- Lonial, S.; Grosicki, S.; Hus, M.; Song, K.W.; Facon, T.; Callander, N.S.; Ribrag, V.; Uttervall, K.; Quach, H.; Vorobyev, V.I.; et al. Synergistic Effects of Low-Dose Belantamab Mafodotin in Combination with a Gamma-Secretase Inhibitor (Nirogacestat) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM): DREAMM-5 Study. JCO 2022, 40, 8019. [Google Scholar] [CrossRef]
- Fang, J.; Zhou, F. BCMA-Targeting Chimeric Antigen Receptor T Cell Therapy for Relapsed and/or Refractory Multiple Myeloma. Ann. Hematol. 2024, 103, 1069–1083. [Google Scholar] [CrossRef]
- Martin, T.G.; Madduri, D.; Pacaud, L.; Usmani, S.Z. Cilta-Cel, a BCMA-Targeting CAR-T Therapy for Heavily Pretreated Patients with Relapsed/Refractory Multiple Myeloma. Future Oncol. 2023, 19, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Jackson, C.C.; Schecter, J.M.; Lendvai, N.; Sun, H.; Akram, M.; Patel, N.; Martin, T.G. Cilta-Cel, a BCMA-Targeting CAR-T Therapy for Patients with Multiple Myeloma. Expert Opin. Biol. Ther. 2024, 24, 339–350. [Google Scholar] [CrossRef]
- Attar, N.; Cirstea, D.; Branagan, A.R.; Yee, A.J.; Frigault, M.J.; Raje, N. Use of Cilta-Cel CAR T Cells Following Previous Use of a BCMA-Directed CAR T-Cell Product in Heavily Treated Patients with Relapsed/Refractory Multiple Myeloma: A Single Institution Case Series. Blood 2023, 142, 6924. [Google Scholar] [CrossRef]
- Freyer, C.W.; Porter, D.L. CAR T Toxicity Management: Cytokine Release Syndrome and Neurotoxicity. In Blood and Marrow Transplant Handbook: Comprehensive Guide for Patient Care; Maziarz, R.T., Slater, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 915–928. ISBN 978-3-030-53626-8. [Google Scholar]
- Gaffney, K.J.; Davis, J.A.; McGann, M.; Edwards, K.; Ahmed, Z.; Butcher, C.; Greenwell, B.; Hess, B.T.; Hashmi, H. Early versus Standard Management of Chimeric Antigen Receptor Therapy Toxicities and Management’s Impact on Safety and Efficacy. J. Oncol. Pharm. Pract. 2024, 30, 151–158. [Google Scholar] [CrossRef]
- Murthy, H.S.; Yassine, F.; Iqbal, M.; Alotaibi, S.; Moustafa, M.A.; Kharfan-Dabaja, M.A. Management of CAR T-Cell Related Toxicities: What Did the Learning Curve Teach Us so Far? Hematol. Oncol. Stem Cell Ther. 2022, 15, 100–111. [Google Scholar] [CrossRef]
- Walton, Z.E.; Frigault, M.J.; Maus, M.V. Current and Emerging Pharmacotherapies for Cytokine Release Syndrome, Neurotoxicity, and Hemophagocytic Lymphohistiocytosis-like Syndrome Due to CAR T Cell Therapy. Expert Opin. Pharmacother. 2024, 25, 263–279. [Google Scholar] [CrossRef]
- Singh, V.; Master, S. Incidence of Parkinsonism As a Complication of Anti-BCMA CAR-T Cell Therapy in Multiple Myeloma. Blood 2023, 142, 6937. [Google Scholar] [CrossRef]
- Graham, C.; Parker, A.; Sun, X.; Haradhvala, N.J.; Dolaher, M.; Lindell, K.; Harris, D.; Lee, W.-H.; Graham, K.J.; Nolan, H.K.; et al. Biological Correlatives of Efficacy and Toxicity of BCMA Targeting CAR T Cell Therapy in a Real-World Setting. Blood 2024, 144, 7126. [Google Scholar] [CrossRef]
- Gul, A.; Afaq, Y.; Khan, T.; Keen, M.A.; Ahmad, S.; Khan, M.A.; Hussain, R.; Inayat, A.; Safi, D.; Safi, S. Risk of Infection: Real-World Analysis of (Ide-Cel) Idecabtagene Vicleucel and (Cilta-Cel) Ciltacabtagene Autoleucel in Relapsed/Refractory Multiple Myeloma. Blood 2024, 144, 5160. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Chohan, K.L.; Siegler, E.L.; Kenderian, S.S. CAR-T Cell Therapy: The Efficacy and Toxicity Balance. Curr. Hematol. Malig. Rep. 2023, 18, 9–18. [Google Scholar] [CrossRef]
- Wesson, W.; Dima, D.; Suleman, N.; Saif, M.S.I.; Tabak, C.; Logan, E.; Davis, J.A.; McGann, M.; Furqan, F.; Mohan, M.; et al. Timing of Toxicities and Non-Relapse Mortality Following CAR T Therapy in Myeloma. Transplant. Cell. Ther. 2024, 30, 876–884. [Google Scholar] [CrossRef]
- Freyer, C.W.; Porter, D.L. Cytokine Release Syndrome and Neurotoxicity Following CAR T-Cell Therapy for Hematologic Malignancies. J. Allergy Clin. Immunol. 2020, 146, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, K.; Jain, M.D.; Smith, E.L. Mechanisms of Resistance and Treatment of Relapse after CAR T-Cell Therapy for Large B-Cell Lymphoma and Multiple Myeloma. Transplant. Cell. Ther. 2023, 29, 418–428. [Google Scholar] [CrossRef]
- Ravi, G.; Costa, L.J. Bispecific T-cell Engagers for Treatment of Multiple Myeloma. Am. J. Hematol. 2023, 98, S13–S21. [Google Scholar] [CrossRef]
- Verkleij, C.P.M.; Frerichs, K.A.; Broekmans, M.; Absalah, S.; Maas-Bosman, P.W.C.; Kruyswijk, S.; Nijhof, I.S.; Mutis, T.; Zweegman, S.; Van De Donk, N.W.C.J. T-Cell Redirecting Bispecific Antibodies Targeting BCMA for the Treatment of Multiple Myeloma. Oncotarget 2020, 11, 4076–4081. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.-Q.; Nie, L.; Zhu, S.-S.; Li, N.; Wu, Z.-H.; Wang, Q.; Qi, J.; Wu, B.-Y.; Chen, S.-Q.; et al. BR109, a Novel Fully Humanized T-Cell-Engaging Bispecific Antibody with GPRC5D Binding, Has Potent Antitumor Activities in Multiple Myeloma. Cancers 2023, 15, 5774. [Google Scholar] [CrossRef]
- Dekhtiarenko, I.; Lelios, I.; Jacob, W.; Schneider, M.; Weisser, M.; Carlo-Stella, C.; Manier, S.; Harrison, S.J.; Popat, R.; Hasselbalch Riley, A.C.; et al. Co-Expression of GPRC5D, FcRH5 and BCMA Suggests That Targeting More Than One Cell Surface Marker May Be a Viable Strategy in Relapsed/Refractory Multiple Myeloma (RRMM): Biomarker Results from the Phase I Study of Forimtamig, a GPRC5DxCD3 Bispecific Antibody. Blood 2023, 142, 1948. [Google Scholar] [CrossRef]
- An, G.; Xing, L.; Chen, W.; Zhang, Y.; Gao, W.; Qiu, L.-G.; Wu, G.; Ning, J.; Wei, M.; Li, F. MBS314, a G Protein-Coupled Receptor Family C Group 5 Member D (GPRC5D) x B-Cell Maturation Antigen (BCMA) x CD3 Trispecific Antibody, in Relapsed and/or Refractory Multiple Myeloma (RRMM): Preliminary Results from a Phase I, First-in-Human, Open-Label, Dose Escalation Study. Blood 2024, 144, 3356. [Google Scholar] [CrossRef]
- Eckmann, J.; Fauti, T.; Biehl, M.; Zabaleta, A.; Blanco, L.; Lelios, I.; Gottwald, S.; Rae, R.; Lechner, S.; Bayer, C.; et al. Forimtamig, a Novel GPRC5D-Targeting T-Cell Bispecific Antibody with a 2+1 Format, for the Treatment of Multiple Myeloma. Blood 2025, 145, 202–219. [Google Scholar] [CrossRef]
- Pillarisetti, R.; Yang, D.; Yao, J.; Smith, M.; Luistro, L.; Vulfson, P.; Testa, J.; Packman, K.; Brodeur, S.; Attar, R.M.; et al. Characterization of JNJ-79635322, a Novel BCMAxGPRC5DxCD3 T-Cell Redirecting Trispecific Antibody, for the Treatment of Multiple Myeloma. Blood 2023, 142, 456. [Google Scholar] [CrossRef]
- Tan, Y.; Li, X.; Yu, F.; Xu, J.; Qian, Z.; Cao, Y.; Yang, X.; Du, Q.; Peng, F.; Han, S.; et al. Abstract LB128: A Novel Tri-Specific T Cell Engager Targeting BCMA and GPRC5D for Treatment of Multiple Myeloma. Cancer Res. 2024, 84, LB128. [Google Scholar] [CrossRef]
- Heuck, C.J.; Jethava, Y.; Khan, R.; Van Rhee, F.; Zangari, M.; Chavan, S.; Robbins, K.; Miller, S.E.; Matin, A.; Mohan, M.; et al. Inhibiting MEK in MAPK Pathway-Activated Myeloma. Leukemia 2016, 30, 976–980. [Google Scholar] [CrossRef]
