Abstract
Background/Objectives: Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the BCR–ABL fusion gene, whose constitutive tyrosine kinase activity drives leukemogenesis. Although tyrosine kinase inhibitors (TKIs) have revolutionized treatment, drug resistance and leukemic stem cell persistence remain major challenges. Natural compounds such as polyphenols have shown potential in modulating key oncogenic pathways in CML. Results: Polyphenols such as resveratrol, quercetin, curcumin, and epigallocatechin gallate (EGCG) demonstrated significant antiproliferative and pro-apoptotic effects in CML cell lines, including imatinib-resistant models. These effects were mediated through the modulation of signaling pathways, including PI3K/Akt, STAT5, and MAPK; inhibition of BCR–ABL expression; induction of oxidative stress; and the enhancement of apoptosis via mitochondrial and caspase-dependent mechanisms. Some polyphenols also showed synergistic activity with TKIs, potentiating their efficacy and overcoming resistance. Conclusions: Preclinical evidence supports the role of polyphenols as potential adjuvants in CML therapy, particularly in drug-resistant contexts. Their pleiotropic molecular actions and low toxicity profile make them promising candidates for integrative oncology. Nonetheless, clinical translation requires further investigation through well-designed trials assessing efficacy, safety, and pharmacokinetics.
1. Introduction
Chronic myeloid leukemia (CML) is a hematological malignancy characterized by the presence of the Philadelphia chromosome (Ph) and the BCR–ABL fusion protein [1]. This disease serves as a paradigm for understanding the molecular mechanisms of carcinogenesis and for the development of targeted therapies. Despite significant therapeutic advances achieved with tyrosine kinase inhibitors (TKIs), drug resistance and the persistence of leukemic stem cells (LSCs) remain major clinical challenges.
CML originates from a pluripotent hematopoietic stem cell that acquires a reciprocal translocation between chromosomes 9 and 22, resulting in the formation of the Ph chromosome and the consequent production of the constitutively active BCR–ABL1 tyrosine kinase [2]. This altered protein triggers an aberrant signaling cascade that promotes uncontrolled proliferation, inhibits apoptosis, and disrupts cell adhesion and immune response [3]. The clinical management of CML has been revolutionized by TKIs; however, approximately 20–30% of patients develop resistance or intolerance, and LSCs, often refractory to TKIs, constitute a pathological reservoir that is difficult to eradicate [4].
Polyphenols, a class of bioactive compounds of plant origin, have garnered increasing attention for their antioxidant, anti-inflammatory, and antitumor properties [5,6,7]. Recent scientific evidence suggests that these compounds may represent a complementary therapeutic strategy in the treatment of CML by modulating molecular mechanisms involved in cell proliferation, apoptosis, and BCR–ABL expression [8].
In particular, polyphenols such as resveratrol, quercetin, curcumin, and epigallocatechin gallate have been investigated for their ability to inhibit leukemic cell growth in preclinical models. They exert their effects through the modulation of signaling pathways such as PI3K/Akt, STAT5, and MAPK, which are frequently dysregulated in CML. Some of these compounds also exhibit synergistic effects with TKIs, enhancing their efficacy and overcoming certain forms of drug resistance. Moreover, their antioxidant activity helps to reduce the burden of reactive oxygen species (ROS), which are often elevated in leukemic cells, thereby mitigating oxidative stress associated with disease progression [9]. Interest in polyphenols is further reinforced by their abundance in the Mediterranean diet, a dietary pattern known for its protective effects against various chronic diseases, including cancer [10].
Among the various alternative approaches explored to overcome the limitations of TKI therapy, polyphenols stand out due to their multifaceted biological activities, low toxicity profile, and ability to target multiple oncogenic pathways simultaneously. Unlike many synthetic agents, polyphenols can modulate both BCR–ABL-dependent and independent mechanisms, addressing not only drug resistance but also the persistence of LSCs. Importantly, their relevance extends beyond TKI-resistant contexts: polyphenols have shown promising activity in general CML models, supporting their potential role in disease prevention, cytoreduction, and long-term disease control. Moreover, their ability to enhance the efficacy of existing TKIs without adding significant side effects makes them particularly appealing as adjunct therapies.
2. Chronic Myeloid Leukemia: Clinical and Molecular Characteristics
CML is a myeloproliferative neoplasm arising from the clonal transformation of a hematopoietic stem cell, characterized by the presence of the Philadelphia chromosome, resulting from the reciprocal translocation t(9;22)(q34;q11) and the consequent formation of the BCR–ABL fusion gene [11].
During chromosomal translocation, the ABL gene on chromosome 9 may break at one of three sites. However, in all cases, the alternative first exons (1b, 1a) of ABL are spliced out. The BCR gene on chromosome 22 contains three major breakpoint cluster regions: the major (M-BCR), minor (m-BCR), and micro (μ-BCR) [12,13,14].
The BCR–ABL gene encodes a chimeric 210-kDa protein (p210 BCR–ABL) with constitutive tyrosine kinase activity. It contains the BCR coiled-coil domain and ABL’s SH3, SH2, and kinase domains. Loss of ABL’s regulatory cap domain leads to constitutive kinase activation [15]. Two major isoforms of BCR–ABL exist: the p210 form, typically associated with CML and some cases of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), and the p190 form, more commonly found in Ph+ ALL [16].
BCR–ABL activates STAT, RAS, RAF, JNK, MYC, and AKT, promoting proliferation and survival. Members of the Src family kinases, especially Hck, play a key role in coupling BCR–ABL to STAT5 activation. It recruits inactive Hck via SH3/SH2, facilitating STAT5 interaction. BCR–ABL phosphorylates Hck, which then activates STAT5 via Tyr699/694 [17,18].
Persistent activity increases ROS, causing DNA damage and resistance [9]. Rac2 disrupts mitochondrial function via MRC-cIII, raising ROS in stem/progenitor cells. ROS derived from MRC-cIII promote oxidative DNA damage, triggering genomic instability and resulting in chromosomal aberrations and BCR–ABL mutations conferring TKI resistance [19]. Additionally, BCR–ABL alters metabolism and the marrow microenvironment to support leukemia [20]. In particular, it triggers a Warburg-like effect, with increased glucose uptake and lactate secretion [21].
Importantly, BCR–ABL was one of the first tyrosine kinases identified as directly involved in the pathogenesis of a human malignancy. Its kinase activity is critical for both the initiation and maintenance of the leukemic phenotype, and mutations impairing enzymatic function abolish its transforming potential, thereby blocking neoplastic proliferation [22].
Within the bone marrow microenvironment, leukemic progression is associated with dynamic alterations in stromal populations, including the expansion of arteriolar endothelial cells, chondrocytes, and mesenchymal stromal cells (MSCs), and the loss of sinusoidal endothelial and osteo-associated cells (Figure 1) [23].
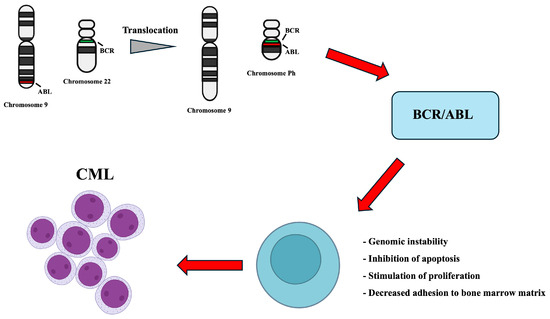
Figure 1.
Schematic representation of the reciprocal translocation between chromosomes 9 and 22 resulting in the BCR–ABL fusion gene. The chimeric BCR–ABL protein exhibits constitutive tyrosine kinase activity, aberrantly activating multiple intracellular signaling pathways. This leads to uncontrolled cell proliferation, apoptosis inhibition, genomic instability and altered cell adhesion, promoting oncogenic transformation, and the persistence of LSCs and MSCs.
