Intrachromosomal Amplification of Chromosome 21 (iAMP21) Impacts Event-Free Survival but Not Overall Survival Among Pediatric Patients with Acute Lymphoblastic Leukemia: A Single-Center Experience Using an Asparaginase-Intensified Spanish Regimen
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics, Risk-Group Assignment, and Early Treatment Response Evaluation
3.2. Outcome and Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| BCP | B-cell precursor |
| BM | Bone marrow |
| CIR | Cumulative incidence of relapse |
| CNS | Central nervous system |
| CR | Complete remission |
| CR2 | Second complete remission |
| EFS | Event-free survival |
| FISH | Fluorescence in situ hybridization |
| FLAG-Ida | Fludarabine, cytarabine, and idarubicin |
| FC | Flow cytometry |
| HR | High risk |
| iAMP21 | Intrachromosomal amplification of chromosome 21 |
| IR | Intermediate risk |
| NCI | National Cancer Institute |
| MRD | Measurable residual disease |
| MLPA | Multiplex ligation-dependent probe amplification |
| MRD | Measurable residual disease |
| OS | Overall survival |
| PCR | Polymerase chain reaction |
| NCI | National Cancer Institute |
| NGS | Next-generation sequencing |
| PEG | Pegylated |
| SCT | Allogeneic stem cell transplantation |
| SEHOP | Spanish Society of Pediatric Hematology and Oncology |
| SR | Standard risk |
| TRM | Treatment-related mortality |
| VAF | Variant allele frequencies |
| WBC | White blood cell count |
| WHO | World Health Organization |
References
- Hunger, S.P.; Mullighan, C.G. Acute lymphoblastic leukemia in children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Haas, O.; Harbott, J.; Biondi, A.; Stanulla, M.; Trka, J.; Izraeli, S. Detection of prognostically relevant genetic abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: Recommendations from the Biology and Diagnosis Committee of the International Berlin-Frankfurt-Munster study group. Br. J. Haematol. 2010, 151, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J. Blood Spotlight on iAMP21 acute lymphoblastic leukemia (ALL), a high-risk pediatric disease. Blood 2015, 125, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.V.; Ensor, H.M.; Richards, S.M.; Chilton, L.; Schwab, C.; Kinsey, S.E.; Vora, A.; Mitchell, C.D.; Harrison, C.J. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: Results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010, 11, 429–438. [Google Scholar] [CrossRef]