- Chang-Yew Leow, C.; Gerondakis, S.; Spencer, A. MEK Inhibitors as a Chemotherapeutic Intervention in Multiple Myeloma. Blood Cancer J. 2013, 3, e105. [Google Scholar] [CrossRef]
- Steinbrunn, T.; Stühmer, T.; Sayehli, C.; Chatterjee, M.; Einsele, H.; Bargou, R.C. Combined Targeting of MEK/MAPK and PI3K/AKt Signalling in Multiple Myeloma. Br. J. Haematol. 2012, 159, 430–440. [Google Scholar] [CrossRef]
- Trudel, S.; Bahlis, N.J.; Venner, C.P.; Hay, A.E.; Kis, O.; Chow, S.; Li, Z.H.; Wei, E.N.; Wang, L.; Tran, C.; et al. Biomarker Driven Phase II Clinical Trial of Trametinib in Relapsed/Refractory Multiple Myeloma with Sequential Addition of the AKT Inhibitor, GSK2141795 at Time of Disease Progression to Overcome Treatment Failure: A Trial of the Princess Margaret Phase II Consortium. Blood 2016, 128, 4526. [Google Scholar] [CrossRef]
- Hideshima, T.; Akiyama, M.; Hayashi, T.; Richardson, P.; Schlossman, R.; Chauhan, D.; Anderson, K.C. Targeting P38 MAPK Inhibits Multiple Myeloma Cell Growth in the Bone Marrow Milieu. Blood 2003, 101, 703–705. [Google Scholar] [CrossRef]
- Reddy, M.; Ma, J.Y.; Kerr, I.; Henjarappa, N.; Navas, T.; Chakravarty, J.; Dugar, S.; Higgins, L.S.; Protter, A.A.; Medicherla, S. P38 Alpha MAP Kinase Inhibitor Inhibits Human Myeloma Cell Growth in Vivo. Blood 2004, 104, 4936. [Google Scholar] [CrossRef]
- Fu, J.; Li, S.; Liu, G.; Ma, H.; Marcireau, C.; Mapara, M.; Lentzsch, S. Synergistic Anti-Tumor Effects in RAS Mut Multiple Myeloma By Targeting MAP4K2 and RAS Pathways. Blood 2023, 142, 6618. [Google Scholar] [CrossRef]
- Malamos, P.; Papanikolaou, C.; Gavriatopoulou, M.; Dimopoulos, M.A.; Terpos, E.; Souliotis, V.L. The Interplay between the DNA Damage Response (DDR) Network and the Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway in Multiple Myeloma. IJMS 2024, 25, 6991. [Google Scholar] [CrossRef] [PubMed]
- Alagpulinsa, D.A.; Ayyadevara, S.; Yaccoby, S.; Shmookler Reis, R.J. A Cyclin-Dependent Kinase Inhibitor, Dinaciclib, Impairs Homologous Recombination and Sensitizes Multiple Myeloma Cells to PARP Inhibition. Mol. Cancer Ther. 2016, 15, 241–250. [Google Scholar] [CrossRef]
- Botrugno, O.A.; Bianchessi, S.; Zambroni, D.; Frenquelli, M.; Belloni, D.; Bongiovanni, L.; Girlanda, S.; Di Terlizzi, S.; Ferrarini, M.; Ferrero, E.; et al. ATR Addiction in Multiple Myeloma: Synthetic Lethal Approaches Exploiting Established Therapies. haematologica 2019, 105, 2440–2447. [Google Scholar] [CrossRef]
- Xu, A.; Mazumder, A.; Borowiec, J.A. Cell Line-Dependent Synergy Between the PARP Inhibitor Veliparib and the Proteasome Inhibitor Bortezomib in the Killing of Myeloma Cells. Blood 2015, 126, 5556. [Google Scholar] [CrossRef]
- Bernasconi, E.; Gaudio, E.; Lejeune, P.; Tarantelli, C.; Cascione, L.; Kwee, I.; Spriano, F.; Rinaldi, A.; Mensah, A.A.; Chung, E.; et al. Preclinical Evaluation of the BET Bromodomain Inhibitor BAY 1238097 for the Treatment of Lymphoma. Br. J. Haematol. 2017, 178, 936–948. [Google Scholar] [CrossRef]
- Carew, J.S.; Espitia, C.M.; Zhao, W.; Visconte, V.; Kelly, K.R.; Nawrocki, S.T. Antagonizing HDAC6 Activity: A New Strategy to Maximize the Anti-Myeloma Effects of BET Inhibition. Blood 2015, 126, 3022. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, N.; Pan, Z.; Li, Z.; Sheng, C. BET–HDAC Dual Inhibitors for Combinational Treatment of Breast Cancer and Concurrent Candidiasis. J. Med. Chem. 2023, 66, 1239–1253. [Google Scholar] [CrossRef]
- Bekri, S.; Rodney-Sandy, R.; Gruenstein, D.; Mei, A.; Bogen, B.; Castle, J.; Levey, D.; Cho, H.J. Neoantigen Vaccine-Induced CD4 T Cells Confer Protective Immunity in a Mouse Model of Multiple Myeloma through Activation of CD8 T Cells against Non-Vaccine, Tumor-Associated Antigens. J. Immunother. Cancer 2022, 10, e003572. [Google Scholar] [CrossRef]
- Kodysh, J.; Marron, T.; Rubinsteyn, A.; O’Donnell, T.; Finnigan, J.; Blazquez, A.; Saxena, M.; Meseck, M.; Friedlander, P.; Bhardwaj, N. Abstract CT173: PGV-001: A Phase I Trial of a Multipeptide Personalized Neoantigen Vaccine in the Adjuvant Setting. Cancer Res. 2020, 80, CT173. [Google Scholar] [CrossRef]
- Marron, T.U.; Saxena, M.; Bhardwaj, N.; Meseck, M.; Rubinsteyn, A.; Finnigan, J.; Kodysh, J.; Blazquez, A.; O’Donnel, T.; Galsky, M.; et al. Abstract LB048: An Adjuvant Personalized Neoantigen Peptide Vaccine for the Treatment of Malignancies (PGV-001). Cancer Res. 2021, 81, LB048. [Google Scholar] [CrossRef]
- Perumal, D.; Imai, N.; Laganà, A.; Finnigan, J.; Melnekoff, D.; Leshchenko, V.V.; Solovyov, A.; Madduri, D.; Chari, A.; Cho, H.J.; et al. Mutation-Derived Neoantigen-Specific T-Cell Responses in Multiple Myeloma. Clin. Cancer Res. 2020, 26, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.G.; Ahmad, S.M.; Abildgaard, N.; Straten, P.T.; Svane, I.M.; Andersen, M.H.; Knudsen, L.M. Peptide Vaccination against Multiple Myeloma Using Peptides Derived from Anti-Apoptotic Proteins: A Phase I Trial. Stem Cell Investig. 2016, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Smith, R.; Daley, J.; Mimura, N.; Tai, Y.-T.; Anderson, K.C.; Munshi, N.C. Myeloma-Specific Multiple Peptides Able to Generate Cytotoxic T Lymphocytes: A Potential Therapeutic Application in Multiple Myeloma and Other Plasma Cell Disorders. Clin. Cancer Res. 2012, 18, 4850–4860. [Google Scholar] [CrossRef]
- Bekri, S.; Uduman, M.; Gruenstein, D.; Mei, A.H.-C.; Tung, K.; Rodney-Sandy, R.; Bogen, B.; Buell, J.; Stein, R.; Doherty, K.; et al. Neoantigen Synthetic Peptide Vaccine for Multiple Myeloma Elicits T Cell Immunity in a Pre-Clinical Model. Blood 2017, 130, 1868. [Google Scholar]
- Bae, J.; Cowens, K.; Kimmelman, A.; Rowell, S.; Lazo, S.; Keskin, D.; Munshi, N.; Anderson, K. OP01-5 Combination Immunotherapy Targeting XBP1, CD138 and CS1 with Cancer Vaccine, Checkpoint Inhibitors, Immune Modulator, and Epigenetic Regulator to Treat Patients with Myeloma or Solid Tumours: Clinical Applications. ESMO Open 2023, 8, 102126. [Google Scholar] [CrossRef]
- Gorgun, G.T.; Whitehill, G.; Anderson, J.L.; Hideshima, T.; Laubach, J.P.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. Myeloid Derived Suppressor Cells (MDSCs) Regulate Tumor Growth, Immune Response and Regulatory T Cell (Treg) Development in the Multiple Myeloma Bone Marrow Microenvironment. Blood 2012, 120, 565. [Google Scholar] [CrossRef]
- Malek, E.; De Lima, M.; Letterio, J.J.; Kim, B.-G.; Finke, J.H.; Driscoll, J.J.; Giralt, S.A. Myeloid-Derived Suppressor Cells: The Green Light for Myeloma Immune Escape. Blood Rev. 2016, 30, 341–348. [Google Scholar] [CrossRef]
- Topal Gorgun, G.; Ohguchi, H.; Hideshima, T.; Tai, Y.-T.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Laubach, J.P.; Anderson, K.C. Inhibition Of Myeloid Derived Suppressor Cells (MDSC) In The Multiple Myeloma Bone Marrow Microenvironment. Blood 2013, 122, 3089. [Google Scholar] [CrossRef]
- Botta, C.; Gullà, A.; Correale, P.; Tagliaferri, P.; Tassone, P. Myeloid-Derived Suppressor Cells in Multiple Myeloma: Pre-Clinical Research and Translational Opportunities. Front. Oncol. 2014, 4, 348. [Google Scholar] [CrossRef]
