Rare Coexistence of Atypical Chronic Lymphocytic Leukemia and B-Acute Lymphoblastic Leukemia in a Patient Followed Up for Monoclonal B-Cell Lymphocytosis
Abstract
1. Introduction
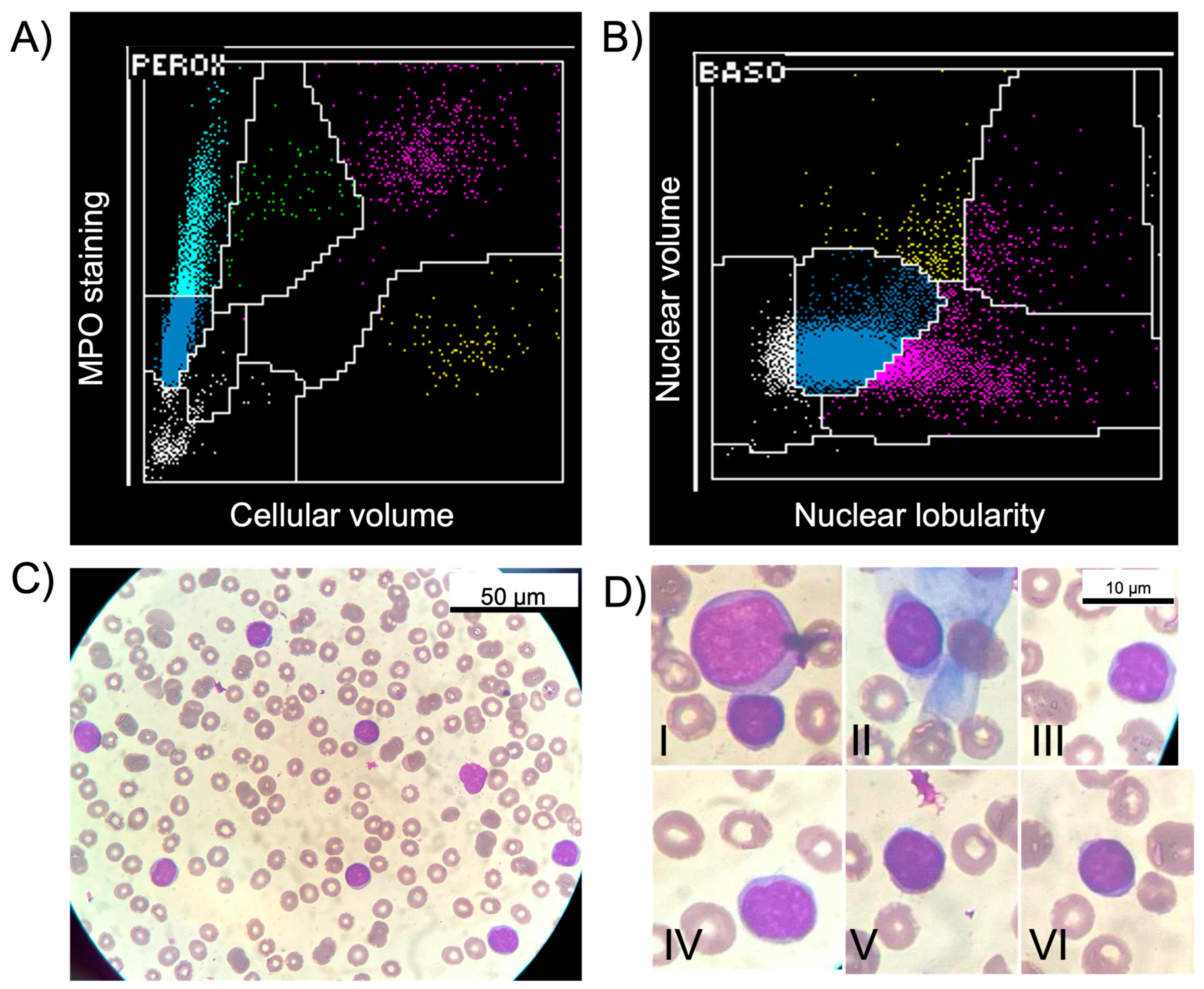
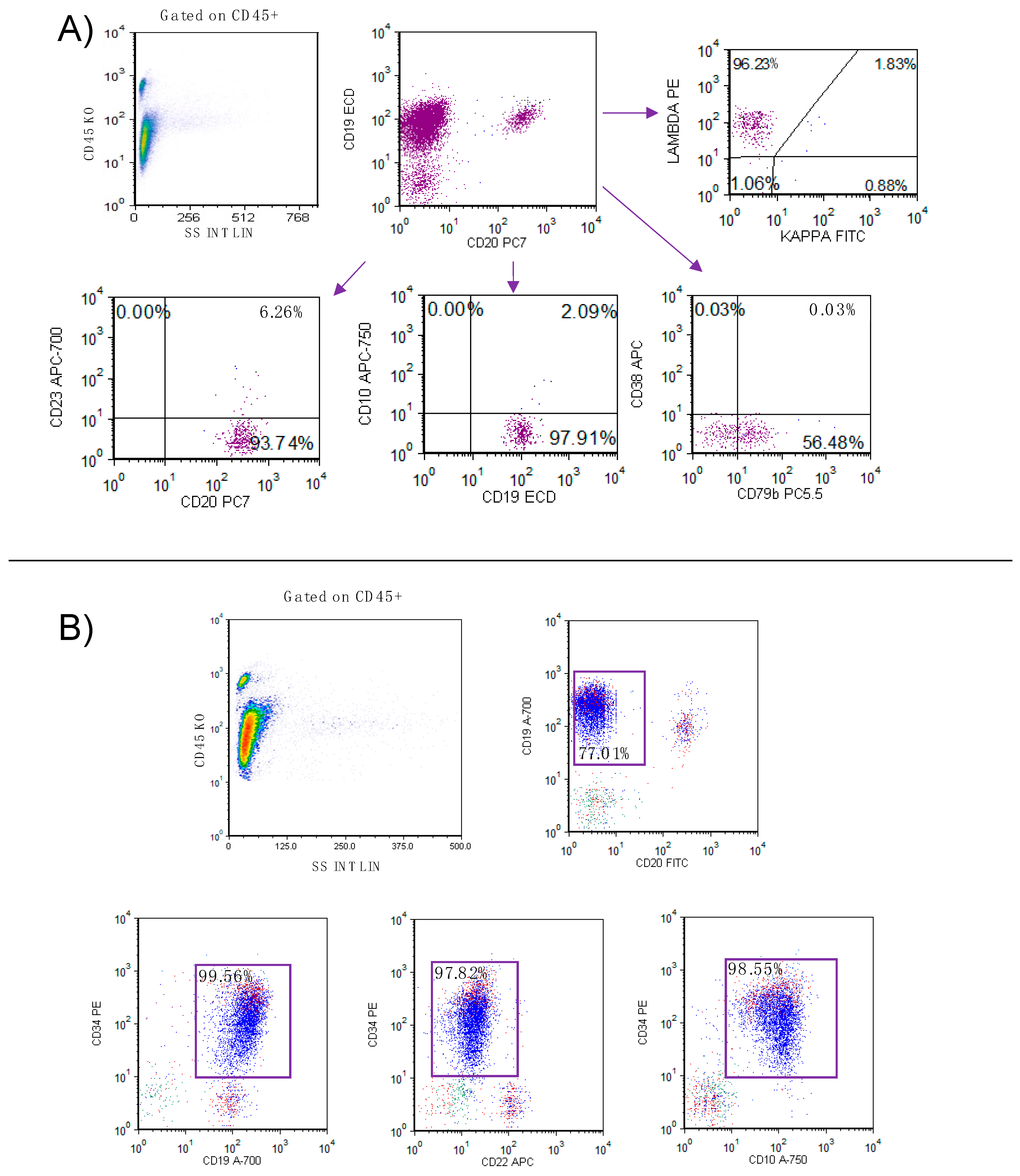
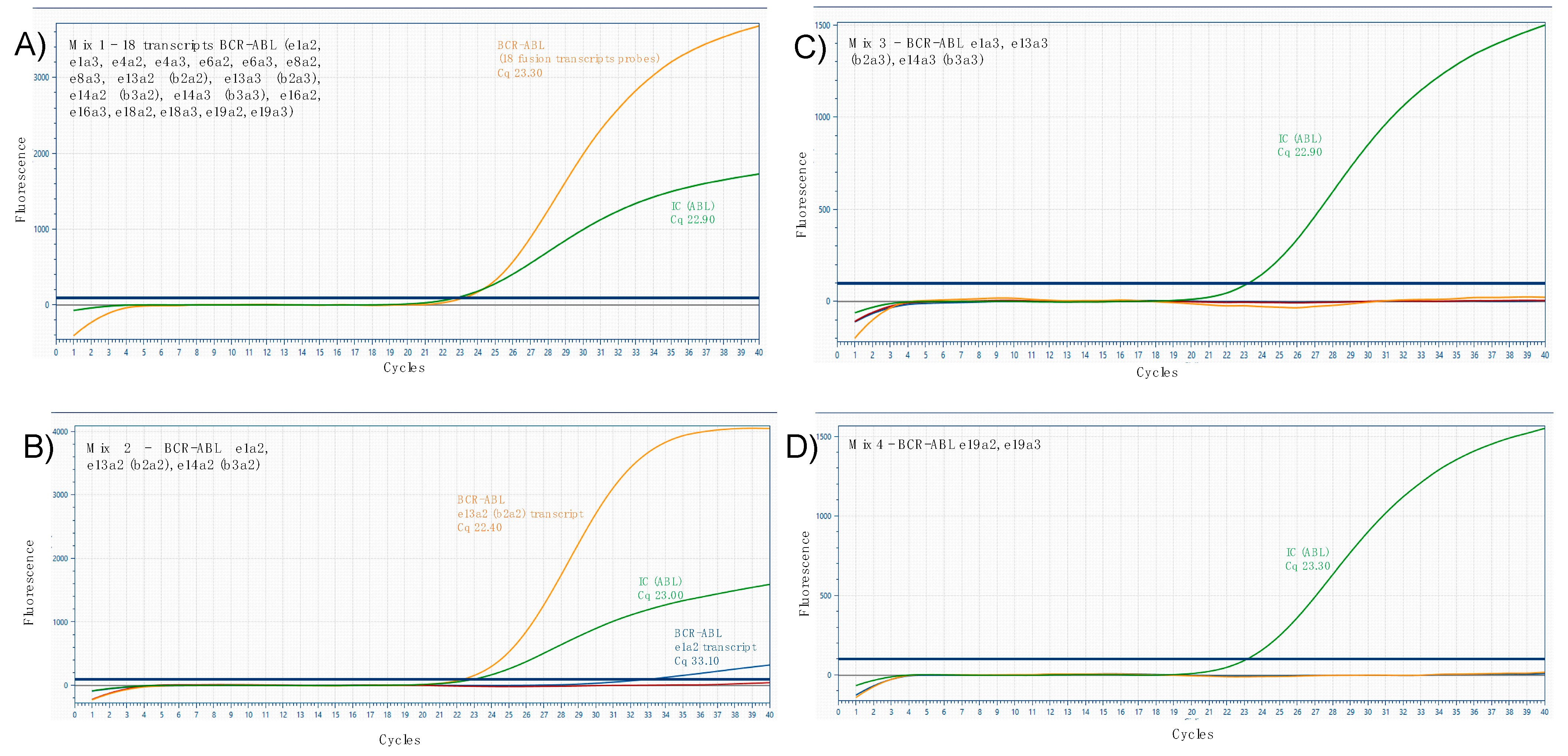
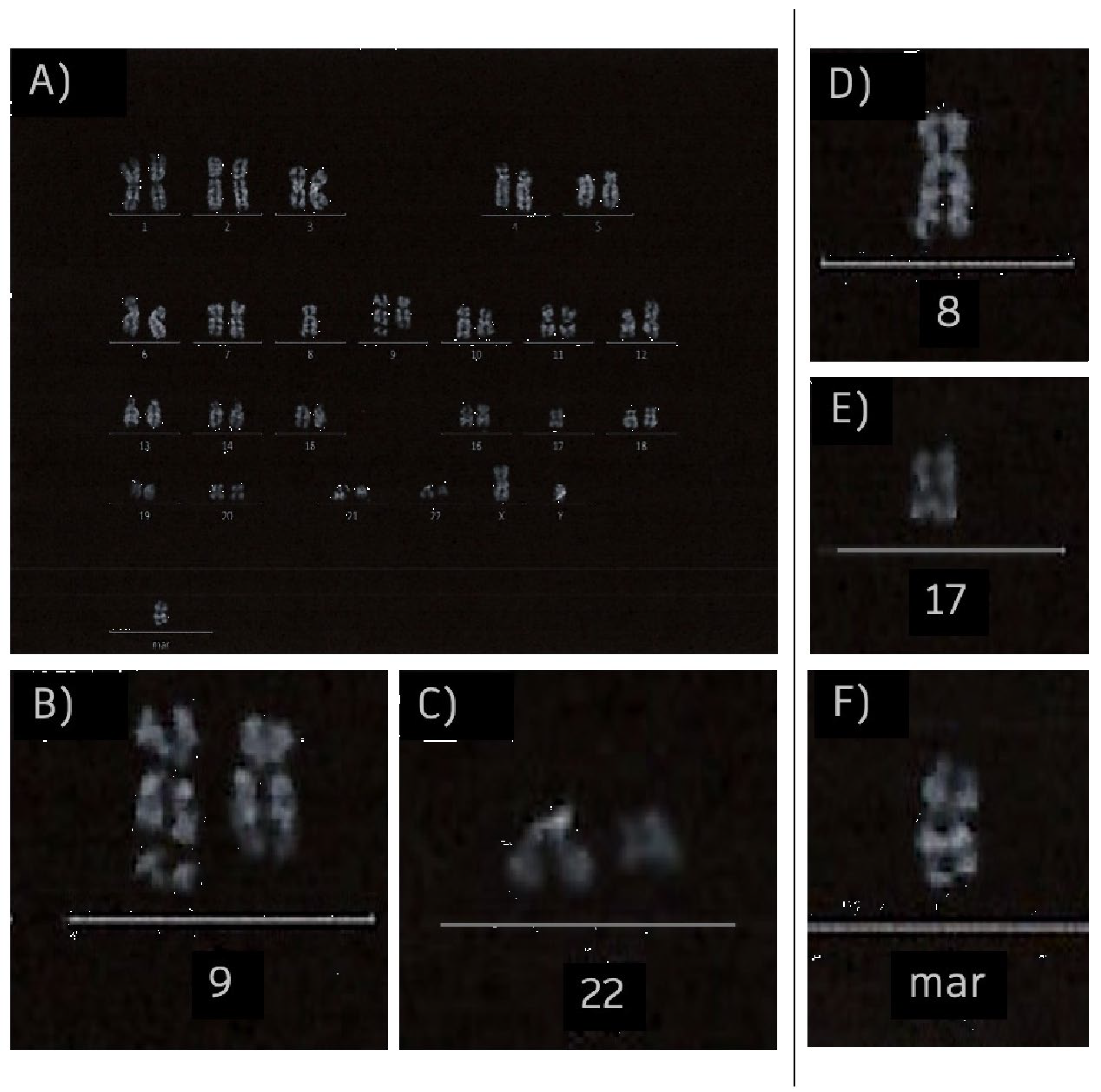
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marti, G.E.; Rawstron, A.C.; Ghia, P.; Hillmen, P.; Houlston, R.S.; Kay, N.; Schleinitz, T.A.; Caporaso, N.; The International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br. J. Haematol. 2005, 130, 325–332. [Google Scholar] [CrossRef]
- Caporaso, N.E.; Marti, G.E.; Landgren, O.; Azzato, E.; Weinberg, J.B.; Goldin, L.; Shanafelt, T. Monoclonal B cell lymphocytosis: Clinical and population perspectives. Cytom. Part B Clin. Cytom. 2010, 78B, S115–S119. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Bennett, F.L.; O’COnnor, S.J.; Kwok, M.; Fenton, J.A.; Plummer, M.; de Tute, R.; Owen, R.G.; Richards, S.J.; Jack, A.S.; et al. Monoclonal B-Cell Lymphocytosis and Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2008, 359, 575–583. [Google Scholar] [CrossRef]