- Heerema, N.A.; Carroll, A.J.; Devidas, M.; Loh, M.L.; Borowitz, M.J.; Gastier-Foster, J.M.; Larsen, E.C.; Mattano, L.A., Jr.; Maloney, K.W.; Willman, C.L.; et al. Intrachromosomal amplification of chromosome 21 is associated with inferior outcomes in children with acute lymphoblastic leukemia treated in contemporary standard-risk children’s oncology group studies: A report from the children’s oncology group. J. Clin. Oncol. 2013, 31, 3397–3402. [Google Scholar] [CrossRef]
- Moorman, A.V.; Robinson, H.; Schwab, C.; Richards, S.M.; Hancock, J.; Mitchell, C.D.; Goulden, N.; Vora, A.; Harrison, C.J. Risk-directed treatment intensification significantly reduces the risk of relapse among children and adolescents with acute lymphoblastic leukemia and intrachromosomal amplification of chromosome 21: A comparison of the MRC ALL97/99 and UKALL2003 trials. J. Clin. Oncol. 2013, 31, 3389–3396. [Google Scholar] [CrossRef]
- Campana, D.; Pui, C.H. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood 2017, 129, 1913–1918. [Google Scholar] [CrossRef]
- Vora, A.; Goulden, N.; Wade, R.; Mitchell, C.; Hancock, J.; Hough, R.; Rowntree, C.; Richards, S. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2013, 14, 199–209. [Google Scholar] [CrossRef]
- Vora, A.; Goulden, N.; Mitchell, C.; Hancock, J.; Hough, R.; Rowntree, C.; Moorman, A.V.; Wade, R. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2014, 15, 809–818. [Google Scholar] [CrossRef]
- Pieters, R.; de Groot-Kruseman, H.; Van der Velden, V.; Fiocco, M.; van den Berg, H.; de Bont, E.; Egeler, R.M.; Hoogerbrugge, P.; Kaspers, G.; Van der Schoot, E.; et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J. Clin. Oncol. 2016, 34, 2591–2601. [Google Scholar] [CrossRef]