- Albu, D.I.; Wang, Z.; Huang, K.-C.; Wu, J.; Twine, N.; Leacu, S.; Ingersoll, C.; Parent, L.; Lee, W.; Liu, D.; et al. EP4 Antagonism by E7046 Diminishes Myeloid Immunosuppression and Synergizes with Treg-Reducing IL-2-Diphtheria Toxin Fusion Protein in Restoring Anti-Tumor Immunity. OncoImmunology 2017, 6, e1338239. [Google Scholar] [CrossRef]
- Giannotta, C.; Autino, F.; Massaia, M. The Immune Suppressive Tumor Microenvironment in Multiple Myeloma: The Contribution of Myeloid-Derived Suppressor Cells. Front. Immunol. 2023, 13, 1102471. [Google Scholar] [CrossRef]
- Park, J.J.H.; Hsu, G.; Siden, E.G.; Thorlund, K.; Mills, E.J. An Overview of Precision Oncology Basket and Umbrella Trials for Clinicians. CA Cancer J. Clin. 2020, 70, 125–137. [Google Scholar] [CrossRef]
- Kesari, H.V.; Ravi, R. Novel Study Designs in Precision Medicine—Basket, Umbrella and PlatformTrials. CRCEP 2022, 17, 114–121. [Google Scholar] [CrossRef]
- González-Calle, V.; Keane, N.; Braggio, E.; Fonseca, R. Precision Medicine in Myeloma: Challenges in Defining an Actionable Approach. Clin. Lymphoma Myeloma Leuk. 2017, 17, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.C.; Guinigundo, A.S. Biomarker-Driven Oncology Clinical Trials: Novel Designs in the Era of Precision Medicine. JADPRO 2023, 14, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Richter, J. Where We Stand with Precision Therapeutics in Myeloma: Prosperity, Promises, and Pipedreams. Front. Oncol. 2022, 11, 819127. [Google Scholar] [CrossRef]
- Zweegman, S.; Engelhardt, M.; Larocca, A. Elderly Patients with Multiple Myeloma: Towards a Frailty Approach? Curr. Opin. Oncol. 2017, 29, 315–321. [Google Scholar] [CrossRef]
- Sutanto, H. Tackling Polypharmacy in Geriatric Patients: Is Increasing Physicians’ Awareness Adequate? Arch. Gerontol. Geriatr. Plus 2025, 2, 100185. [Google Scholar] [CrossRef]
- Möller, M.-D.; Gengenbach, L.; Graziani, G.; Greil, C.; Wäsch, R.; Engelhardt, M. Geriatric Assessments and Frailty Scores in Multiple Myeloma Patients: A Needed Tool for Individualized Treatment? Curr. Opin. Oncol. 2021, 33, 648–657. [Google Scholar] [CrossRef]
- Larocca, A.; Palumbo, A. Frail Patients with Newly Diagnosed Multiple Myeloma. In Neoplastic Diseases of the Blood; Wiernik, P.H., Dutcher, J.P., Gertz, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 539–549. ISBN 978-3-319-64263-5. [Google Scholar]
- Bao, L.; Wang, Y.-T.; Liu, P.; Lu, M.-Q.; Zhuang, J.-L.; Zhang, M.; Xia, Z.-J.; Li, Z.-L.; Yang, Y.; Yan, Z.-Y.; et al. Ixazomib-Based Frontline Therapy Followed by Ixazomib Maintenance in Frail Elderly Newly Diagnosed with Multiple Myeloma: A Prospective Multicenter Study. EClinicalMedicine 2024, 68, 102431. [Google Scholar] [CrossRef]
- Salazar, A.S.; Recinos, L.M.; Mian, H.S.; Stoll, C.; Simon, L.E.; Sekhon, S.; Colditz, G.A.; Wildes, T.M. Geriatric Assessment and Frailty Scores Predict Mortality in Myeloma: Systematic Review and Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2019, 19, 488–496.e6. [Google Scholar] [CrossRef]
- Smits, F.; Nasserinejad, K.; Levin, M.-D.; Timmers, G.J.; De Waal, E.G.M.; Regelink, J.C.; Velders, G.A.; Westerman, M.; Durdu-Rayman, N.; Last-Koopmans, S.; et al. Dynamics of Geriatric Impairments Are Associated with Risk of Treatment Discontinuation in Elderly Patients with Multiple Myeloma. Blood 2024, 144, 3330. [Google Scholar] [CrossRef]
- Nathwani, N.; Kurtin, S.E.; Lipe, B.; Mohile, S.G.; Catamero, D.D.; Wujcik, D.; Birchard, K.; Davis, A.; Dudley, W.; Stricker, C.T.; et al. Integrating Touchscreen-Based Geriatric Assessment and Frailty Screening for Adults with Multiple Myeloma to Drive Personalized Treatment Decisions. JCO Oncol. Pract. 2020, 16, e92–e99. [Google Scholar] [CrossRef]
- Greenberg, A.J.; Vachon, C.M.; Rajkumar, S.V. Disparities in the Prevalence, Pathogenesis and Progression of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma between Blacks and Whites. Leukemia 2012, 26, 609–614. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Jacobus, S.; Sexton, R.; Stewart, A.K.; Dispenzieri, A.; Hussein, M.A.; Zonder, J.A.; Crowley, J.; Hoering, A.; Barlogie, B.; et al. Disease and Outcome Disparities in Multiple Myeloma: Exploring the Role of Race/Ethnicity in the Cooperative Group Clinical Trials. Blood Cancer J. 2018, 8, 67. [Google Scholar] [CrossRef]
- Kazandjian, D.; Hill, E.; Hultcrantz, M.; Rustad, E.H.; Yellapantula, V.; Akhlaghi, T.; Korde, N.; Mailankody, S.; Dew, A.; Papaemmanuil, E.; et al. Molecular Underpinnings of Clinical Disparity Patterns in African American vs. Caucasian American Multiple Myeloma Patients. Blood Cancer J. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.L.; Han, L.; Kodali, S.; Aptekmann, A.; Carter-Bawa, L.; Feinman, R.; Tycko, B.; Siegel, D.S.; Goy, A.; Kaplan, P.; et al. Genetic Ethnicity and Hypertension Epistatic Interaction Underlying Racial Disparities in US Multiple Myeloma Susceptibility. medRxiv 2024. [Google Scholar] [CrossRef]
- Pierre, A.; Williams, T. African American Patients with Multiple Myeloma: Optimizing Care to Decrease Racial Disparities. CJON 2020, 24, 439–443. [Google Scholar] [CrossRef]
- Guadamuz, J.S.; Rohrer, R.; Mohyuddin, G.R.; Chiu, B.C.-H.; Patel, P.R.; Sweiss, K.; Seymour, E.; Sborov, D.; Calip, G.S. Abstract 3669: Racial/Ethnic Disparities in Treatment with Bone-Modifying Agents among Newly Diagnosed Multiple Myeloma Patients. Cancer Res. 2022, 82, 3669. [Google Scholar] [CrossRef]
- Jasaraj, R.; Gaire, S.; Proskuriakova, E.; Jasaraj, R.; Keruakous, A.R. Racial Bias in Multiple Myeloma Clinical Trials: Racial Minorities Continue to Miss out on Treatment Advances. JCO 2023, 41, e20009. [Google Scholar] [CrossRef]
- Kelly, K.R.; Yang, D.; Sharma, M.; Roy, V.; Chanan-Khan, A.A.; Ailawadhi, S. Persistent Racial/Ethnic Disparities in Outcomes for Multiple Myeloma: A SEER-Database Update. Blood 2016, 128, 1191. [Google Scholar] [CrossRef]
- Jain, T.; Fonseca, R.; Chen, R.; Patel, R.; Jani, P.; De La Calle, V.G.; Meghji, Z.; Hoffman, J.E.; Alencar, A.J.; Kelly, K.R.; et al. Racial Differences in Disease Characteristics: Understanding Multiple Myeloma in Hispanics. Blood 2017, 130, 864. [Google Scholar] [CrossRef]
- Moreau, P.; Kumar, S.K.; San Miguel, J.; Davies, F.; Zamagni, E.; Bahlis, N.; Ludwig, H.; Mikhael, J.; Terpos, E.; Schjesvold, F.; et al. Treatment of Relapsed and Refractory Multiple Myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021, 22, e105–e118. [Google Scholar] [CrossRef]
- Miguel, J.F.S.; Mateos, M.-V. Current Challenges in the Management of Patients with Relapsed/Refractory Multiple Myeloma. Oncology 2011, 25 (Suppl. S2), 10–18. [Google Scholar]
- Offidani, M.; Corvatta, L.; Morabito, F.; Gentile, M.; Musto, P.; Leoni, P.; Palumbo, A. How to Treat Patients with Relapsed/Refractory Multiple Myeloma: Evidence-Based Information and Opinions. Expert Opin. Investig. Drugs 2011, 20, 779–793. [Google Scholar] [CrossRef]