Globally, the incidence of CML is estimated at 0.7–1.0 cases per 100,000 individuals annually, with a median age at diagnosis of 57–60 years and a male-to-female ratio of 1.2–1.7 [24,25].
CML typically progresses through three clinical phases: chronic, accelerated, and blast crisis. Chronic phase often has mild or no symptoms like fatigue, splenomegaly, and leukocytosis [26,27].
Progression to advanced phases brings more blasts, cytopenias, and resistance. It is driven by additional mutations, gene inactivation, or BCR–ABL amplification. The blast phase mimics acute leukemia and is associated with poor prognosis [28]. Common side effects include chronic fatigue, metabolic disturbances, gastrointestinal symptoms, skin rash, and cardiovascular dysfunction. Long-term adherence to therapy may also pose challenges for some patients [29].
In this context, there is growing interest in integrative approaches, including the use of natural bioactive compounds such as polyphenols. Due to their antioxidant and regulatory properties, polyphenols may help mitigate chronic oxidative stress induced by both the disease and its treatment, improving therapy tolerability and potentially reducing the risk of disease progression.
3. Polyphenols: Structure and Biological Functions
Polyphenols are a broad and heterogeneous class of plant-derived organic compounds, characterized by the presence of one or more aromatic rings directly bound to hydroxyl groups (–OH), which confer high reactivity towards ROS [30]. In plants, polyphenols act as secondary metabolites involved in defense against environmental stress, pathogens, and UV radiation [31]. In the human diet, they are widely found in fruits, vegetables, legumes, whole grains, tea, red wine, extra virgin olive oil, and other Mediterranean diet components [32,33,34].
Structurally, polyphenols can be grouped into main subclasses, including flavonoids, phenolic acids, stilbenes, and tannins. Flavonoids are the most abundant and include compounds such as quercetin, catechins, anthocyanins, and isoflavones [35]. They share a common C6–C3–C6 backbone and their biological activity is influenced by the degree and position of hydroxylation and methylation [36]. For example, hydroxyl groups on aromatic rings generally enhance bioactivity, while methylation can reduce it, except in some flavonols, where it may increase stability and efficacy [37].
Phenolic acids, such as gallic and caffeic acid, are simpler structures and often act as biosynthetic precursors for more complex polyphenols. Their anti-AGE activity is closely related to their hydroxylation patterns [38].
Stilbenes, including resveratrol and pterostilbene, have shown antioxidant, anti-inflammatory, and anticancer properties. Resveratrol, found in grapes and red wine, is particularly studied for its capacity to modulate redox balance and signaling pathways relevant to cancer [39,40]. Stilbenes have specific acid dissociation constants (pKa) affecting their biological activity [41].
Tannins are high molecular weight polyphenols, classified into hydrolyzable tannins (e.g., ellagic acid) and condensed tannins (proanthocyanidins). These compounds are abundant in berries, tea, and wine, and contribute to antioxidant activity through radical scavenging and metal chelation (Figure 2) [42,43].
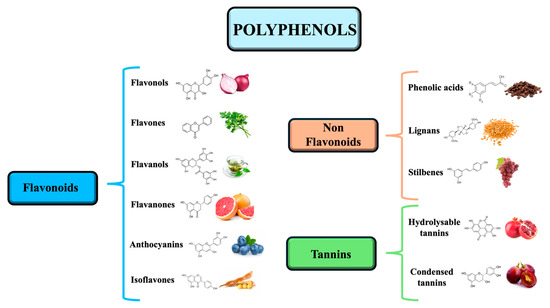
Figure 2.
The three main categories of polyphenols include flavonoids divided into flavonols, flavones, flavanols, flavanones, anthocyanins, and isoflavones; non flavonoids, divided into phenolic acids, lignans, and stilbenes; and tannins, divided into hydrolyzable tannins and condensed tannins.
Polyphenols exert antioxidant effects through several mechanisms: neutralizing free radicals via electron donation, chelating pro-oxidant metals like iron and copper, inhibiting oxidative enzymes (e.g., NADPH oxidase), and regenerating endogenous antioxidants such as glutathione and vitamin E [44].
Beyond direct radical scavenging, polyphenols modulate cellular redox homeostasis by activating Nrf2 (nuclear factor erythroid 2-related factor 2), a transcription factor that induces the expression of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase [45,46]. Under basal conditions, Nrf2 is inhibited by Keap1, but polyphenols can relieve this inhibition and promote the nuclear translocation of Nrf2. Moreover, they regulate the bioavailability of iron, limiting ROS formation through Fenton reactions [47].
Numerous epidemiological, preclinical, and clinical studies have documented the health benefits of polyphenols, especially in cardiovascular protection [48]. They improve endothelial function, stimulate nitric oxide (NO) production, reduce low-density lipoprotein (LDL) oxidation, inhibit platelet aggregation, and modulate vascular inflammation [49].
In recent years, hydroxytyrosol, a phenolic compound abundant in extra virgin olive oil, has gained interest for its cardioprotective properties [50,51]. It reduces the exposure of phosphatidylserine on erythrocyte membranes, a key event in programmed red blood cell death (eryptosis) [52,53,54,55]. By inhibiting eryptosis, hydroxytyrosol may help preserve erythrocyte integrity and longevity, potentially protecting against oxidative stress-related hematologic and cardiovascular disorders [56,57].
Many polyphenols cross the blood–brain barrier and show protective effects against neurodegenerative diseases such as Alzheimer’s and Parkinson’s [58,59]. Clinical studies with phytochemicals like quercetin, caffeine, and ginkgolides reveal anti-inflammatory, antioxidant, and neuroprotective effects, mitigating neuroinflammation, neuronal oxidation, and synaptic dysfunction [60].
At the metabolic level, polyphenols contribute to glycemic control by inhibiting digestive enzymes, enhancing insulin sensitivity, and modulating glucose uptake and hepatic output, thereby supporting the prevention of type 2 diabetes [61,62].
Lastly, polyphenols have shown strong anti-inflammatory and antimicrobial properties. They inhibit proinflammatory pathways like NF-κB, reduce cytokine production (e.g., TNF-α, IL-6), and promote beneficial gut microbiota, selectively enhancing commensal bacteria such as Lactobacillus and Bifidobacterium while inhibiting pathogens [63].
In oncology, including hematologic malignancies such as chronic myeloid leukemia, polyphenols show antiproliferative, pro-apoptotic, and chemosensitizing properties [64,65].
4. Scientific Evidence for Polyphenols in the Treatment of Chronic Myeloid Leukemia
CML represents a paradigmatic oncological model in which the introduction of targeted therapies has revolutionized treatment. However, the emergence of resistance and the side effects of conventional drugs call for the exploration of alternative or complementary therapeutic strategies. In this context, polyphenols, known for their antioxidant and pro-apoptotic properties, are emerging as promising bioactive compounds [66,67]. A review that summarizes the available evidence on the effect of polyphenols in CML is therefore essential to identify new treatment pathways and to promote targeted clinical studies.
In a study by Luzi et al. the in vitro effects of resveratrol on the apoptotic pathway in human K562 and acute lymphoblastic (HSB-2) cells were investigated. Treatment with resveratrol in both cell types significantly and irreversibly inhibited their growth, which was associated with extensive apoptosis and an increase in hypodiploid cells. Resveratrol-induced apoptosis was associated with the increased expression of Bax and a marked release of cytochrome c from the mitochondria. Interestingly, K562 cells showed a basal glutathione content 10 times higher than that of HSB-2 cells, which increased after 24–48 h of exposure to resveratrol, along with an increase in glutathione reductase and peroxidase activity. These data are highly encouraging for the use of these natural substances in the treatment of specific pathologies such as CML [68]. Supporting these results, further studies have investigated the multiple effects of resveratrol on leukemic cells.