- Criado, I.; Rodríguez-Caballero, A.; Gutiérrez, M.L.; Pedreira, C.E.; Alcoceba, M.; Nieto, W.; Teodosio, C.; Bárcena, P.; Romero, A.; Fernández-Navarro, P.; et al. Low-count monoclonal B-cell lymphocytosis persists after seven years of follow up and is associated with a poorer outcome. Haematologica 2018, 103, 1198–1208. [Google Scholar] [CrossRef]
- Urbaniak, M.; Iskierka-Jażdżewska, E.; Majchrzak, A.; Robak, T. Atypical immunophenotype of chronic lymphocytic leukemia. Acta Haematol. Pol. 2022, 53, 48–52. [Google Scholar] [CrossRef]
- Robak, T.; Krawczyńska, A.; Cebula-Obrzut, B.; Urbaniak, M.; Iskierka-Jażdżewska, E.; Robak, P. Atypical Chronic Lymphocytic Leukemia—The Current Status. Cancers 2023, 15, 4427. [Google Scholar] [CrossRef]
- Frater, J.L.; McCarron, K.F.; Hammel, J.P.; Shapiro, J.L.; Miller, M.L.; Tubbs, R.R.; Pettay, J.; Hsi, E.D. Typical and atypical chronic lymphocytic leukemia differ clinically and immunophenotypically. Am. J. Clin. Pathol. 2001, 116, 655–664. [Google Scholar] [CrossRef]