- Ni, B.; Hou, J. Promising Therapeutic Approaches for Relapsed/Refractory Multiple Myeloma. Hematology 2022, 27, 343–352. [Google Scholar] [CrossRef]
- Richardson, P.G.; Laubach, J.P.; Schlossman, R.L.; Ghobrial, I.M.; Redman, K.C.; Mckenney, M.; Warren, D.; Noonan, K.; Lunde, L.; Doss, D.; et al. The Potential Benefits of Participating in Early-phase Clinical Trials in Multiple Myeloma: Long-term Remission in a Patient with Relapsed Multiple Myeloma Treated with 90 Cycles of Lenalidomide and Bortezomib. Eur. J. Haematol. 2012, 88, 446–449. [Google Scholar] [CrossRef]
- Clark, C.A.; Cornell, R.F.; Scott, E.C.; Chung, J.; Costa, L.J. Management of Relapsed and Refractory Multiple Myeloma in Modern Times: Incorporating New Agents into Decision-making. Am. J. Hematol. 2016, 91, 1044–1051. [Google Scholar] [CrossRef]
| Feature | Linear Evolution | Branching Evolution |
|---|---|---|
| Definition | Stepwise accumulation of mutations in a single dominant clone | Multiple subclones diverge from a common ancestor and evolve in parallel |
| Clonal dynamics | One clone sequentially replaces the previous one (clonal sweeps) | Several clones coexist and compete; dominance can shift over time |
| Genetic trajectory | Unidirectional with sequential driver mutations | Diverse mutational paths; subclones may acquire different mutations independently |
| Temporal pattern | Progressive and ordered | Non-linear and dynamic |
| Driver mutation Distribution | Accumulated in a linear fashion within a single lineage | Found in distinct subclones; can appear independently |
| Influence of therapy | Selective pressure can accelerate emergence of the next dominant clone | Selective pressure alters subclone proportions; resistant clones may expand |
| Clinical presentation | Often associated with more aggressive disease progression and shorter survival | More heterogeneous presentation; some subclones may respond differently to therapy |
| Frequency in MM | Less common (~15–25%) | More common (~60–70%) |
| Detection | Easier to detect using bulk sequencing due to dominant clone | Requires single-cell sequencing or longitudinal sampling to fully resolve |
| Example markers | TP53 inactivation, high mutational load | Divergent RAS mutations in separate subclones |
| Prognostic implication | Often associated with poor prognosis and rapid relapse | Variable prognosis; can enable early detection of resistance pathways |
| Therapeutic implications | May benefit from aggressive upfront treatment strategies | Supports adaptive or combination therapy approaches to target multiple clones |
| Component | Cell Type/Molecule | Role in MM Pathogenesis | Key Molecular Mechanisms/Pathways |
|---|---|---|---|
| Stromal cells | Bone marrow stromal cells (BMSCs) | Promote MM cell survival, proliferation, and drug resistance | IL-6, SDF-1/CXCL12 → CXCR4, adhesion molecules (VCAM-1, VLA-4), NF-κB activation |
| Osteoclasts | Multinucleated bone-resorbing cells | Promote bone destruction and release growth factors for MM cells | RANK/RANKL, M-CSF, IL-6, TGF-β; release of IGF-1 and calcium enhances MM growth |
| Osteoblasts | Bone-forming cells | Suppressed in MM, leading to bone lesions | Inhibition via DKK1, sFRP2, and activin A; Wnt pathway inhibition |
| Endothelial cells | Vascular endothelial cells | Promote angiogenesis and MM progression | VEGF, FGF-2, HIF-1α pathways; MM cells stimulate angiogenic switch |
| Immune cells—Myeloid lineage | Myeloid-derived suppressor cells (MDSCs) | Suppress anti-MM immunity, support MM cell survival | Arginase-1, ROS, IL-10, TGF-β; inhibit cytotoxic T cells and NK cells |
| Immune cells—Lymphoid lineage | Regulatory T cells (Tregs) | Inhibit effective immune responses against MM | IL-10, TGF-β, CTLA-4; suppression of CD8+ T-cell activation |
| Dendritic cells | Antigen-presenting cells | Impaired antigen presentation; promote immune tolerance in MM | Downregulation of costimulatory molecules, IL-6, IDO expression |
| Macrophages | Tumor-associated macrophages (TAMs) | Support MM growth and immune evasion | IL-6, TNF-α, CD163 expression; M2 polarization promotes tumor-supportive environment |
| Mesenchymal stem cells (MSCs) | Multipotent stromal progenitors | Give rise to BMSCs; modulate inflammation and MM niche | Secretion of IL-6, CXCL12; direct interactions with MM cells via adhesion molecules |
| Adipocytes | Bone marrow fat cells | Provide metabolic support and cytokines for MM cells | Leptin, adiponectin, IL-6, lipolysis-derived fatty acids; affect metabolism and resistance |
| Extracellular matrix (ECM) | Fibronectin, collagen, laminin, hyaluronan | Promotes MM adhesion, survival, and drug resistance | Integrins (VLA-4/VCAM-1), FAK, PI3K/AKT, ERK signaling; CAM-DR (cell adhesion-mediated drug resistance) |
| Cytokines and chemokines | IL-6, IL-10, TGF-β, SDF-1 (CXCL12), TNF-α | Mediate pro-survival, pro-migratory, and immunosuppressive signals | Activation of JAK/STAT3, MAPK, NF-κB, and PI3K/AKT pathways |
| Growth factors | VEGF, IGF-1, BAFF, APRIL, FGF | Enhance angiogenesis, MM cell proliferation, and immune evasion | Binding to receptors on MM cells (e.g., IGF-1R, VEGFR), MAPK/ERK and AKT activation |
| Exosomes/extracellular vesicles | Secreted by MM cells and stromal cells | Mediate intercellular communication, resistance, and immune modulation | Transfer of miRNAs, proteins; enhance angiogenesis, suppress T-cell function |
| Feature | Hyperdiploid Multiple Myeloma (H-MM) | Non-Hyperdiploid Multiple Myeloma (NH-MM) |
|---|---|---|
| Definition | Presence of trisomies (usually odd-numbered chromosomes) | Characterized by structural abnormalities, especially IgH translocations |
| Common cytogenetic features | Trisomies of chromosomes 3, 5, 7, 9, 11, 15, 19, 21 | IgH translocations: t(11;14), t(4;14), t(14;16), t(14;20), t(6;14) |
| Frequency | ~50–60% of newly diagnosed MM | ~30–40% of newly diagnosed MM |
| Oncogenic mechanism | Gene dosage effect from trisomies | Oncogene activation via enhancer hijacking (e.g., CCND1, FGFR3/MMSET, MAF) |
| Gene expression profile | Enrichment in genes linked to protein synthesis and oxidative phosphorylation | Enrichment in cell cycle, proliferation, and oncogenic transcription factors |
| MYC deregulation | Less frequent | More frequent, especially in t(4;14), t(14;16) subtypes |
| Cyclin D deregulation | Via gene dosage (CCND1, CCND2) | Via IgH translocations (e.g., t(11;14) → CCND1 overexpression) |
| Prognosis | Generally more favorable | Variable, often poorer (especially with t(4;14), del(17p), or 1q gain) |
| Associated risk abnormalities | Fewer high-risk features | More frequent del(17p), 1q gain, and complex karyotypes |
| Response to therapy | Good response to standard therapy | Often requires risk-adapted or novel agents (e.g., bortezomib in t(4;14)) |
| Microenvironmental interaction | Less dependent on niche signaling | Greater dependence on IL-6 and stromal support in early clones |
| Progression pattern | Typically slower progression from MGUS | Can have rapid evolution from SMM or de novo aggressive disease |