A study by Alhawamdeh et al. also explored the effects of resveratrol on apoptosis, cell cycle regulation, and DNA fragmentation in K562 cells. The compound induced a time-dependent reduction in cell viability, with IC50 values decreasing from 282.2 µM at 24 h to 107.1 µM and 102.4 µM at 48 and 72 h, respectively. Its pro-apoptotic activity was confirmed by marked DNA fragmentation in 55 ± 5% of treated cells, which was absent in control cells. Gene expression analysis showed an upregulation of pro-apoptotic genes (BCL-2, AIF, BAX, VDAC1) and a slight downregulation of genes associated with cell survival (CASP3, PGC1α, NDUFA9, Cyclin-D1, p53), suggesting a dual role of resveratrol in inducing apoptosis and inhibiting survival signals [8].
Further investigating the efficacy of resveratrol under the conditions of drug resistance, Can et al. evaluated its effect on both imatinib-sensitive and resistant K562 cells (K562/IMA-3). The concentrations of resveratrol required to inhibit cell growth by 50% (IC50) were found to be 85 and 122 μM, respectively. The loss of mitochondrial membrane potential increased 1.91, 7.42, and 14.73 times in K562 cells and 2.21, 3.30, and 7.65 times in K562/IMA-3 cells in response to increasing concentrations of resveratrol (10–100 μM). Caspase-3 activity also showed a dose-dependent increase, with apoptosis rates reaching 58.7% in K562 cells and 43.3% in K562/IMA-3 cells treated with 100 μM resveratrol. These results suggest the potential usefulness of resveratrol even in cases of primary or acquired resistance to imatinib [69].
In addition to its direct effects on the cell cycle and apoptosis, resveratrol also acts on specific molecular pathways. In particular, histone H2AX phosphorylation has been proposed as a crucial event in resveratrol-induced apoptosis. Treatment of K562 cells with concentrations between 20 and 100 μmol/L induced apoptosis and phosphorylation of H2AX at Ser139 in a dose- and time-dependent manner, while simultaneously reducing histone H3 phosphorylation at Ser10. Resveratrol activated the MAPK family members p38 and JNK, while blocking ERK activation. Pharmacological or genetic inhibition of p38 and JNK reduced H2AX phosphorylation and apoptosis induced by treatment. H2AX overexpression amplified the apoptotic response, whereas Ser139 mutation or H2AX silencing conferred resistance, suggesting an essential role for H2AX phosphorylation at Ser139 [70].
Another relevant molecular target is STAT5, a transcription factor constitutively activated in several neoplasms, including CML. The study by Li et al. showed that resveratrol inhibits STAT5 activation in K562 and KU812 cell lines, indicating possible therapeutic utility also through this pathway [71].
It has also been observed that resveratrol acts through AMPK activation and p62/SQSTM1 expression mediated by JNK, as reported by Puissant et al., adding further insight into its cytotoxic effects [72].
Among the mechanisms of therapy resistance, the high expression of heat shock protein 70 (Hsp70) is a key factor in CML. Resveratrol has shown the ability to suppress Hsp70 at the transcriptional and protein levels, with a reduction in heat shock factor 1 (HSF1) activity, suggesting that Hsp70 downregulation may enhance chemosensitivity [73].
The efficacy of resveratrol is amplified when combined with other chemotherapeutic agents such as imatinib mesylate or arsenic trioxide. In combination with imatinib mesylate, it resulted in a greater inhibition of cell growth and an increased apoptotic rate compared to single-agent treatment [74]. Similarly, resveratrol enhanced the pro-apoptotic effect of arsenic trioxide, suggesting a potential use in targeting early leukemic cells [75].
Other polyphenols beyond resveratrol also show promising effects. Quercetin, in particular, has shown significant anti-leukemic activity in CML cells. In in vitro models with the K562 cell line, the combination of quercetin and curcumin induced synergistic apoptosis, characterized by increased ROS levels, glutathione reduction, and a loss of mitochondrial membrane potential. The apoptotic action occurred mainly via the mitochondrial pathway, with cytochrome c release and activation of caspase-9 and PARP. The molecular mechanism involves the coordinated modulation of multiple pathways: the p53 pathway; inflammation (NFκB); the TGFα growth factor pathway, including cell cycle regulatory genes such as p21, p27, BTG2, and FAS; and the downregulation of AKT1 and IFNγ. Furthermore, in a comparison of eight polyphenols (quercetin, chrysin, apigenin, emodin, aloe-emodin, rhein, cis-stilbene, and trans-stilbene) on hematologic cells, quercetin, emodin, and cis-stilbene proved the most potent, with IC50 values in the 8–33 μM range for quercetin. Even more resistant cell lines, such as KG 1a and K562, responded significantly, suggesting efficacy even in resistant contexts [76].
Another study showed that quercetin displayed significant activity against imatinib-sensitive and resistant K562 cells, with IC50 values of 85 and 122 μM, respectively. The 100 μM dose induced apoptosis in 58.7% of the sensitive K562 cells and 43.3% of the resistant ones. The effect was mediated by mitochondrial potential loss and caspase-3 activation, encouraging its use as a potential adjuvant strategy in TKI resistance cases [77].
Curcumin has also shown promising results both in vitro and in vivo. In K562 cells, treatment with 20–40 μM resulted in a dose-dependent reduction of BCR–ABL mRNA by 20–45%; this effect was mediated by increased miR-196b, known to suppress BCR–ABL. Curcumin also affects the PTEN/AKT pathway through the downregulation of miR-21, leading to increased miR-21 export in exosomes and the inhibition of leukemic growth. Moreover, the authors studied curcumin’s effects on a CML xenograft in SCID mice. Data showed that animals treated with curcumin developed smaller tumors compared to control mice [78].
Recent studies have shown that curcumin, in nanomicellar formulations, reduces BCR–ABL expression in imatinib-sensitive and resistant cells (fold change ≈ 0.54–0.50), and induces apoptosis in K562 [79]. One study compared curcumin sensitivity between chronic (K562) and acute (HL-60) myeloid lines. K562 showed greater sensitivity, with vitality reduction < 80% at 10 μM and mortality peaks up to 90% with treatment. However, caspase activation was more incomplete in K562, suggesting differences in the mechanisms of action [80].
Khatamsaz et al. also showed that curcumin, particularly a curcumin-based nanodrug, induced apoptosis in CML K562 cells. IC50 values for curcumin and the nanodrug were 50 and 25 μg/mL, respectively. Apoptosis in the K562 cell line occurred 48 h after treatment with 25 μg/mL curcumin and 12.5 μg/mL nanodrug. The increased cytotoxicity of curcumin and the nanodrug was directly related to drug concentration and administration time. Interestingly, the nanodrug showed greater cytotoxic effects on cell viability and stimulated more apoptosis than curcumin alone [81].
A study by Jia et al. also showed that curcumin inhibited K562 cell viability in a dose- and time-dependent manner. Moreover, curcumin-induced cell death was associated with apoptosome complex formation, mitochondrial membrane potential collapse, and caspase-3 activation. Curcumin treatment also induced Bid cleavage and reduced Bcl-2 protein expression. Surprisingly, despite these apoptotic features, the authors demonstrated that curcumin stimulated autophagy, as evidenced by the immunoreactivity of microtubule-associated protein light chain 3. Curcumin also increased Beclin-1 protein levels. Overall, these results suggested that curcumin induces both autophagic and apoptotic death in K562 cells [82].
