Simultaneous Central Nervous System and Cutaneous Relapse in Acute Myeloid Leukemia
Abstract
1. Introduction
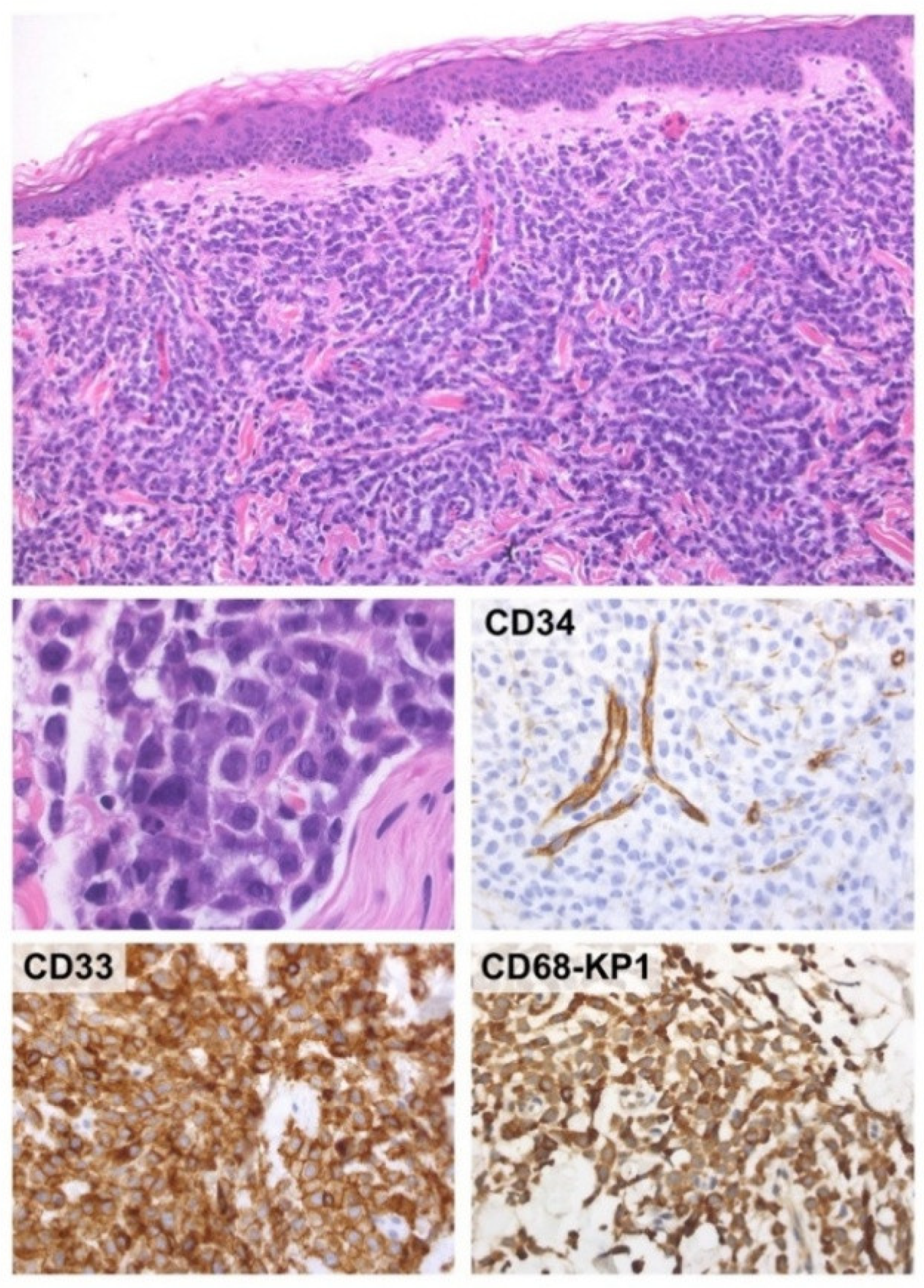
2. Case-Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mrózek, K.; Kohlschmidt, J.; Blachly, J.S.; Nicolet, D.; Carroll, A.J.; Archer, K.J.; Mims, A.S.; Larkin, K.T.; Orwick, S.; Oakes, C.C.; et al. Outcome prediction by the 2022 European LeukemiaNet genetic-risk classification for adults with acute myeloid leukemia: An Alliance study. Leukemia 2023, 37, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, H.A.; Çınar, O.E.; Köylü, N.Y.; Barışta, İ.; Göker, H.; Büyükaşık, Y. Central nervous system involvement in patients with acute myeloid leukemia. Turk. J. Med. Sci. 2021, 51, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Jiao, C.; Yu, J.; Gong, Y.; Jin, D.; Ma, X.; Cui, J.; Wu, Z.; Zhou, J.; Wang, H.; et al. Assessment of 2022 European LeukemiaNet risk classification system in real-world cohort from China. Cancer Med. 2023, 12, 21615–21626. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Long, N.; Saultz, J.; Gandhi, A.; Newell, L.F.; Hayes-Lattin, B.; Maziarz, R.T.; Leonard, J.; Bottomly, D.; McWeeney, S.; et al. Comparison and validation of the 2022 European LeukemiaNet guidelines in acute myeloid leukemia. Blood Adv. 2023, 7, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, X.; Zhu, W.; Cai, X.; Wu, J.; Sun, Z. Tailored central nervous system-directed treatment strategy for isolated CNS recurrence of adult acute myeloid leukemia. Hematology 2014, 19, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Mau, C.; Ghali, M.G.Z.; Styler, M.; Malysz, J.; Specht, C.S.; Rizk, E. Neuroleukemiosis: Diagnosis and management. Clin. Neurol. Neurosurg. 2019, 184, 105340. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T.; Benouaich-Amiel, A.; Bairey, O. Neurologic complications of acute myeloid leukemia. Diagn. Approach Ther. Modalities Blood Rev. 2022, 53, 100910. [Google Scholar]
- Rozovski, U.; Ohanian, M.; Ravandi, F.; Garcia-Manero, G.; Faderl, S.; Pierce, S.; Cortes, J.; Estrov, Z. Incidence of and risk factors for involvement of the central nervous system in acute myeloid leukemia. Leuk. Lymphoma 2015, 56, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Del Principe, M.I.; Buccisano, F.; Soddu, S.; Maurillo, L.; Cefalo, M.; Piciocchi, A.; Consalvo, M.I.; Paterno, G.; Sarlo, C.; De Bellis, E.; et al. Involvement of central nervous system in adult patients with acute myeloid leukemia: Incidence and impact on outcome. Semin. Hematol. 