| Common clinical features | Older age, indolent course | Younger patients more common; higher tumor burden and extramedullary disease |
| Translocation | Partner Gene/Locus | Frequency | Molecular Consequence | Associated Clinical Features/Risk |
|---|---|---|---|---|
| t(11;14)(q13;q32) | CCND1 (cyclin D1) | ~15–20% | Overexpression of cyclin D1, promoting G1/S cell cycle entry | Standard-risk; enriched in light chain amyloidosis, lymphoplasmacytic morphology, and BCL-2 dependency (venetoclax-responsive) |
| t(4;14)(p16;q32) | FGFR3/MMSET (NSD2) | ~15% | Dual activation: FGFR3 (MAPK signaling) and MMSET (epigenetic modulation) | High-risk; associated with poor prognosis, early relapse, and benefit from bortezomib-based therapy |
| t(14;16)(q32;q23) | MAF (v-maf musculoaponeurotic fibrosarcoma) | ~5–7% | Upregulation of MAF transcription factor, altering adhesion and migration genes | High-risk; associated with extramedullary disease and plasma cell leukemia-like features |
| t(14;20)(q32;q12) | MAFB | <2% | Overexpression of MAFB, disrupting plasma cell differentiation | High-risk; rare but aggressive, with poor response to conventional therapy |
| t(6;14)(p21;q32) | CCND3 (cyclin D3) | <1–2% | Overexpression of cyclin D3, promoting cell cycle progression | Rare; clinical significance not fully established; intermediate prognosis |
| t(8;14)(q24;q32) | MYC (secondary event) | ~15–30% (progression) | Enhancer hijacking leads to MYC overexpression | Secondary translocation in disease progression; associated with high tumor burden and relapse |
| t(9;14)(p13;q32) | PAX5 | Very rare | Disrupts B-cell differentiation pathway | Extremely rare in MM; more common in lymphomas |
| Feature | KRAS Mutation | NRAS Mutation | BRAF Mutation |
|---|---|---|---|
| Gene location | Chromosome 12p12.1 | Chromosome 1p13.2 | Chromosome 7q34 |
| Frequency in MM | ~20–25% | ~20–25% | ~4–8% |
| Common mutation hotspots | G12D, G12V, G13D, Q61H | Q61K, Q61R, G12D, G13R | V600E (most common), K601N |
| Activation effect | Constitutive activation of MAPK and PI3K/AKT | Preferential activation of MAPK signaling | Strong MAPK pathway activation via MEK/ERK |
| Pathway preference | Both MAPK and PI3K pathways | MAPK (ERK-driven proliferation) | MAPK pathway exclusively |
| Mutual exclusivity | Generally mutually exclusive with NRAS/BRAF | Rare co-occurrence with KRAS/BRAF | Usually exclusive of RAS mutations |
| Prognostic impact | Variable; may be associated with relapse | May be associated with early progression | V600E associated with poor prognosis and high-risk disease |
| Clonal distribution | Often subclonal at diagnosis, may become clonal at relapse | Often clonal and persistent through disease course | Can be early or late event |
| Functional role in MM | Promotes cell proliferation, resistance to apoptosis | Drives proliferation and survival | Promotes rapid proliferation and metabolic changes |
| Response to MEK inhibitors | Variable; better response in dual PI3K/MEK inhibition | Similar to KRAS; requires combination therapy | More responsive to BRAF/MEK inhibitors (e.g., vemurafenib + cobimetinib) |
| Preclinical targetability | Requires combined targeting of PI3K and MEK | Limited success with MEK inhibitors alone | V600E mutation shows sensitivity to BRAF inhibitors in vitro |
| Clinical trial implications | Enriched in biomarker-driven MEK/AKT inhibitor studies | Evaluated in MAPK pathway-targeted trials | Enrolled in BRAF inhibitor trials [64] (e.g., vemurafenib [VEM] + cobimetinib [COBI]) |
| Feature | MGUS | SMM | MM |
|---|---|---|---|
| Clonal bone marrow plasma cells (BMPCs) | <10% | ≥10% to <60% | ≥10% or biopsy-proven plasmacytoma |
| M-protein in serum (if present) | <3 g/dL | ≥3 g/dL (if present) or urinary M-protein ≥500 mg/24 h | Any level (often >3 g/dL) |
| Myeloma-defining Events | Absent | Absent | Present (≥1 CRAB or SLiM criteria, see below) |
| CRAB features (myeloma-defining) | None | None | At least one of the following: |
| - C: hypercalcemia (Ca >11 mg/dL or >1 mg/dL above normal) | |||
| - R: renal insufficiency (Cr > 2 mg/dL or CrCl < 40 mL/min) | |||
| - A: anemia (Hb < 10 g/dL or >2 g/dL below normal) | |||
| - B: bone lesions (≥1 osteolytic lesion on imaging) | |||
| SLiM biomarkers (added in 2014) | Not applicable | Not applicable | Presence of any of the following (even without CRAB): |
| - S: ≥60% clonal BMPCs | |||
| - Li: involved/uninvolved serum free light chain (FLC) ratio ≥100 | |||
| - M: >1 focal lesion ≥5 mm on MRI | |||
| End-organ damage | Absent | Absent | Present or imminent (CRAB or SLiM features) |
| Progression risk (2-year) | ~1% | ~10% (standard SMM) to ~50% (high-risk SMM) | 100% (active disease requiring treatment) |
| Recommended management | Observation; no treatment | Observation or clinical trial (if high-risk) | Initiate systemic therapy |
| Feature | International Staging System (ISS) | Revised International Staging System (R-ISS) | R2-ISS/NGS-Enhanced or FISH-Enhanced Systems [110] |
|---|---|---|---|
| Year Introduced | 2005 | 2015 | Emerging (post-2020), based on genomic insights |
| Primary Purpose | Estimate prognosis using basic lab values | Refine prognosis by integrating cytogenetics and LDH | Improve risk stratification using NGS and/or advanced FISH data |
| Parameters Used | - Serum β2-microglobulin - Serum albumin | - ISS components - Chromosomal abnormalities (via FISH) - LDH level | - R-ISS components - TP53 mutation status - 1q gain, del(1p), RAS/BRAF mutations, others |
| Genetic/cytogenetic input | None | Yes (FISH): del(17p), t(4;14), t(14;16) | Yes (NGS/FISH): includes extended high-risk markers (e.g., 1q gain, biallelic TP53 loss) |
| LDH incorporation | No | Yes | Yes |
| Stages defined | - Stage I: β2M < 3.5 mg/L and serum albumin ≥ 3.5 g/dL - Stage II: β2M < 3.5 mg/L; serum albumin < 3.5 g/dL; or β2M 3.5 to 5.5 mg/L, irrespective of serum albumin - Stage III: β2M ≥ 5.5 mg/L | - Stage I: ISS I + no high-risk FISH + normal LDH - Stage II: not R-ISS stage I or III - Stage III: ISS III + high-risk FISH and/or high LDH | - More granular stratification (e.g., 4 groups in R2-ISS) - Assigns scores to each risk marker for cumulative risk |
| Risk categories | 3 tiers (I, II, III) | 3 tiers (I, II, III) | Often 4 tiers (e.g., R2-ISS groups I–IV) |
| Prognostic discrimination | Moderate | Improved over ISS | Superior; captures genetic heterogeneity and clonal complexity |
| Clinical usefulness | Still used for baseline staging | Widely adopted in clinical trials and risk stratification | Emerging in precision medicine; useful in high-risk MM trials |
| Limitations | Lacks genetic insight | Does not account for 1q gain, TP53 mutation, or multiple abnormalities | Requires access to NGS and extended FISH; not yet fully standardized |
| Examples of added markers | N/A | FISH only: del(17p), t(4;14), t(14;16) | NGS/FISH: TP53 mutations, biallelic inactivation, gain(1q21), del(1p), RAS/BRAF mutations, complex karyotype |