- D’arena, G.; Vitale, C.; Pietrantuono, G.; Villani, O.; Mansueto, G.; D’auria, F.; Statuto, T.; D’agostino, S.; Sabetta, R.; Tarasco, A.; et al. What Does Atypical Chronic Lymphocytic Leukemia Really Mean? A Retrospective Morphological and Immunophenotypic Study. Cancers 2024, 16, 469. [Google Scholar] [CrossRef] [PubMed]
- Dalivand, M.M.; Tizro, P.; Partain, J.; Aggarwal, A.; Lichy, J.; Ara, F.M.; Nava, V.E.; Tauro, S. Concurrent B Cell Acute Lymphoblastic Lymphoma/Leukemia and Monoclonal B Cell Lymphocytosis: A Case Report with Extensive Molecular Analysis. Case Rep. Hematol. 2022, 2022, 1132544. [Google Scholar] [CrossRef] [PubMed]
- Condoluci, A.; Rossi, D. Richter Syndrome. Richter Syndrome. Curr. Oncol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Gaidano, G.; Mouhssine, S. Immunological Aspects of Richter Syndrome: From Immune Dysfunction to Immunotherapy. Cancers 2023, 15, 1015. [Google Scholar] [CrossRef]
- Favini, C.; Talotta, D.; Almasri, M.; Andorno, A.; Rasi, S.; Adhinaveni, R.; Kogila, S.; Awikeh, B.; Schipani, M.; Boggione, P.; et al. Clonally unrelated Richter syndrome are truly de novo diffuse large B-cell lymphomas with a mutational profile reminiscent of clonally related Richter syndrome. Br. J. Haematol. 