- Harewood, L.; Robinson, H.; Harris, R.; Al-Obaidi, M.J.; Jalali, G.R.; Martineau, M.; Moorman, A.V.; Sumption, N.; Richards, S.; Mitchell, C.; et al. Amplification of AML1 on a duplicated chromosome 21 in acute lymphoblastic leukemia: A study of 20 cases. Leukemia 2003, 17, 547–553. [Google Scholar] [CrossRef]
- Moorman, A.V.; Richards, S.M.; Robinson, H.M.; Strefford, J.C.; Gibson, B.E.; Kinsey, S.E.; Eden, T.O.B.; Vora, A.J.; Mitchell, C.D.; Harrison, C.J.; et al. Prognosis of children with acute lymphoblastic leukemia (ALL) and intrachromosomal amplification of chromosome 21 (iAMP21). Blood 2007, 109, 2327–2330. [Google Scholar] [CrossRef]
- Attarbaschi, A.; Mann, G.; Panzer-Grumayer, R.; Rottgers, S.; Steiner, M.; Konig, M.; Csinady, E.; Dworzak, M.N.; Seidel, M.; Janousek, D.; et al. Minimal residual disease values discriminate between low and high relapse risk in children with B-cell precursor acute lymphoblastic leukemia and an intrachromosomal amplification of chromosome 21: The Austrian and German acute lymphoblastic leukemia Berlin-Frankfurt-Munster (ALL-BFM) trials. J. Clin. Oncol. 2008, 26, 3046–3050. [Google Scholar] [PubMed]
- Rand, V.; Parker, H.; Russell, L.J.; Schwab, C.; Ensor, H.; Irving, J.; Jones, L.; Masic, D.; Minto, L.; Morrison, H.; et al. Genomic characterization implicates iAMP21 as a likely primary genetic event in childhood B-cell precursor acute lymphoblastic leukemia. Blood 2011, 117, 6848–6855. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Moorman, A.V.; Schwab, C.; Carroll, A.J.; Raetz, E.A.; Devidas, M.; Strehl, S.; Nebral, K.; Harbott, J.; Teigler-Schlegel, A.; et al. An international study of intrachromosomal amplification of chromosome 21 (iAMP21): Cytogenetic characterization and outcome. Leukemia 2014, 28, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.L.; Matheson, E.; Grossmann, V.; Sinclair, P.; Bashton, M.; Schwab, C.; Towers, W.; Partington, M.; Elliott, A.; Minto, L.; et al. The role of the RAS pathway in iAMP21-ALL. Leukemia 2016, 30, 1824–1831. [Google Scholar] [CrossRef]
- Irving, J.A.E.; Enshaei, A.; Parker, C.A.; Sutton, R.; Kuiper, R.P.; Erhorn, A.; Minto, L.; Venn, N.C.; Law, T.; Yu, J.; et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood 2016, 128, 911–922. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2009; pp. 109–138. [Google Scholar]