| Progression-free survival (PFS) and OS prediction | Limited precision | Better at stratifying high-risk patients | Most accurate; enables personalized risk-adapted therapy |
| Feature | Next-Generation Sequencing (NGS) | Next-Generation Flow Cytometry (NGF) | Allele-Specific Oligonucleotide PCR (ASO-PCR) | Imaging (PET/CT, MRI) |
|---|---|---|---|---|
| Technology basis | DNA sequencing of unique IgH rearrangements | Multiparameter flow cytometry with >8–10 color panels | PCR amplification of patient-specific IgH gene rearrangement | Functional/metabolic (PET/CT) and anatomical (MRI) imaging |
| Sample type | Bone marrow (BM) aspirate | Bone marrow aspirate | Bone marrow aspirate | Whole-body (bone marrow and extramedullary sites) |
| Sensitivity | Up to 10−6 (1 cell in 1 million) | Up to 10−5 to 10−6 | ~10−5 | ~10−2 to 10−3 (lower sensitivity) |
| Standardization | Highly standardized (e.g., Adaptive ClonoSEQ®) | Standardized by EuroFlow consortium | Requires individualized primers (complex setup) | Variable depending on protocol and scanner |
| Turnaround time | Moderate (few days to 1–2 weeks) | Fast (24–48 h) | Slow (requires pretreatment sample) | Immediate (PET/CT ~same day; MRI may take longer) |
| Clonality requirement | Requires identification of clone at diagnosis | Not needed | Requires diagnostic sample for primer design | Not applicable |
| Applicability | Applicable in ≥90% of patients | Applicable in virtually all patients | Applicable in ~60–70% | Applicable in all patients |
| Cost and accessibility | Expensive; limited to specialized labs | Moderate cost; increasingly available in tertiary centers | Cost-effective but labor-intensive | Expensive; PET/CT requires radiotracers |
| Advantages | Highest sensitivity; highly quantitative; standardized | Real-time detection; rapid; does not require diagnostic sample | Highly sensitive; cost-effective for known clone | Detects extramedullary disease and focal lesions missed by BM sampling |
| Limitations | Requires diagnostic sample; BM only; not real-time | Limited to BM; may miss patchy or extramedullary disease | Technically demanding; not standardized; limited utility | Low sensitivity for minimal disease; can yield false positives due to inflammation |
| Detects extramedullary disease | No | No | No | Yes |
| Role in clinical trials | Used in multiple trials as MRD endpoint (e.g., FORTE, GRIFFIN) | Widely used in European MRD studies | Rarely used in modern trials | Used for imaging-based response and disease mapping |
| Regulatory approval | FDA-cleared (ClonoSEQ for MRD in MM) | CE-marked (EuroFlow) | Not FDA-approved for MRD | Standard clinical use for imaging; not specific for MRD |
| Integration with IMWG MRD criteria | Yes | Yes | Partially (less common) | Yes (for imaging-defined MRD negativity) |
| Feature | PET/CT (FDG-PET/CT) | MRI (Whole-Body or Spine/Pelvis) | WBLDCT (Whole-Body Low-Dose CT) |
|---|---|---|---|
| Imaging modality | Functional (metabolic) + anatomical imaging | Anatomical + functional (tissue contrast, edema detection) | Anatomical (bone structure) only |
| Radiotracer or contrast | 18F-FDG (fluorodeoxyglucose) | None required (but gadolinium can be used) | No contrast or radiotracer required |
| Radiation exposure | Moderate (higher than WBLDCT) | None | Low-dose radiation |
| Sensitivity for bone lesions | Moderate to high (especially for active lesions) | Highest for diffuse and focal marrow infiltration | High for osteolytic lesions ≥5 mm |
| Sensitivity for early disease | Less sensitive for non-metabolically active lesions | Best modality for early bone marrow involvement | Poor at detecting marrow infiltration before bone destruction |
| Detection of extramedullary disease | Excellent | Limited to soft tissue near bones | Not reliable |
| Detection of focal lesions | Yes (when metabolically active) | Yes (even before bone destruction) | Yes (if lytic and ≥5 mm) |
| Detection of diffuse infiltration | Limited | Yes (via signal intensity on STIR/DWI sequences) | No |
| Detection of healing or inactive lesions | No (FDG-negative) | Yes (but may not distinguish active from inactive) | Yes (can see healed lesions, but not activity) |
| Use in MRD evaluation | Yes (for imaging-defined MRD negativity) | Yes (e.g., ≥1 focal lesion ≥5 mm = SLiM criterion) | No (not part of IMWG MRD criteria) |
| Turnaround time | Moderate (requires radiotracer synthesis and uptake time) | Fast (30–60 min; more with contrast) | Fast (10–15 min) |
| Availability | Moderate (depends on PET facility) | Widely available in tertiary centers | Widely available |
| Limitations | False negatives in low-FDG-avid MM; false positives from inflammation | Expensive; time-consuming; contraindicated in severe renal failure (if contrast used) | Can miss early or non-lytic disease |
| Advantages | Identifies active lesions and extramedullary disease | Best for early marrow disease, ideal for SLiM criteria | Best for osteolytic lesion detection; quick and accessible |
| Regimen | Components | Patient Population | Mechanism of Action | Key Clinical Trials | Response Rates/PFS | Special Considerations |
|---|---|---|---|---|---|---|
| VRd | Bortezomib (V) + lenalidomide (R) + dexamethasone (d) | Transplant-eligible and -ineligible | PI + IMiD + steroid | SWOG S0777, IFM 2009 | ORR ~90%, CR ~30–40%, median PFS ~43–50 months | Standard of care; neuropathy with bortezomib SC > IV preferred |
| Dara-VRd | Daratumumab (Dara) + VRd | Transplant-eligible (per FDA/EMA) | Anti-CD38 mAb + PI + IMiD + steroid | GRIFFIN, PERSEUS | sCR ~60%, MRD negativity ≥60%, PFS not reached (superior to VRd) | More infusion time initially; improved depth of response |
| KRd | Carfilzomib (K) + lenalidomide (R) + dexamethasone (d) | Transplant-eligible and fit patients | 2nd-gen PI + IMiD + steroid | FORTE, ENDURANCE | ORR >90%, CR ~40%, MRD negativity ~30–50% | Less neuropathy; more cardiovascular risk than bortezomib |
| Dara-KRd | Daratumumab + KRd | Transplant-eligible (under investigation) | Anti-CD38 + PI + IMiD + steroid | MASTER, MANHATTAN | MRD negativity >80% in MASTER trial | Deepest responses to date; still investigational in frontline |
| VTD | Bortezomib + thalidomide + dexamethasone | Transplant-eligible (Europe) | PI + IMiD + steroid | IFM studies | ORR ~85%, CR ~25%, median PFS ~30–35 months | Thalidomide: more neurotoxicity; replaced by lenalidomide in many settings |
| Dara-VTD | Daratumumab + VTD | Transplant-eligible (Europe) | Anti-CD38 + PI + IMiD + steroid | CASSIOPEIA | sCR 39% vs. 26%; MRD negativity 64% with Dara-VTD | Approved in Europe as standard frontline for transplant-eligible patients |
| Rd | Lenalidomide + dexamethasone | Transplant-ineligible, frail, or elderly patients | IMiD + steroid | FIRST (MM-020) | PFS ~26 months (continuous Rd) | Better tolerated than triplets; standard for frail or elderly |
| Dara-Rd | Daratumumab + Rd | Transplant-ineligible (standard of care) | Anti-CD38 + IMiD + steroid | MAIA | ORR ~93%, MRD negativity >30%, PFS not reached (5+ years) | Superior to Rd; preferred triplet for non-transplant-eligible patients |