2022, 198, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.G. Genetics and prognosis of ALL in children vs adults. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 137–145. [Google Scholar] [CrossRef]
- Preudhomme, C.; Lepelley, P.; Lovi, V.; Zandecki, M.; Cosson, A.; Fenaux, P. T-cell acute lymphoblastic leukemia occurring in the course of b cell chronic lymphocytic leukemia: A case report. Leuk. Lymphoma 1995, 18, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Haznedaroğlu, İ.C.; Savaş, M.C.; Benekli, M.; Sayinalp, N.M.; Özcebe, O.I.; Dündar, S. Acute lymphoblastic leukemia occurring in a case of chronic lymphocytic leukemia. Hematology 1997, 2, 87–89. [Google Scholar] [CrossRef]
- Kajtár, B.; Rajnics, P.; Egyed, M.; Alizadeh, H. Case report: Concomitant chronic lymphocytic leukaemia and cytogenetically normal de novo acute leukaemia in a patient. Ann. Clin. Lab. Sci. 2015, 45, 602–606. [Google Scholar]
- Maurer, C.; Langerbeins, P.; Bahlo, J.; Cramer, P.; Fink, A.M.; Pflug, N.; Engelke, A.; von Tresckow, J.; Kovacs, G.; Stilgenbauer, S.; et al. Effect of first-line treatment on second primary malignancies and Richter’s transformation in patients with CLL. Leukemia. 2016, 10, 2019–2025. [Google Scholar] [CrossRef]