Another widely studied natural polyphenol in oncology is (−)-epigallocatechin-3-gallate (EGCG), the main catechin in green tea. A study by Della Via et al. showed that EGCG, a gallate ester obtained from gallic acid condensation with the (3R)-hydroxyl group of (−)-epigallocatechin, has multiple effects on signaling pathways and enzymatic activity, which may enhance apoptosis and suppress cell proliferation, invasion, angiogenesis, and metastasis in tumors. Specifically, hematologic analysis in this study revealed that EGCG treatment reversed leukocytosis, anemia, and thrombocytopenia and prolonged the survival of PML/RARα mice. Notably, EGCG reduced immature leukemia cells and promyelocytes in the bone marrow while increasing mature myeloid cells, probably by inducing cell differentiation. Additionally, EGCG has been reported to inhibit PIN1, a peptidyl isomerase overexpressed and/or overactivated in human tumors, described as a key target in PML/RARα. Overall, the data in this study supported further evaluation of EGCG in clinical studies on acute myeloid leukemia [83].
Xiao et al. also studied EGCG’s effect on the growth of Bcr/Abl+ CML cell lines, including imatinib-resistant lines and primary CML cells. The results revealed that EGCG could inhibit cell growth and induce apoptosis in CML cells. The mechanisms involved include inhibition of the Bcr/Abl oncoprotein and regulation of its downstream pathways p38 MAPK/JNK and JAK2/STAT3/AKT. In conclusion, the data showed EGCG’s anti-CML effects in Bcr/Abl+ cells sensitive and resistant to imatinib, particularly in cells with the T315I mutation [84].
An epidemiological study also showed that green tea consumption is associated with a reduced risk of hematopoietic neoplasms. In particular, the authors demonstrated the activation of acid sphingomyelinase induced by EGCG and the clustering of lipid rafts in CML cells. The ASM inhibitor desipramine significantly reduced EGCG-induced cell death. Protein kinase Cδ, a well-known kinase, plays an important role in ASM activation. Furthermore, the authors observed EGCG-induced phosphorylation of protein kinase Cδ at Ser664. Importantly, EGCG-induced ASM activation was significantly reduced by the pre-treatment of CML cells with the soluble guanylate cyclase inhibitor NS2028, suggesting that EGCG induces ASM activation via a cyclic guanosine monophosphate (cGMP)-dependent pathway. Indeed, pharmacological inhibition of a negative cGMP regulator enhanced EGCG’s anti-CML effect. These results indicated that EGCG-induced cell death occurs through the cGMP/ASM pathway in CML cells [85].
An additional study by Iwasaki et al. demonstrated that EGCG predominantly causes necrotic-type cell death through a caspase-independent mechanism in CML, K562, and C2F8 cells, whereas imatinib induces typical apoptotic cell death. Moreover, this caspase-independent cell death partially mediated the release of apoptosis-inducing factor and the serine protease HtrA2/Omi from the mitochondria to the cytosol. Furthermore, EGCG enhanced the imatinib-induced cell death, resulting in additive cell death in K562 cells, and EGCG alone effectively reduced the viability of imatinib-resistant K562 cells. The authors therefore suggested that catechin is a potential candidate as an antitumor agent causing cell death in CML cells via a caspase-independent mechanism [86].
EGCG has also been studied in combination with ponatinib, a drug used in the treatment of CML. This study also aimed to detect changes in the expression levels of genes related to cell cycle regulation after the combination of ponatinib and EGCG in the CML K562 cell line. The IC50 values for ponatinib and EGCG were 87.13 nM and 50 μM respectively, and the combination showed a synergistic effect. Cyclin D1 and CDC25A were downregulated by ponatinib–EGCG by 2.49 and 2.63 times, respectively. TGF-β2 was upregulated 4.57 times. The authors suggested that EGCG could synergistically cooperate with ponatinib’s growth inhibitory activity against CML cells. Furthermore, according to the authors, apoptosis mediated by ponatinib–EGCG may be associated with the upregulation of the TGF-β2 gene and the downregulation of the cyclin D1 and CDC25A genes [87].
Finally, epidemiological evidence supports the rationale for the use of additional polyphenols in the prevention and treatment of CML. Anthocyanin-rich extracts (cyanidin 3-rutinoside, delphinidin) reduced the proliferation of murine Ph+ ALL cells (BM185), reaching efficacy comparable to standard agents such as imatinib [88].
Triterpenoids, particularly a pentacyclic derivative of gypenoside (compound 1c), also showed an inhibition of ABL1 (IC50 ≈ 9.3 μM) and improved selectivity compared to imatinib on normal cells. Notably, compound 1c exhibited a different inhibitory profile against eight kinases compared to imatinib. The interaction between the ATP binding site of ABL and compound 1c was examined via molecular docking studies, revealing a binding mode distinct from that of imatinib and next-generation inhibitors. Additionally, compound 1c suppressed downstream BCR–ABL signaling. This study suggested that plant extracts could be a source for CML treatment and offer a strategy to overcome drug resistance to known BCR–ABL inhibitors [89]. The polyphenolic tri-vanillic compound (13c) also inhibited the phosphorylation of JAK2V617F and BCR–ABL; downregulating P STAT3/5, Mcl-1, and c-Myc; and inducing apoptosis in CML cells [90].
Interestingly, while some clover species are known for beneficial effects in human diseases, little is known about the activity of the forage plant Trifolium repens. However, a 2020 study showed that its phytochemical components, specifically its isoflavonoid-rich fraction, exhibited strong cytotoxic effects in CML K562 cells, with IC50 values of 1.67 and 0.092 mg/mL, respectively. Cell growth arrest was associated with the total inhibition of BCR–ABL/STAT5 and activation of the p38 signaling pathways. Conversely, these strong cytotoxic effects did not occur in normal cells. These results suggested that developing new compounds derived from phytochemical molecules in Trifolium could lead to the identification of new therapeutic agents active against CML [91].
Carnosic acid, an important polyphenol mainly isolated from the well-known spice and medicinal plant Rosmarinus officinalis, also showed antitumor effects in CML. Specifically, in this study using the KBM-7 cell line, carnosic acid demonstrated significant antitumor activity against KBM-7 CML cells with an IC50 of 25 μM. The antitumor activity was due to apoptosis induction and cell cycle arrest. Additionally, carnosic acid inhibited the proliferation and invasion of KBM-7 CML cells, which could be mainly attributed to the downregulation of microRNA-780 expression, as indicated by quantitative RT-PCR analysis. Overall, the authors proposed that carnosic acid could prove to be a potential lead compound in CML treatment [92].
A 2019 study aimed to manipulate the apoptotic pathway of tumor necrosis factor-related apoptosis-inducing ligand using hydroxychavicol, a polyphenol extracted from Piper betel leaves, to induce apoptosis in TRAIL-resistant CML cells. This ligand, a member of the cytokine superfamily, induces apoptosis in various tumor cells through activation of the extrinsic apoptotic pathway but shows little to no cytotoxicity toward normal cells. However, some tumor cells are intrinsically resistant to TRAIL-mediated apoptosis, a challenge in establishing TRAIL as a potential chemotherapeutic drug. When imatinib-resistant K562 cells were treated with hydroxychavicol, they became sensitive to TRAIL. It was observed that hydroxychavicol reduced the expression of anti-apoptotic proteins XIAP and FLIP, while expression of the TRAIL receptors DR4 and DR5 remained unchanged. Data from this study demonstrated that the combined treatment with TRAIL and hydroxychavicol represents a promising alternative therapeutic approach for treating leukemia resistant to imatinib and TRAIL [93].