| VMP | Bortezomib + melphalan + prednisone | Transplant-ineligible (Europe, elderly) | PI + alkylator + steroid | VISTA | PFS ~24 months | Used mostly in countries without lenalidomide access |
| Dara-VMP | Daratumumab + VMP | Transplant-ineligible | Anti-CD38 + PI + alkylator + steroid | ALCYONE | sCR ~43%, MRD negativity ~27%, median PFS ~36 months | Approved for elderly/NTI; limited in U.S. use |
| Ixazomib-Rd | Ixazomib (oral PI) + Rd | Transplant-ineligible, elderly patients | Oral PI + IMiD + steroid | TOURMALINE-MM2 (did not meet PFS endpoint) | Slightly better PFS than Rd; not statistically significant | All-oral regimen; more convenient but less effective than Dara-Rd |
| Therapy Type | Regimen/Drug | Patient Population | Mechanism of Action | Key Trials | Outcomes (PFS/OS) | Special Considerations |
|---|---|---|---|---|---|---|
| Maintenance | Lenalidomide | Post-ASCT; also in non-transplant patients | IMiD; enhances T-cell/NK function, anti-angiogenic | CALGB 100104, IFM 2005-02, Myeloma XI | Median PFS benefit: +18–24 months; OS benefit confirmed | Most widely used; risk of secondary malignancies (SPMs ~7–9%) |
| Maintenance | Bortezomib (every 2 weeks SC) | High-risk cytogenetics (e.g., del(17p), t(4;14)) | Proteasome inhibition → apoptosis | HOVON-65/GMMG-HD4 | Improved PFS and OS in high-risk MM | Better tolerability SC vs. IV; neuropathy risk |
| Maintenance | Ixazomib (oral) | Post-ASCT, especially for convenience | Oral proteasome inhibitor | TOURMALINE-MM3/MM4 | PFS improved (~26.5 vs. 21.3 months); no OS benefit yet | All-oral; fewer logistics; mild side effect profile |
| Maintenance | Daratumumab (monotherapy or with IMiD) | Emerging; studied in high-risk and standard-risk | Anti-CD38 monoclonal antibody | CASSIOPEIA part 2, AURIGA (ongoing) | Early data promising; deepens MRD negativity | SC formulation preferred; infusion reactions mainly in induction |
| Maintenance | Combination (lenalidomide + bortezomib) | High-risk patients post-ASCT | Dual targeting: IMiD + PI | FORTE, EMN02/HO95 | Better MRD negativity; improved PFS in high-risk patients | Considered for double-hit or high-risk cytogenetics |
| Consolidation | VRd (2–4 cycles post-ASCT) | Transplant-eligible, especially with suboptimal response | PI + IMiD + steroid | IFM/DFCI 2009, EMN02/HO95 | Improved depth of response; modest PFS benefit | Useful if no CR/sCR post-ASCT |
| Consolidation | KRd (carfilzomib-based) | Post-ASCT, high-risk or standard-risk | 2nd-gen PI + IMiD + steroid | FORTE (KRd vs. no consolidation) | Higher MRD negativity, PFS advantage | Better tolerated in younger/fit patients |
| Consolidation | Dara-VTd/Dara-KRd | Under investigation post-ASCT | Anti-CD38-based combinations | GMMG-CONCEPT, MASTER, MANHATTAN | Deep MRD negativity (>80% in MASTER) | Still investigational; tailored to MRD-guided therapy |
| Maintenance (SMM) | Lenalidomide ± dexamethasone | High-risk smoldering MM | Delays progression to active MM | ECOG E3A06, ASCENT-SMM | 3-year PFS ~90% vs. ~60% (control) | For selected high-risk SMM patients under clinical trial protocols |
| Aspect | Standard-Risk MM | High-Risk MM |
|---|---|---|
| Definition | Absence of high-risk cytogenetic features or clinical markers | Presence of high-risk cytogenetics (e.g., del(17p), t(4;14), t(14;16), gain 1q), or double-hit MM (e.g., biallelic TP53) |
| Common cytogenetic features | Trisomies (hyperdiploid), t(11;14), no 1q gain | del(17p), t(4;14), t(14;16), gain(1q21), del(1p), complex karyotype, TP53 mutation |
| Initial risk assessment tools | FISH, R-ISS | R2-ISS, NGS, GEP, FISH |
| Frontline induction therapy | Triplet regimens (e.g., VRd, Dara-VRd) | Intensified regimens (e.g., KRd, Dara-KRd, quadruplets) |
| Stem cell transplant | Single ASCT | Early ASCT recommended; tandem ASCT considered in some high-risk cases |
| Consolidation therapy | Optional; may use short course of VRd post-ASCT | Recommended (e.g., KRd, Dara-KRd) to deepen response |
| Maintenance therapy | Lenalidomide monotherapy | Bortezomib-based (e.g., bortezomib ± lenalidomide); consider dual-agent maintenance |
| MRD monitoring | MRD monitoring used, but not always guiding therapy | Essential; MRD status guides continuation or intensification |
| Treatment goals | Durable remission; manageable toxicity | Achieve and sustain MRD negativity; delay clonal evolution |
| Role of clinical trials | Optional; used for access to novel agents | Strongly encouraged to access cutting-edge immunotherapy/targeted regimens |
| Preferred immunotherapy options | Anti-CD38 mAbs (e.g., daratumumab) in standard regimens | Early use of immunotherapy (e.g., Dara, BiTEs, CAR-T in trials) |
| Relapse management | Sequential therapy (PI/IMiD/monoclonal) | Early use of novel agents (e.g., selinexor, CAR-T, bispecifics) |
| Prognosis | Median PFS > 5 years; OS > 8–10 years | Median PFS ~2–3 years; OS ~4–6 years (may vary by subtype) |
| Monitoring frequency | Routine labs every 1–3 months | More frequent labs, imaging, and MRD assessments |
| Emerging approaches | MRD-guided de-escalation (investigational) | MRD-guided intensification, genomics-based precision therapy |
| Feature | Daratumumab | Isatuximab | Elotuzumab |
|---|---|---|---|
| Target Antigen | CD38 | CD38 | SLAMF7 (CS1) |
| Type of Antibody | Fully human IgG1κ | Chimeric IgG1 | Humanized IgG1 |
| Mechanism of Action | - Direct apoptosis (crosslinking) - CDC, ADCC, ADCP - Immune modulation (Treg depletion) | - Direct apoptosis (even without crosslinking) - ADCC, CDC, ADCP - Inhibits CD38 ectoenzyme | - Enhances NK cell activation via SLAMF7 - ADCC (no direct cytotoxicity) |
| FDA Approval (MM) | Yes (monotherapy and in combinations, frontline and relapsed) | Yes (in combination regimens for relapsed MM) | Yes (only in combination with IMiDs for relapsed MM) |
| Common Combinations | - Dara-Rd (MAIA) - Dara-VRd (GRIFFIN) - Dara-KRd - Dara-Pd (APOLLO) | - Isa-Rd (IKEMA) - Isa-Kd (IKEMA) | - Elo-Rd (ELOQUENT-2) - Elo-Pd (ELOQUENT-3) |
| Line of Use | - Frontline (NTI and transplant-eligible) - Relapsed/refractory (RRMM) | - RRMM (second line and beyond) | - RRMM (second line and beyond, not approved for frontline) |
| Route of Administration | IV and SC (subcutaneous) | IV only | IV only |
| Infusion Time | IV: ~3–6 h; SC: ~5 min | IV: ~3 h | IV: ~2–3 h |
| Infusion Reactions | ~40% (mostly first dose); lower with SC | ~35% first-dose reactions | ~10–15% (generally mild) |
| Effect on MRD | High MRD negativity when combined with IMiDs/PI | Comparable MRD negativity in IKEMA study | Not MRD-focused; less depth of response |
| Efficacy in High-Risk MM | Active; used in high-risk combinations | Active; included in some high-risk trials | Less data in high-risk subsets |
| Unique Features | - SC formulation reduces infusion burden - Depletes Tregs and Bregs | - Inhibits CD38 enzymatic function - More potent direct killing | - Synergizes with NK cells; no single-agent activity |