2018, 55, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kostic, I.; Ruiz, M.; Branca, A.; Nabergoj, M.; Piazza, F.; Semenzato, G.; Gurrieri, C.; Briani, C. Possible neuroleukemiosis in two patients with acute myeloid leukemia in complete bone marrow remission. J. Neurol. Sci. 2018, 392, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Fozza, C.; Dore, F.; Isoni, M.A.; Longu, F.; Dessì, L.; Coppola, L.; Contini, S.; Longinotti, M. Strabismus and diplopia in a patient with acute myeloid leukemia. Am. J. Case Rep. 2014, 15, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Gold, H.L.; Grynspan, D.; Kanigsberg, N. Leukemia cutis and facial nerve palsy as presenting symptoms of acute lymphoblastic leukemia. Pediatr. Dermatol. 2014, 31, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Ergin, H.; Özdemir, Ö.M.A.; Karaca, A.; Türk, N.Ş.; Düzcan, F.; Ergin, Ş.; Kazancı, E.; Vergin, C.; Erbay, A. A Newborn with Congenital Mixed Phenotype Acute Leukemia After In Vitro Fertilization. Pediatr. Neonatol. 2015, 56, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Bilavsky, E.; Scheuerman, O.; Marcus, N.; Hoffer, V.; Garty, B.Z. Facial paralysis as a presenting symptom of leukemia. Pediatr. Neurol. 2006, 34, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Salutari, P.; Sica, S.; Micciulli, G.; Rutella, S.; Di Mario, A.; Leone, G. Extramedullary relapse after allogeneic bone marrow transplantation plus buffy-coat in two high risk patients. Haematologica 1996, 81, 182–185. [Google Scholar] [PubMed]
- Shen, H.; Zhao, Y.; Shi, Y.; Sun, J.; Zhou, D.; Li, L.; Ye, X.; Xie, W. The diagnostic and prognostic value of MRI in central nervous system involvement of acute myeloid leukemia: A retrospective cohort of 84 patients. Hematology 2020, 25, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Deak, D.; Gorcea-Andronic, N.; Sas, V.; Teodorescu, P.; Constantinescu, C.; Iluta, S.; Pasca, S.; Hotea, I.; Turcas, C.; Moisoiu, V.; et al. A narrative review of central nervous system involvement in acute leukemias. Ann. Transl. Med. 2021, 9, 68. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerantola, E.; Forlani, L.; Pizzi, M.; Manara, R.; Alaibac, M.; Lessi, F.; Dei Tos, A.P.; Briani, C.; Gurrieri, C. Simultaneous Central Nervous System and Cutaneous Relapse in Acute Myeloid Leukemia. Hemato 2025, 6, 25. https://doi.org/10.3390/hemato6030025
Cerantola E, Forlani L, Pizzi M, Manara R, Alaibac M, Lessi F, Dei Tos AP, Briani C, Gurrieri C. Simultaneous Central Nervous System and Cutaneous Relapse in Acute Myeloid Leukemia. Hemato. 2025; 6(3):25. https://doi.org/10.3390/hemato6030025
Chicago/Turabian StyleCerantola, Eros, Laura Forlani, Marco Pizzi, Renzo Manara, Mauro Alaibac, Federica Lessi, Angelo Paolo Dei Tos, Chiara Briani, and Carmela Gurrieri. 2025. "Simultaneous Central Nervous System and Cutaneous Relapse in Acute Myeloid Leukemia" Hemato 6, no. 3: 25. https://doi.org/10.3390/hemato6030025
APA StyleCerantola, E., Forlani, L., Pizzi, M., Manara, R., Alaibac, M., Lessi, F., Dei Tos, A. P., Briani, C., & Gurrieri, C. (2025). Simultaneous Central Nervous System and Cutaneous Relapse in Acute Myeloid Leukemia. Hemato, 6(3), 25. https://doi.org/10.3390/hemato6030025