- Raza, S.; Hampel, P.J.; Firwana, B.; Wang, Z.; Shad, Y.; Aluri, L.M.; Naidzionak, U.; Doll, D.C. Chronic lymphocytic leukemia-associated chromosomal abnormalities and risk of secondary malignancies. Blood 2014, 124, 5636. [Google Scholar] [CrossRef]
- Mellemgaard, A.; Geisler, C.H.; Storm, H.H. Risk of kidney cancer and other second solid malignancies in patients with chronic lymphocytic leukemia. Eur. J. Haematol. 1994, 53, 218–222. [Google Scholar] [CrossRef]
- Song, A.; Li, J.Y. Unexpected concurrent B-lymphoblastic leukaemia and untreated chronic lymphocytic leukaemia presenting as worsening thrombocytopenia: A rare case report. Pathology 2025, 57, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q. Cocirculating precursor B acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2013, 122, 2148. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertani, F.; Faiella, F.; Di Franco, C.; Milani, R.; Gualdoni, A.; Fulceri, C.; Costa, E. Rare Coexistence of Atypical Chronic Lymphocytic Leukemia and B-Acute Lymphoblastic Leukemia in a Patient Followed Up for Monoclonal B-Cell Lymphocytosis. Hemato 2025, 6, 27. https://doi.org/10.3390/hemato6030027