| Adverse Effects | Cytopenias, infections, IRRs, fatigue | Similar to daratumumab | Fatigue, diarrhea, cough, IRRs |
| Use with IMiDs or PIs | Works well with both IMiDs and PIs | Primarily used with IMiDs | Only approved with IMiDs (lenalidomide, pomalidomide) |
| Trial Highlights | - MAIA (Dara-Rd) - GRIFFIN (Dara-VRd) - CASSIOPEIA (Dara-VTd) | - IKEMA (Isa-Kd) - ICARIA-MM (Isa-Pd) | - ELOQUENT-2 (Elo-Rd) - ELOQUENT-3 (Elo-Pd) |
| Feature | Idecabtagene Vicleucel (ide-cel; Abecma®) | Ciltacabtagene Autoleucel (cilta-cel; Carvykti®) |
|---|---|---|
| Target antigen | B-cell maturation antigen (BCMA) | BCMA |
| CAR structure | Single-chain variable fragment (scFv) | Two BCMA-binding domains (dual-epitope targeting scFvs) |
| CAR-T-cell generation | Lentiviral vector, autologous T cells | Lentiviral vector, autologous T cells |
| FDA approval | March 2021 | February 2022 |
| Indication | Triple-class refractory MM (≥4 prior lines) | Triple-class refractory MM (≥4 prior lines) |
| Key clinical trial | KarMMa (Phase 2) | CARTITUDE-1 (Phase 1b/2) |
| Overall response rate (ORR) | ~73% | ~98% |
| Stringent complete response (sCR) | ~33% | ~80% |
| Median progression-free survival | ~8.8 months (median follow-up 13.3 months) | Not reached at 27 months; PFS at 2 years: ~61% |
| Median overall survival | ~24.8 months | Not reached at 2 years; OS ~74% |
| Time to response | ~1 month | ~1 month |
| Time from apheresis to infusion | ~4–5 weeks | ~4–6 weeks |
| Toxicity—CRS (cytokine release syndrome) | ~84% (mostly Grade 1–2); Grade ≥3 in ~5% | ~95%; mostly Grade 1–2; Grade ≥3 in ~4% |
| Toxicity—ICANS (neurotoxicity) | ~18% (Grade ≥3: 3%) | ~21% (Grade ≥3: 10%) |
| Unique features | First FDA-approved BCMA CAR-T; robust data in heavily pretreated patients | Dual-epitope binding = higher avidity; very deep and durable responses |
| Dosing regimen | Single infusion of 150–450 × 106 CAR+ T cells | Single infusion of 0.75 × 106 CAR+ T cells/kg |
| Emerging directions | Used in KarMMa-3 (earlier lines); potential combinations | CARTITUDE-4: early relapse; being explored for frontline high-risk MM |
| Grade | Fever ≥ 38 °C | Hypotension | Hypoxia |
|---|---|---|---|
| Grade 1 | Present | None | None |
| Grade 2 | Present | Does not require vasopressors; responds to fluids | Requires low-flow oxygen (nasal cannula ≤6 L/min) |
| Grade 3 | Present | Requires vasopressors or multiple boluses | Requires high-flow oxygen, CPAP, or BiPAP |
| Grade 4 | Present | Requires multiple vasopressors ± ventilatory support | Requires mechanical ventilation |
| Grade | ICE Score | Level of Consciousness | Seizure | Motor Findings | Cerebral Edema |
|---|---|---|---|---|---|
| Grade 1 | 7–9 | Awaken spontaneously | None | None | None |
| Grade 2 | 3–6 | Awaken to voice | None | None | None |
| Grade 3 | 0–2 | Arousable with tactile stimulation | Any seizure | None | Focal/local edema in neuroimaging |
| Grade 4 | 0 | Unarousable or obtunded; stupor or coma | Life-threatening prolonged seizure | Deep focal motor weakness, such as hemiparesis or paraparesis | Diffuse cerebral edema in neuroimaging; decerebrate or decorticate |
| Vaccine Name/Platform | Antigen Target(s) | Vaccine Type | Clinical Phase/Status | Mechanism of Action | Key Studies/Notes |
|---|---|---|---|---|---|
| PVX-410 | XBP1, CD138, CS1 (SLAMF7) | Peptide-based multi-epitope vaccine | Phase I (completed) | Induces CD8+ T-cell responses against MM-associated antigens | Safe and immunogenic in SMM; studied alone and with lenalidomide (NCT01718899) |
| Bcl-2 family peptide vaccine | Bcl-2, Bcl-XL, Mcl-1 | Peptide-based vaccine | Phase I | Stimulates cytotoxic T cells targeting anti-apoptotic proteins | Combined with bortezomib; immunogenic with minimal toxicity |
| MAGE-A3 peptide vaccine | MAGE-A3 | Tumor antigen-specific peptide vaccine | Early phase/exploratory | Targets cancer–testis antigen expressed in MM and other malignancies | Used in pilot studies and as a possible post-ASCT strategy |
| Idiotype (Id) protein vaccine | Patient-specific paraprotein (Id) | Personalized protein vaccine | Phase I/II (historical interest) | Induces helper and cytotoxic T-cell responses against myeloma idiotype | Limited efficacy; used in post-ASCT settings; declining interest |
| DC/MM fusion vaccine | Whole-tumor antigens from patient myeloma cells | Dendritic cell (DC)-tumor fusion vaccine | Phase I/II | Enhances antigen presentation by fusing DCs with autologous MM cells | Shown to induce tumor-specific immunity post-ASCT (NCT02728102, NCT01067287) |
| Neoantigen-based vaccines | Patient-specific neoantigens | Personalized peptide/RNA vaccine | Preclinical/early phase | Stimulates T cells against mutation-specific targets unique to each patient | Requires whole-exome sequencing and epitope prediction |
| Gp96-Ig vaccine (ImPACT®) | Broad tumor antigen spectrum | Heat shock protein-based chaperone vaccine | Preclinical/exploratory | Cross-presents multiple antigens via HLA to CD8+ T cells | Used in MM and other hematologic malignancies |
| mRNA-based vaccines (exploratory) | Personalized neoantigens or MM-associated antigens | mRNA encoding tumor antigens | Preclinical | Induces cellular immunity via in vivo expression of encoded antigens | Emerging platform post-COVID-19 vaccine success |
| WT1 peptide vaccine | Wilms’ tumor 1 (WT1) | Peptide vaccine | Pilot/early phase | Targets overexpressed WT1 protein in MM | Studied in combination with other immunotherapies |
| NY-ESO-1 vaccine | NY-ESO-1 | Cancer–testis antigen peptide vaccine | Early phase | Induces T-cell response against highly immunogenic antigen | Under study in hematologic and solid tumors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutanto, H.; Romadhon, P.Z.; Fatmawati, V.R.; Waitupu, A.; Ansharullah, B.A.; Rachma, B.; Elisa, E.; Pratiwi, L.; Adytia, G.J. Multiple Myeloma and Precursor Plasma Cell Disorders: From Emerging Driver Mutations to Current and Future Therapeutic Strategies. Hemato 2025, 6, 29. https://doi.org/10.3390/hemato6030029
Sutanto H, Romadhon PZ, Fatmawati VR, Waitupu A, Ansharullah BA, Rachma B, Elisa E, Pratiwi L, Adytia GJ. Multiple Myeloma and Precursor Plasma Cell Disorders: From Emerging Driver Mutations to Current and Future Therapeutic Strategies. Hemato. 2025; 6(3):29. https://doi.org/10.3390/hemato6030029
Chicago/Turabian StyleSutanto, Henry, Pradana Zaky Romadhon, Vembi Rizky Fatmawati, Alief Waitupu, Bagus Aditya Ansharullah, Betty Rachma, Elisa Elisa, Laras Pratiwi, and Galih Januar Adytia. 2025. "Multiple Myeloma and Precursor Plasma Cell Disorders: From Emerging Driver Mutations to Current and Future Therapeutic Strategies" Hemato 6, no. 3: 29. https://doi.org/10.3390/hemato6030029
APA StyleSutanto, H., Romadhon, P. Z., Fatmawati, V. R., Waitupu, A., Ansharullah, B. A., Rachma, B., Elisa, E., Pratiwi, L., & Adytia, G. J. (2025). Multiple Myeloma and Precursor Plasma Cell Disorders: From Emerging Driver Mutations to Current and Future Therapeutic Strategies. Hemato, 6(3), 29. https://doi.org/10.3390/hemato6030029