- Bene, M.C.; Castoldi, G.; Knapp, W.; Ludwig, W.D.; Matutes, E.; Orfao, A.; van’t Veer, M.B. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 1995, 9, 1783–1786. [Google Scholar]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Gao, Q.; Ryan, S.L.; Iacobucci, I.; Ghate, P.S.; Cranston, R.E.; Schwab, C.; Elsayed, A.H.; Shi, L.; Pounds, S.B.; Lei, S.; et al. The genomic landscape of acute lymphoblastic leukemia with intrachromosomal amplification of chromosome 21. Blood 2023, 142, 711–723. [Google Scholar] [CrossRef]
- Koleilat, A.; Smadbeck, J.B.; Zepeda-Mendoza, C.J.; Williamson, C.M.; Pitel, B.A.; Golden, C.L.; Xu, X.; Greipp, P.T.; Ketterling, R.P.; Hoppman, N.L.; et al. Characterization of unusual iAMP21 B-lymphoblastic leukemia (iAMP21-ALL) from the Mayo Clinic and Children’s Oncology Group. Genes Chromosomes Cancer 2022, 61, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.D.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Hormann, F.M.; Østergaard, A.; van den Broek, S.; Boeree, A.; van de Ven, C.; Escherich, G.; Sonneveld, E.; Boer, J.M.; den Boer, M.L. Secondary lesions and sensitivity to signaling inhibitors in iAMP21 acute lymphoblastic leukemia. Hemasphere 2025, 9, e70069. [Google Scholar] [CrossRef] [PubMed]
- Izraeli, S. Application of genomics for risk stratification of childhood acute lymphoblastic leukaemia: From bench to bedside? Br. J. Haematol. 2010, 151, 119–131. [Google Scholar] [CrossRef]
- Gottschalk Højfeldt, S.; Grell, K.; Abrahamsson, J.; Lund, B.; Vettenranta, K.; Jónsson, Ó.G.; Frandsen, T.L.; Wolthers, B.O.; Marquart, H.V.H.; Vaitkeviciene, G.; et al. Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood 2021, 137, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Gómez, G.; Minguela, A.; Tazón-Vega, B.; Ribera, J.; Galián, J.A.; Martínez-Banaclocha, H.; García-Garay, M.; Velasco, P.; Fuster-Soler, J.L.; Armengol, G.; et al. Clonal heterogeneity and genomic evolution in intrachromosomal amplification of chromosome 21: A case report Case Report. Br. J. Haematol. 2024, 204, 2512–2515. [Google Scholar] [CrossRef]