Collectively, preclinical evidence indicates that polyphenols act on multiple molecular targets in CML: from microRNA modulation to regulation of the cell cycle and caspases, as well as influencing resistance to TKIs. Synergistic combinations of polyphenols may reduce necessary doses, improve efficacy, and mitigate resistance, opening innovative therapeutic perspectives. However, valid clinical translation is still lacking; well-designed clinical trials are needed to validate these compounds as nutraceutical supports or co-therapeutic agents in CML (Table 1).

Table 1.
Main effects of polyphenols in MCL model systems.
5. Discussion
The collected preclinical evidence consistently demonstrates the effectiveness of polyphenols, particularly resveratrol, quercetin, curcumin, and EGCG, in significantly modulating the proliferation and survival of CML cells, including cell lines resistant to TKIs such as imatinib [94]. Their effects are mediated through multiple and converging mechanisms: the induction of mitochondrial apoptosis, the inhibition of key oncoproteins (such as BCR–ABL and STAT5), regulation of the cell cycle, and the activation of cellular stress pathways (MAPK, AMPK, JNK) [71,90].
In particular, resveratrol stands out for its robust pro-apoptotic activity, mediated by cytochrome c release, caspase-3 activation, and histone H2AX phosphorylation, as well as its ability to overcome resistance mechanisms related to Hsp70 overexpression and mitochondrial dysfunction [70]. Its effects also extend to the transcriptional regulation of pro- and anti-apoptotic genes, highlighting a potential epigenetic or signal transduction role in modulating the cellular response.
Similarly, quercetin shows synergistic activity with other polyphenols and chemo-targeted agents, exerting cytotoxic effects even on drug-resistant cell lines. The coordinated involvement of pathways related to ROS, inflammation and cell cycle regulation (p21, p27) suggests a broad spectrum of action, which could also prove useful in combination with conventional therapies or personalized approaches [77,95].
Curcumin, in addition to confirming its pro-apoptotic and anti-proliferative activity, displays unique characteristics, including the ability to induce autophagic cell death and modulate the expression of onco-miRNAs (such as miR-21 and miR-196b), strengthening the hypothesis that its effects are linked to fine-tuned regulation of the molecular networks within leukemic cells. Nanomicellar formulations of curcumin have further enhanced its bioavailability and therapeutic efficacy, offering a promising rationale for clinical development [78,82].
Finally, EGCG is notable for its ability to induce non-conventional cell death through caspase-independent necrotic mechanisms, the activation of acid sphingomyelinase, and modulation of the cGMP pathway. Its synergistic effect with imatinib and its activity against mutant lines (e.g., T315I) reinforce its therapeutic relevance, suggesting it may serve as a valuable adjuvant or alternative strategy, particularly in refractory cases [83,85].
Despite the consistency and biological plausibility of these preclinical findings, the translational impact of polyphenols in the clinical setting remains limited. No clinical trials have yet been initiated in CML patients to test the efficacy of these compounds, and this gap underscores several challenges that still need to be addressed. First, although the results are promising, they are still preliminary and largely restricted to in vitro and selected in vivo models, which do not fully capture the complexity of the disease in humans. Furthermore, the limited bioavailability, rapid metabolism, and systemic instability of many polyphenols significantly reduce their therapeutic potential in clinical contexts, unless advanced delivery systems or structural modifications are employed.
Another critical limitation concerns the lack of standardized formulations and dosing regimens, which hampers the reproducibility of results and complicates the design of interventional studies. Additionally, the pleiotropic nature of polyphenols, while advantageous from a mechanistic standpoint, introduces difficulties in identifying specific molecular targets and clinically measurable endpoints, which are essential for drug development and regulatory approval.
Taken together, these factors help explain the absence of clinical trials to date and highlight the need for a more integrated approach, combining pharmaceutical innovation with translational research, in order to unlock the full therapeutic potential of polyphenols in CML.
6. Conclusions
Polyphenols represent a class of bioactive compounds of great interest in the treatment of CML, due to their ability to selectively modulate key processes such as apoptosis, proliferation, oxidative stress, and oncogenic signaling. The collected preclinical evidence indicates significant potential, both as monotherapy and in combination with TKIs, particularly in resistant or refractory cases.
The integration of polyphenols into standard therapeutic protocols could represent an evolution in CML management, promoting a more personalized and less toxic approach. However, to support their clinical integration, well-designed translational and clinical studies will be necessary to assess their efficacy, safety, pharmacokinetics, and drug interactions.
In conclusion, further investigation into the role of polyphenols in CML opens new therapeutic perspectives and strengthens the concept of integrated oncology, in which natural compounds can effectively complement conventional treatments, improving patients’ quality of life and treatment adherence.
Author Contributions
Conceptualization: S.D. and P.P.; methodology: C.M., S.D. and P.P.; data curation: S.D., C.D.R. and C.M.; validation: P.P. and S.D.; resources: S.D.; writing—original draft preparation: S.D. and P.P.; writing—review and editing: C.M., S.D. and P.P.; visualization: S.D. and P.P.; supervision: S.D.; project administration: S.D.; funding acquisition: S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by grants from NextGenerationEU in the framework of PRIN 2022 (project code 2022J2NT4L)—CUP I53D23004490006 (S.D.).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AGE | Advanced glycation end product |
| CML | Chronic myeloid leukemia |
| EGCG | (−)-epigallocatechin-3-gallate |
| LSC | Leukemic stem cell |
| MSC | Mesenchymal stromal cell |
| MRC-cIII | Mitochondrial respiratory chain complex III |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ROS | Reactive oxygen species |
| TXNIP | Thioredoxin-interacting protein |
| TKI | Tyrosine kinase inhibitor |
References
- Minciacchi, V.R.; Kumar, R.; Krause, D.S. Chronic Myeloid Leukemia: A Model Disease of the Past, Present and Future. Cells 2021, 10, 117. [Google Scholar] [CrossRef]
- Jain, S.; Abraham, A. BCR-ABL1-like B-Acute Lymphoblastic Leukemia/Lymphoma: A Comprehensive Review. Arch. Pathol. Lab. Med. 2020, 144, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Messina, M.; Foà, R. BCR/ABL1-like Acute Lymphoblastic Leukemia: How to Diagnose and Treat? Cancer 2019, 125, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, S.; Vozniak, M.; Rhodes, J.; Forcello, N.; Olszta, D. BCR-ABL1 Tyrosine Kinase Inhibitors for the Treatment of Chronic Myeloid Leukemia. J. Oncol. Pharm. Pract. 2018, 24, 433–452. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Available online: https://www.ingentaconnect.com/content/ben/cnf/2020/00000016/00000008/art00005 (accessed on 17 February 2025).