Bertani F, Faiella F, Di Franco C, Milani R, Gualdoni A, Fulceri C, Costa E. Rare Coexistence of Atypical Chronic Lymphocytic Leukemia and B-Acute Lymphoblastic Leukemia in a Patient Followed Up for Monoclonal B-Cell Lymphocytosis. Hemato. 2025; 6(3):27. https://doi.org/10.3390/hemato6030027
Chicago/Turabian StyleBertani, Fabio, Francesco Faiella, Claudia Di Franco, Raffaella Milani, Antonella Gualdoni, Cinzia Fulceri, and Elena Costa. 2025. "Rare Coexistence of Atypical Chronic Lymphocytic Leukemia and B-Acute Lymphoblastic Leukemia in a Patient Followed Up for Monoclonal B-Cell Lymphocytosis" Hemato 6, no. 3: 27. https://doi.org/10.3390/hemato6030027
APA StyleBertani, F., Faiella, F., Di Franco, C., Milani, R., Gualdoni, A., Fulceri, C., & Costa, E. (2025). Rare Coexistence of Atypical Chronic Lymphocytic Leukemia and B-Acute Lymphoblastic Leukemia in a Patient Followed Up for Monoclonal B-Cell Lymphocytosis. Hemato, 6(3), 27. https://doi.org/10.3390/hemato6030027