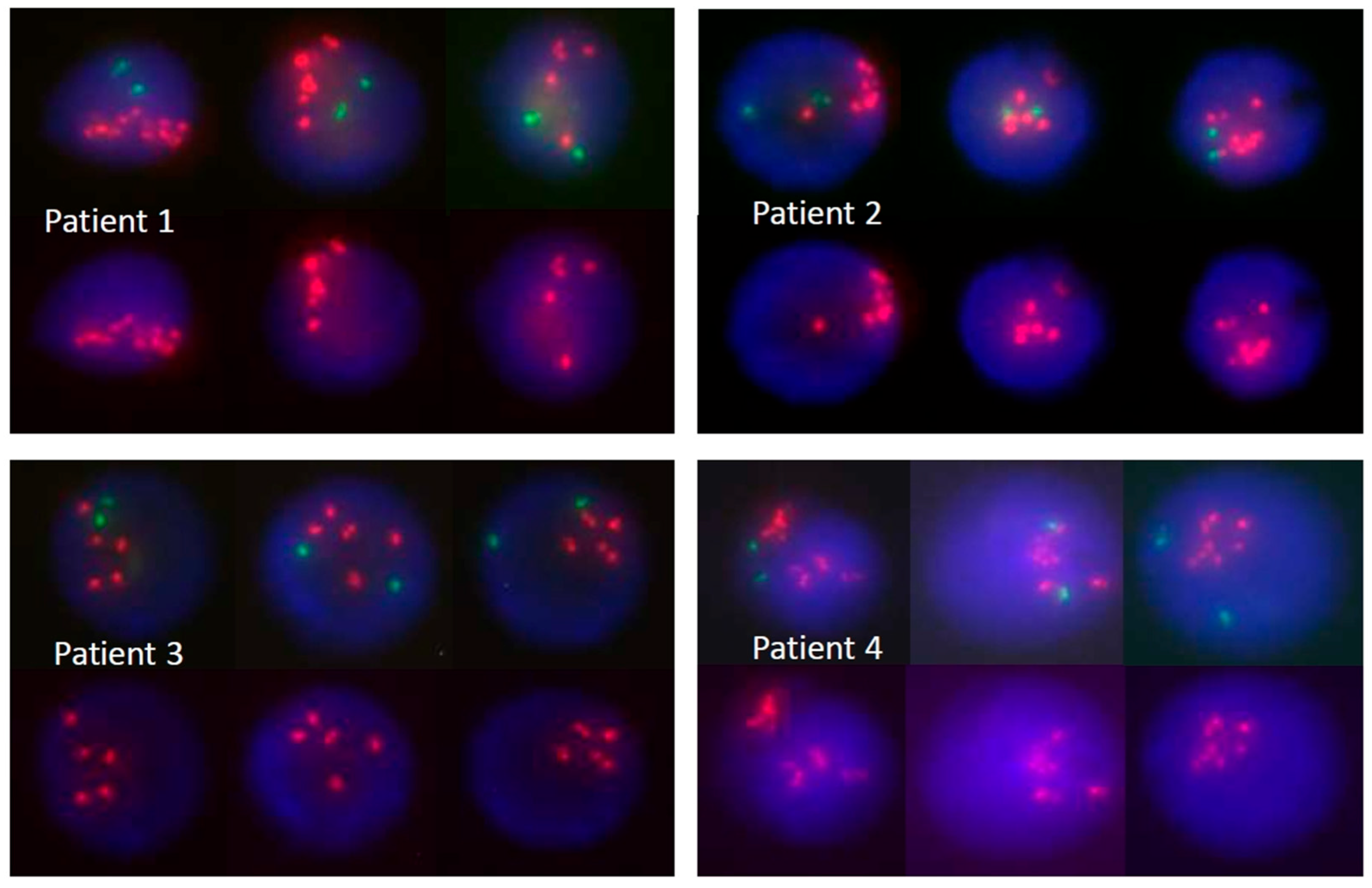
| Biologic Subtype | Diagnostic Method | Comment |
|---|---|---|
| BCR/ABL1 | FISH and/or PCR | Identification of translocation t(9; 22)(q34; q11.2) with FISH and/or BCR/ABL1 fusion with PCR |
| KMT2A-r | FISH and/or PCR | Identification of any translocation t(v; 11q23) with FISH and/or any KMT2A-r with PCR |
| ETV6/RUNX1 | FISH and/or PCR | Identification of translocation t(12; 21)(q13; q22) with FISH and/or ETV6/RUNX1 fusion with PCR |
| TCF3 (E2A)/PBX1 | FISH and/or PCR | Identification of translocation t(1; 19)(q23; p13.3) with FISH and/or TCF3 (E2A)/PBX1 fusion with PCR |
| High hyperdiploidy | Conventional cytogenetics and/or DNA index by FC | 51 to 67 chromosomes and/or DNI index 1.1 to 1.44 by FC |
| Low hypodiploidy | Conventional cytogenetics and/or DNA index by FC | <44 chromosomes and/or a DNA index <0.81 |
| iAMP21 | FISH | Identification of ≥5 copies of RUNX1 in interphase |
| B-other | FC, FISH and PCR | B-cell precursor immunophenotype and negative results from all previous diagnostic markers |
| T-immunophenotype | FC | According to EGIL criteria (see Bene et al., Leukemia-1995, reference number [19]) |
| Unclassifiable | All previous diagnostic methods | Insufficient results available |
| iAMP21-Positive Patients | Non-iAMP21 Patients | p | ||||
|---|---|---|---|---|---|---|
| Total | n = 4 | % | n = 85 | % | ||
| Sex | Male | 1 | 25 | 49 | 57.6 | 0.31 |
| Female | 3 | 75 | 36 | 42.4 | ||
| Age (years) 1 | <10 | 3 | 75 | 61 | 71.7 | 1 |
| ≥10 | 1 | 25 | 24 | 28.3 | ||
| Ethnic group | Caucasian | 3 | 75 | 64 | 75.3 | 1 |
| Hispanic | 1 | 25 | 15 | 17.6 | ||
| Other | 0 | 0 | 6 | 7.1 | ||
| WBC (×109/L) 2 | <50 | 4 | 100 | 67 | 78.8 | 0.58 |
| ≥50 | 0 | 0 | 18 | 21.2 | ||
| Immunophenotype | BCP | 4 | 100 | 70 | 82.4 | 0.43 |
| T | 0 | 0 | 15 | 17.6 | ||
| CNS involvement 3 | CNS1 | 4 | 100 | 78 | 91.8 | 1 |
| CNS2 | 0 | 0 | 3 | 3.5 | ||
| CNS3 | 0 | 0 | 4 | 4.7 | ||
| NCI risk group 4 | Standard | 3 | 75 | 48 | 56.5 | 0.63 |
| High | 1 | 25 | 37 | 43.5 | ||