- Vuoso, D.; Porcelli, M.; Cacciapuoti, G.; D’Angelo, S. Biological Activity of MelAnnurca Flesh Apple Biophenols. Curr. Nutr. Food Sci. 2020, 16, 1149–1162. [Google Scholar] [CrossRef]
- Vuoso, D.C.; D’Angelo, S.; Ferraro, R.; Caserta, S.; Guido, S.; Cammarota, M.; Porcelli, M.; Cacciapuoti, G. Annurca Apple Polyphenol Extract Promotes Mesenchymal-to-Epithelial Transition and Inhibits Migration in Triple-Negative Breast Cancer Cells through ROS/JNK Signaling. Sci. Rep. 2020, 10, 15921. [Google Scholar] [CrossRef]
- Alhawamdeh, L.; Almajali, B.; Atoom, A.M.; Saad, H.K.M.; Madi, R.; Al-Jamal, H.A.N. Resveratrol-Induced Modulation of Key Genes and DNA Fragmentation in Chronic Myeloid Leukemia Cells. Asian Pac. J. Cancer Prev. 2025, 26, 905–911. [Google Scholar] [CrossRef]
- Allegra, A.; Mirabile, G.; Caserta, S.; Stagno, F.; Russo, S.; Pioggia, G.; Gangemi, S. Oxidative Stress and Chronic Myeloid Leukemia: A Balance between ROS-Mediated Pro- and Anti-Apoptotic Effects of Tyrosine Kinase Inhibitors. Antioxidants 2024, 13, 461. [Google Scholar] [CrossRef]
- Perrone, P.; De Rosa, C.; D’Angelo, S. Mediterranean Diet and Agri-Food By-Products: A Possible Sustainable Approach for Breast Cancer Treatment. Antioxidants 2025, 14, 789. [Google Scholar] [CrossRef]
- Zhou, T.; Medeiros, L.J.; Hu, S. Chronic Myeloid Leukemia: Beyond BCR-ABL1. Curr. Hematol. Malig. Rep. 2018, 13, 435–445. [Google Scholar] [CrossRef]
- Sawyers, C.L. Molecular Consequences of the BCR-ABL Translocation in Chronic Myelogenous Leukemia. Leuk. Lymphoma 1993, 11 (Suppl. S2), 101–103. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Tirrò, E.; Stella, S.; Manzella, L.; Pennisi, M.S.; Romano, C.; Vitale, S.R.; Puma, A.; Tomarchio, C.; Di Gregorio, S.; et al. Impact of the Breakpoint Region on the Leukemogenic Potential and the TKI Responsiveness of Atypical BCR-ABL1 Transcripts. Front. Pharmacol. 2021, 12, 669469. [Google Scholar] [CrossRef]
- Yoshimaru, R.; Minami, Y. Genetic Landscape of Chronic Myeloid Leukemia and a Novel Targeted Drug for Overcoming Resistance. Int. J. Mol. Sci. 2023, 24, 13806. [Google Scholar] [CrossRef]
- Panjarian, S.; Iacob, R.E.; Chen, S.; Engen, J.R.; Smithgall, T.E. Structure and Dynamic Regulation of Abl Kinases. J. Biol. Chem. 2013, 288, 5443–5450. [Google Scholar] [CrossRef]
- Tipping, A.J.; Melo, J.V. Comparative Gene Expression Profile of P185(Bcr-Abl) versus P210(Bcr-Abl) Expressing Cells. Leuk. Res. 2004, 28, 219–220. [Google Scholar] [CrossRef]
- Chatain, N.; Ziegler, P.; Fahrenkamp, D.; Jost, E.; Moriggl, R.; Schmitz-Van de Leur, H.; Müller-Newen, G. Src Family Kinases Mediate Cytoplasmic Retention of Activated STAT5 in BCR-ABL-Positive Cells. Oncogene 2013, 32, 3587–3597. [Google Scholar] [CrossRef]
- Klejman, A.; Schreiner, S.J.; Nieborowska-Skorska, M.; Slupianek, A.; Wilson, M.; Smithgall, T.E.; Skorski, T. The Src Family Kinase Hck Couples BCR/ABL to STAT5 Activation in Myeloid Leukemia Cells. EMBO J. 2002, 21, 5766–5774. [Google Scholar] [CrossRef]
- Nieborowska-Skorska, M.; Kopinski, P.K.; Ray, R.; Hoser, G.; Ngaba, D.; Flis, S.; Cramer, K.; Reddy, M.M.; Koptyra, M.; Penserga, T.; et al. Rac2-MRC-cIII-Generated ROS Cause Genomic Instability in Chronic Myeloid Leukemia Stem Cells and Primitive Progenitors. Blood 2012, 119, 4253–4263. [Google Scholar] [CrossRef]
- Nair, R.R.; Tolentino, J.; Hazlehurst, L.A. The Bone Marrow Microenvironment as a Sanctuary for Minimal Residual Disease in CML. Biochem. Pharmacol. 2010, 80, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Ding, R.; Qu, X.; Li, Y.; Shen, T.; Wang, L.; Li, R.; Zhang, J.; Ru, Y.; Bu, X.; et al. BCR-ABL Triggers a Glucose-Dependent Survival Program during Leukemogenesis through the Suppression of TXNIP. Cell Death Dis. 2023, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Schneckenleithner, C.; Hoelbl-Kovacic, A.; Sexl, V. Modeling BCR/ABL-Driven Malignancies in the Mouse. Methods Mol. Biol. 2015, 1267, 263–282. [Google Scholar] [CrossRef]
- Borella, G.; Da Ros, A.; Borile, G.; Porcù, E.; Tregnago, C.; Benetton, M.; Marchetti, A.; Bisio, V.; Montini, B.; Michielotto, B.; et al. Targeting the Plasticity of Mesenchymal Stromal Cells to Reroute the Course of Acute Myeloid Leukemia. Blood 2021, 138, 557–570. [Google Scholar] [CrossRef]
- Saußele, S.; Kohlbrenner, K.; Vogelmann, T.; Schubert, T. Incidence, Prevalence, and Real-World Treatment Patterns in Chronic Myeloid Leukemia: Results from a Population-Representative German Claims Data Analysis. Oncol. Res. Treat. 2022, 45, 400–407. [Google Scholar] [CrossRef]
- Höglund, M.; Sandin, F.; Simonsson, B. Epidemiology of Chronic Myeloid Leukaemia: An Update. Ann. Hematol. 2015, 94 (Suppl. S2), S241–S247. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: A Review. JAMA 2025, 333, 1618–1629. [Google Scholar] [CrossRef]
- How, J.; Venkataraman, V.; Hobbs, G.S. Blast and Accelerated Phase CML: Room for Improvement. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 122–128. [Google Scholar] [CrossRef]
- Senapati, J.; Jabbour, E.; Kantarjian, H.; Short, N.J. Pathogenesis and Management of Accelerated and Blast Phases of Chronic Myeloid Leukemia. Leukemia 2023, 37, 5–17. [Google Scholar] [CrossRef]
- Dereme, J.; Ségot, A.; Friedrich, N.; Tsilimidos, G.; Blum, S. Recognizing and managing side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Rev. Med. Suisse 2023, 19, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Polyphenols: Potential Beneficial Effects of These Phytochemicals in Athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.; Sarıtaş, S.; Duman, H.; Eker, F.; Akdaşçi, E.; Karav, S.; Witkowska, A.M. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients 2024, 16, 2550. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; D’Angelo, S. Original Article Extra Virgin Olive Oil as a Functional Food for Athletes: Recovery, Health, and Performance. J. Phys. Educ. Sport 2025, 25, 370–381. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Hormesis and Health: Molecular Mechanisms and the Key Role of Polyphenols. Food Chem. Adv. 2025, 7, 101030. [Google Scholar] [CrossRef]
- Perrone, P.; Palmieri, S.; Piscopo, M.; Lettieri, G.; Eugelio, F.; Fanti, F.; D’Angelo, S. Antioxidant Activity of Annurca Apple By-Products at Different Ripening Stages: A Sustainable Valorization Approach. Antioxidants 2025, 14, 941. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Chen, M.; Ren, X.; Sun, S.; Wang, X.; Xu, X.; Li, X.; Wang, X.; Li, X.; Yan, X.; Li, R.; et al. Structure, Biological Activities and Metabolism of Flavonoid Glucuronides. Mini Rev. Med. Chem. 2022, 22, 322–354. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Piwowar, A.; Rorbach-Dolata, A.; Fecka, I. The Antiglycoxidative Ability of Selected Phenolic Compounds—An In Vitro Study. Molecules 2019, 24, 2689. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and Related Stilbenes: Their Anti-Aging and Anti-Angiogenic Properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef]
- Lamuela-Raventos, R.M.; Romero-Perez, A.I.; Waterhouse, A.L.; de la Torre-Boronat, M.C. Direct HPLC Analysis of Cis- and Trans-Resveratrol and Piceid Isomers in Spanish Red Vitis Vinifera Wines. J. Agric. Food Chem. 1995, 43, 281–283. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Condensed Tannins-Their Content in Plant Foods, Changes during Processing, Antioxidant and Biological Activities. Adv. Food Nutr. Res. 2024, 110, 327–398. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Hou, C.; Yang, L.; Li, H.; Guo, J.; Huo, C.; Wang, M.; Miao, Y.; Liu, J.; et al. Oleuropein Improves Mitochondrial Function to Attenuate Oxidative Stress by Activating the Nrf2 Pathway in the Hypothalamic Paraventricular Nucleus of Spontaneously Hypertensive Rats. Neuropharmacology 2017, 113, 556–566. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Ahmadi, A.; Jamialahmadi, T.; Sahebkar, A. Polyphenols and Atherosclerosis: A Critical Review of Clinical Effects on LDL Oxidation. Pharmacol. Res. 2022, 184, 106414. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and Metabolism of Hydroxytyrosol, a Natural Antioxidant from Olive Oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- D’Angelo, S.; Ingrosso, D.; Migliardi, V.; Sorrentino, A.; Donnarumma, G.; Baroni, A.; Masella, L.; Tufano, M.A.; Zappia, M.; Galletti, P. Hydroxytyrosol, a Natural Antioxidant from Olive Oil, Prevents Protein Damage Induced by Long-Wave Ultraviolet Radiation in Melanoma Cells. Free Radic. Biol. Med. 2005, 38, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Spinelli, S.; Mantegna, G.; Notariale, R.; Straface, E.; Caruso, D.; Falliti, G.; Marino, A.; Manna, C.; Remigante, A.; et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells 2023, 12, 424. [Google Scholar] [CrossRef]
- Perrone, P.; Notariale, R.; Lettieri, G.; Mele, L.; La Pietra, V.; Piscopo, M.; Manna, C. Protective Effects of Olive Oil Antioxidant Phenols on Mercury-Induced Phosphatidylserine Externalization in Erythrocyte Membrane: Insights into Scramblase and Flippase Activity. Free Radic. Biol. Med. 2024, 227, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Ortega-Luna, R.; Manna, C.; Álvarez-Ribelles, Á.; Collado-Diaz, V. Increased Adhesiveness of Blood Cells Induced by Mercury Chloride: Protective Effect of Hydroxytyrosol. Antioxidants 2024, 13, 1576. [Google Scholar] [CrossRef]
- Notariale, R.; Moriello, C.; Alessio, N.; Del Vecchio, V.; Mele, L.; Perrone, P.; Manna, C. Protective Effect of Hydroxytyrosol against Hyperglycemia-Induced Phosphatidylserine Exposure in Human Erythrocytes: Focus on Dysregulation of Calcium Homeostasis and Redox Balance. Redox Biol. 2025, 85, 103783. [Google Scholar] [CrossRef]
- Notariale, R.; Längst, E.; Perrone, P.; Crettaz, D.; Prudent, M.; Manna, C. Effect of Mercury on Membrane Proteins, Anionic Transport and Cell Morphology in Human Erythrocytes. Cell Physiol. Biochem. 2022, 56, 500–513. [Google Scholar] [CrossRef]
- Notariale, R.; Perrone, P.; Mele, L.; Lettieri, G.; Piscopo, M.; Manna, C. Olive Oil Phenols Prevent Mercury-Induced Phosphatidylserine Exposure and Morphological Changes in Human Erythrocytes Regardless of Their Different Scavenging Activity. Int. J. Mol. Sci. 2022, 23, 5693. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-Virgin Olive Oil in Alzheimer’s Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef]
- Singh, A.; Tripathi, P.; Yadawa, A.K.; Singh, S. Promising Polyphenols in Parkinson’s Disease Therapeutics. Neurochem. Res. 2020, 45, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Kim, T.H.; Kim, M.O. Protective Effects of Anthocyanins against Amyloid Beta-Induced Neurotoxicity in Vivo and in Vitro. Neurochem. Int. 2015, 80, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Ferrara, L.; Joksimovic, M.; D’Angelo, S. Could Polyphenolic Food Intake Help in the Control of Type 2 Diabetes? A Narrative Review of the Last Evidence. Curr. Nutr. Food Sci. 2022, 18, 785–798. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-Oxidant and pro-Apoptotic Activity of Polyphenol Extract from Annurca Apple and Its Underlying Mechanisms in Human Breast Cancer Cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Malus pumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751. [Google Scholar] [CrossRef]
- Izuegbuna, O.O. Polyphenols: Chemoprevention and Therapeutic Potentials in Hematological Malignancies. Front. Nutr. 2022, 9, 1008893. [Google Scholar] [CrossRef]
- Fakhar, F.; Mohammadian, K.; Keramat, S.; Stanek, A. The Potential Role of Dietary Polyphenols in the Prevention and Treatment of Acute Leukemia. Nutrients 2024, 16, 4100. [Google Scholar] [CrossRef]
- Luzi, C.; Brisdelli, F.; Cinque, B.; Cifone, G.; Bozzi, A. Differential Sensitivity to Resveratrol-Induced Apoptosis of Human Chronic Myeloid (K562) and Acute Lymphoblastic (HSB-2) Leukemia Cells. Biochem. Pharmacol. 2004, 68, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Can, G.; Cakir, Z.; Kartal, M.; Gunduz, U.; Baran, Y. Apoptotic Effects of Resveratrol, a Grape Polyphenol, on Imatinib-Sensitive and Resistant K562 Chronic Myeloid Leukemia Cells. Anticancer. Res. 2012, 32, 2673–2678. [Google Scholar]
- Wu, X.; Xiong, M.; Xu, C.; Duan, L.; Dong, Y.; Luo, Y.; Niu, T.; Lu, C. Resveratrol Induces Apoptosis of Human Chronic Myelogenous Leukemia Cells in Vitro through P38 and JNK-Regulated H2AX Phosphorylation. Acta Pharmacol. Sin. 2015, 36, 353–361. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Ma, L.; Bai, X.; Li, X.; Zhao, M.; Sui, T. Resveratrol Inhibits STAT5 Activation through the Induction of SHP-1 and SHP-2 Tyrosine Phosphatases in Chronic Myelogenous Leukemia Cells. Anticancer. Drugs 2018, 29, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Auberger, P. AMPK- and P62/SQSTM1-Dependent Autophagy Mediate Resveratrol-Induced Cell Death in Chronic Myelogenous Leukemia. Autophagy 2010, 6, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.K.; Mustafi, S.B.; Ganguly, S.; Chatterjee, M.; Raha, S. Resveratrol Induces Apoptosis in K562 (Chronic Myelogenous Leukemia) Cells by Targeting a Key Survival Protein, Heat Shock Protein 70. Cancer Sci. 2008, 99, 1109–1116. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.H. Inhibition of Human Chronic Myelogenous Leukemia K562 Cell Growth Following Combination Treatment with Resveratrol and Imatinib Mesylate. Genet. Mol. Res. 2015, 14, 6413–6418. [Google Scholar] [CrossRef]
- Wu, E.J.; Goussetis, D.J.; Beauchamp, E.; Kosciuczuk, E.M.; Altman, J.K.; Eklund, E.A.; Platanias, L.C. Resveratrol Enhances the Suppressive Effects of Arsenic Trioxide on Primitive Leukemic Progenitors. Cancer Biol. Ther. 2014, 15, 473–478. [Google Scholar] [CrossRef]
- Mutlu Altundağ, E.; Yılmaz, A.M.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Synergistic Induction of Apoptosis by Quercetin and Curcumin in Chronic Myeloid Leukemia (K562) Cells. Nutr. Cancer 2018, 70, 97–108. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Hosseinzadeh, E.; Rezapour, S.; Vahedi, G.; Haghnavaz, N.; Marofi, F. Quercetin Promotes Cell Cycle Arrest and Apoptosis and Attenuates the Proliferation of Human Chronic Myeloid Leukemia Cell Line-K562 Through Interaction with HSPs (70 and 90), MAT2A and FOXM1. Anticancer. Agents Med. Chem. 2019, 19, 1523–1534. [Google Scholar] [CrossRef]
- Taverna, S.; Giallombardo, M.; Pucci, M.; Flugy, A.; Manno, M.; Raccosta, S.; Rolfo, C.; De Leo, G.; Alessandro, R. Curcumin Inhibits in Vitro and in Vivo Chronic Myelogenous Leukemia Cells Growth: A Possible Role for Exosomal Disposal of miR-21. Oncotarget 2015, 6, 21918–21933. [Google Scholar] [CrossRef]
- Virany, Z.G.; Bagheri, P.; Khaniki, S.H.; Chehreghani, Z.; Rahimi, H.R. Evaluation of the Effect of Curcumin and Imatinib on BCR-ABL Expression Gene in Chronic Human K562 Cells. Int. J. Med. Lab. 2022, 9, 141–149. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; Villegas-Sepúlveda, N.; Meraz-Rios, M.A.; Hernández-Zavala, A.; Berumen, J.; Coleman, M.A.; Orozco, L.; Cordova, E.J. Curcumin Differentially Affects Cell Cycle and Cell Death in Acute and Chronic Myeloid Leukemia Cells. Oncol. Lett. 2018, 15, 6777–6783. [Google Scholar] [CrossRef]
- Khatamsaz, S.; Hashemi, M. Curcumin and Curcumin-Loaded Nanogel Induce Apoptosis Activity in K562 Chronic Myelogenous Leukemia Cells. Galen. Med. J. 2018, 7, e921. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-L.; Li, J.; Qin, Z.-H.; Liang, Z.-Q. Autophagic and Apoptotic Mechanisms of Curcumin-Induced Death in K562 Cells. J. Asian Nat. Prod. Res. 2009, 11, 918–928. [Google Scholar] [CrossRef]
- Della Via, F.I.; Shiraishi, R.N.; Irene, S.; Ferro, K.P.; Salazar-Terreros, M.; Franchi Junior, G.C.; Rego, E.M.; Olalla Saad, S.T.; Torello, C.O. Epigallocatechin-3-Gallate Induces Cellular Differentiation and Reduces Leukemia Burden in PML/Rarα Mice By Increasing Reactive Oxygen Species and Reducing PIN1 Expression. Blood 2019, 134, 5765. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, K.; Xu, Y.; Peng, H.; Wang, Z.; Liu, S.; Zhang, G. (-)-Epigallocatechin-3-Gallate Induces Cell Apoptosis in Chronic Myeloid Leukaemia by Regulating Bcr/Abl-Mediated P38-MAPK/JNK and JAK2/STAT3/AKT Signalling Pathways. Clin. Exp. Pharmacol. Physiol. 2019, 46, 126–136. [Google Scholar] [CrossRef]
- Huang, Y.; Kumazoe, M.; Bae, J.; Yamada, S.; Takai, M.; Hidaka, S.; Yamashita, S.; Kim, Y.; Won, Y.; Murata, M.; et al. Green Tea Polyphenol Epigallocatechin-O-Gallate Induces Cell Death by Acid Sphingomyelinase Activation in Chronic Myeloid Leukemia Cells. Oncol. Rep. 2015, 34, 1162–1168. [Google Scholar] [CrossRef]
- Iwasaki, R.; Ito, K.; Ishida, T.; Hamanoue, M.; Adachi, S.; Watanabe, T.; Sato, Y. Catechin, Green Tea Component, Causes Caspase-Independent Necrosis-like Cell Death in Chronic Myelogenous Leukemia. Cancer Sci. 2009, 100, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Goker, B.; Caliskan, C.; Onur Caglar, H.; Kayabasi, C.; Balci, T.; Erbaykent Tepedelen, B.; Aygunes, D.; Yilmaz Susluer, S.; Mutlu, Z.; Selvi Gunel, N.; et al. Synergistic Effect of Ponatinib and Epigallocatechin-3-Gallate Induces Apoptosis in Chronic Myeloid Leukemia Cells through Altering Expressions of Cell Cycle Regulatory Genes. J. BUON 2014, 19, 992–998. [Google Scholar] [PubMed]
- Köchling, J.; Schmidt, M.; Rott, Y.; Sagner, M.; Ungefroren, H.; Wittig, B.; Henze, G. Can Anthocyanins Improve Maintenance Therapy of Ph(+) Acute Lymphoblastic Leukaemia? Eur. J. Haematol. 2013, 90, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.I.; Ozturk, S.E.; Ali, T.F.S.; Radwan, M.O.; Tateishi, H.; Koga, R.; Ocak, Z.; Can, M.; Otsuka, M.; Fujita, M. The First Pentacyclic Triterpenoid Gypsogenin Derivative Exhibiting Anti-ABL1 Kinase and Anti-Chronic Myelogenous Leukemia Activities. Biol. Pharm. Bull. 2018, 41, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Trécul, A.; Morceau, F.; Gaigneaux, A.; Orsini, M.; Chateauvieux, S.; Grandjenette, C.; Dicato, M.; Diederich, M. Polyphenol Tri-Vanillic Ester 13c Inhibits P-JAK2V617F and Bcr-Abl Oncokinase Expression in Correlation with STAT3/STAT5 Inactivation and Apoptosis Induction in Human Leukemia Cells. Cancer Lett. 2013, 340, 30–42. [Google Scholar] [CrossRef]
- Sarno, F.; Pepe, G.; Termolino, P.; Carafa, V.; Massaro, C.; Merciai, F.; Campiglia, P.; Nebbioso, A.; Altucci, L. Trifolium Repens Blocks Proliferation in Chronic Myelogenous Leukemia via the BCR-ABL/STAT5 Pathway. Cells 2020, 9, 379. [Google Scholar] [CrossRef]
- Liu, D.; Wang, B.; Zhu, Y.; Yan, F.; Dong, W. Carnosic Acid Regulates Cell Proliferation and Invasion in Chronic Myeloid Leukemia Cancer Cells via Suppressing microRNA-708. J. BUON 2018, 23, 741–746. [Google Scholar] [PubMed]
- Paul, T.; Banerjee, A.; Reddy, S.V.B.; Mahato, S.K.; Biswas, N. Hydroxychavicol Sensitizes Imatinib-Resistant Chronic Myelogenous Leukemia Cells to TRAIL-Induced Apoptosis by ROS-Mediated IAP Downregulation. Anticancer. Drugs 2019, 30, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, F.; Taverna, S.; Alessandro, R.; Fontana, S. SWATH-MS Based Quantitative Proteomics Analysis Reveals That Curcumin Alters the Metabolic Enzyme Profile of CML Cells by Affecting the Activity of miR-22/IPO7/HIF-1α Axis. J. Exp. Clin. Cancer Res. 2018, 37, 170. [Google Scholar] [CrossRef] [PubMed]
- Torello, C.O.; Alvarez, M.C.; Olalla Saad, S.T. Polyphenolic Flavonoid Compound Quercetin Effects in the Treatment of Acute Myeloid Leukemia and Myelodysplastic Syndromes. Molecules 2021, 26, 5781. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).