| Initial risk group 5 | Standard | 0 | 0 | 22 | 25.9 | 0.67 |
| Intermediate | 4 | 100 | 57 | 67 | ||
| High | 0 | 0 | 6 | 7.1 | ||
| Day +8 blast count on PB | <1 × 109/L | 4 | 100 | 79 | 92.9 | 1 |
| ≥1 × 109/L | 0 | 0 | 6 | 7.1 | ||
| Day +15 BM blasts | <25% | 3 | 75 | 71 | 83.5 | 0.55 |
| ≥25% | 1 | 25 | 9 | 10.6 | ||
| NA | 0 | 0 | 5 | 5.9 | ||
| Day +15 MRD | <10% | 3 | 75 | 70 | 82.4 | 0.55 |
| ≥10% | 1 | 25 | 15 | 17.6 | ||
| Day +15 MRD | <0.1% | 3 | 75 | 51 | 60 | 1 |
| ≥0.1% | 1 | 25 | 34 | 40 | ||
| Day +33 BM blasts | <5% | 3 | 75 | 80 | 94.1 | 0.17 |
| ≥5% | 1 | 25 | 3 | 3.5 | ||
| NA | 0 | 0 | 2 | 2.4 | ||
| Day +33 MRD | <1% | 3 | 75 | 78 | 91.8 | 0.28 |
| ≥1% | 1 | 25 | 6 | 7 | ||
| NA | 0 | 0 | 1 | 1.2 | ||
| Day +78 MRD 5 | <0.1% | 3 | 100 | 76 | 89.4 | 1 |
| ≥0.1% | 0 | 0 | 3 | 3.5 | ||
| NA | 1 | - | 6 | 7.1 | ||
| Final risk-group 6 | Standard | 0 | 0 | 14 | 16.5 | 1 |
| Intermediate | 3 | 75 | 52 | 61.2 | ||
| High | 0 | 0 | 19 | 22.3 | ||
| Induction failure | 1 | 25 | 0 |
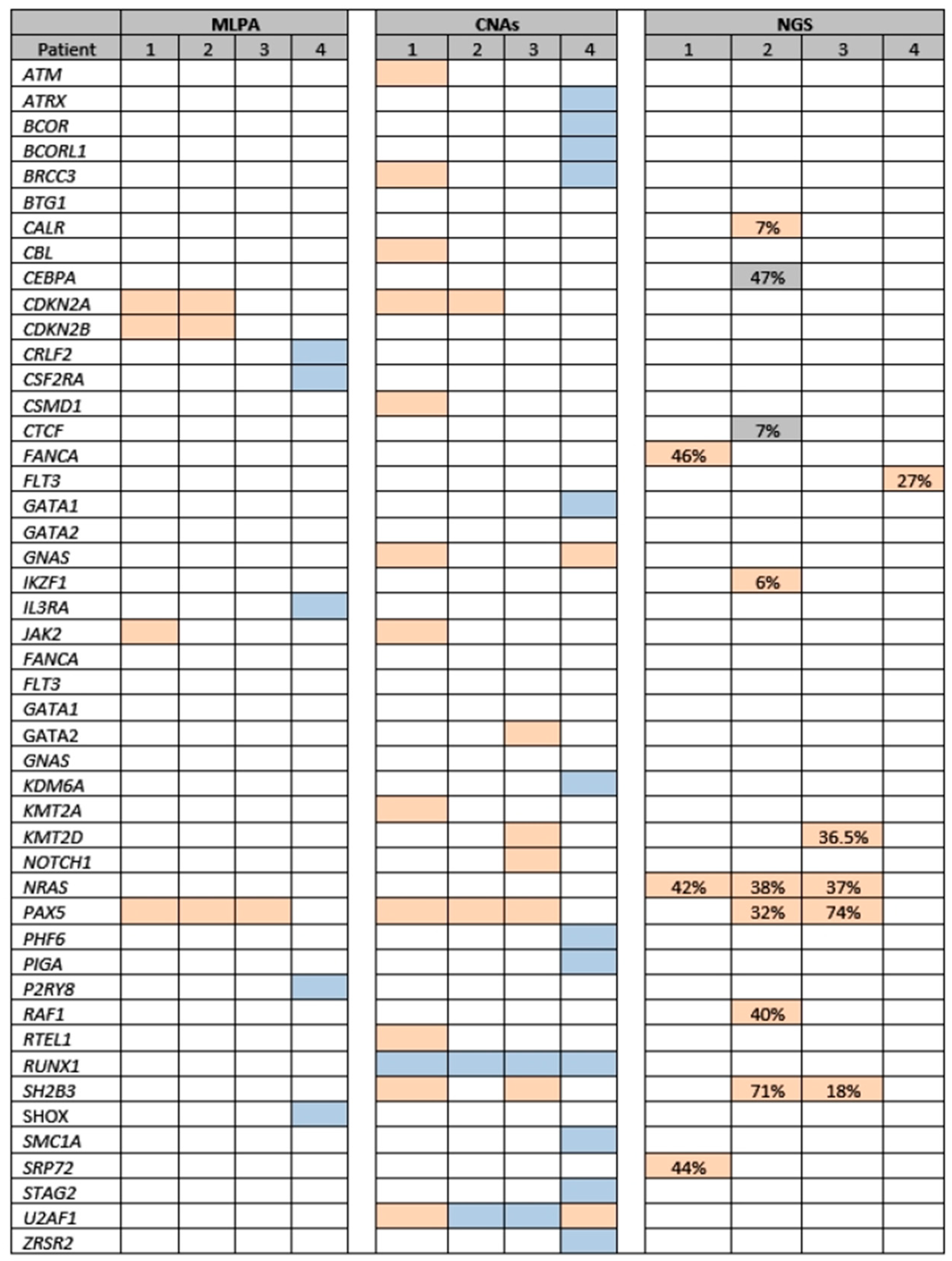
| Pt | MLPA 1 | NGS-CNAs 1 | NGS-SNVs/ INDELS 1 | Event | Time to Event (m) | Tt. After Event | Response | Subsequent Treatment | Outcome (m) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CDKN2A, CDKN2B, JAK2, and PAX5 deletions | CDKN2A, JAK2, PAX5, CSMD1, KMT2A, ATM, CBL, SH2B3, RTEL1, GNAS, U2AF1, and BRCC3 losses; RUNX1 gain | FANCA, NRAS, SRP72 2 | Ref. | 0 | FLAG-Ida | CR1 | SCT | AIR (59) |
| 2 | CDKN2A, CDKN2B, and PAX5 deletions | CDKN2A and PAX5 losses, SH2B, RUNX1, and U2AF1 gains | CEBPA, NRAS, PAX5, SH2B3, IKZF1, CALR, CTCF, RAF1 3 | Rel. | 54 | CT | CR2 | Blina + SCT | AIR (89) |
| 3 | PAX5 deletion | PAX5, SH2B, KMT2D, NOTCH1, and GATA2 losses; RUNX1 and U2AF1 gains | NRAS, PAX5, SH2B3, KMT2D 4 | Rel. | 24 | InO | CR2 | HR + Blina + SCT | AIR (33) |
| 4 | BTG1 Deletion; CRLF2, CSF2RA, IL3RA, P2RY8 and SHOX (PAR1 region) duplications | GNAS and U2AF1 losses; RUNX1, BCORL1, BRCC3, GATA1, ZRSR2, STAG2, PHF6, KDM6A, ATRX, SMC1A, PIGA, and BCOR gains | FLT3 5 | No event | NA | NA | NA | NA | AIR (111) |
| 5-Year EFS (%) | 95% CI | p | ||
|---|---|---|---|---|
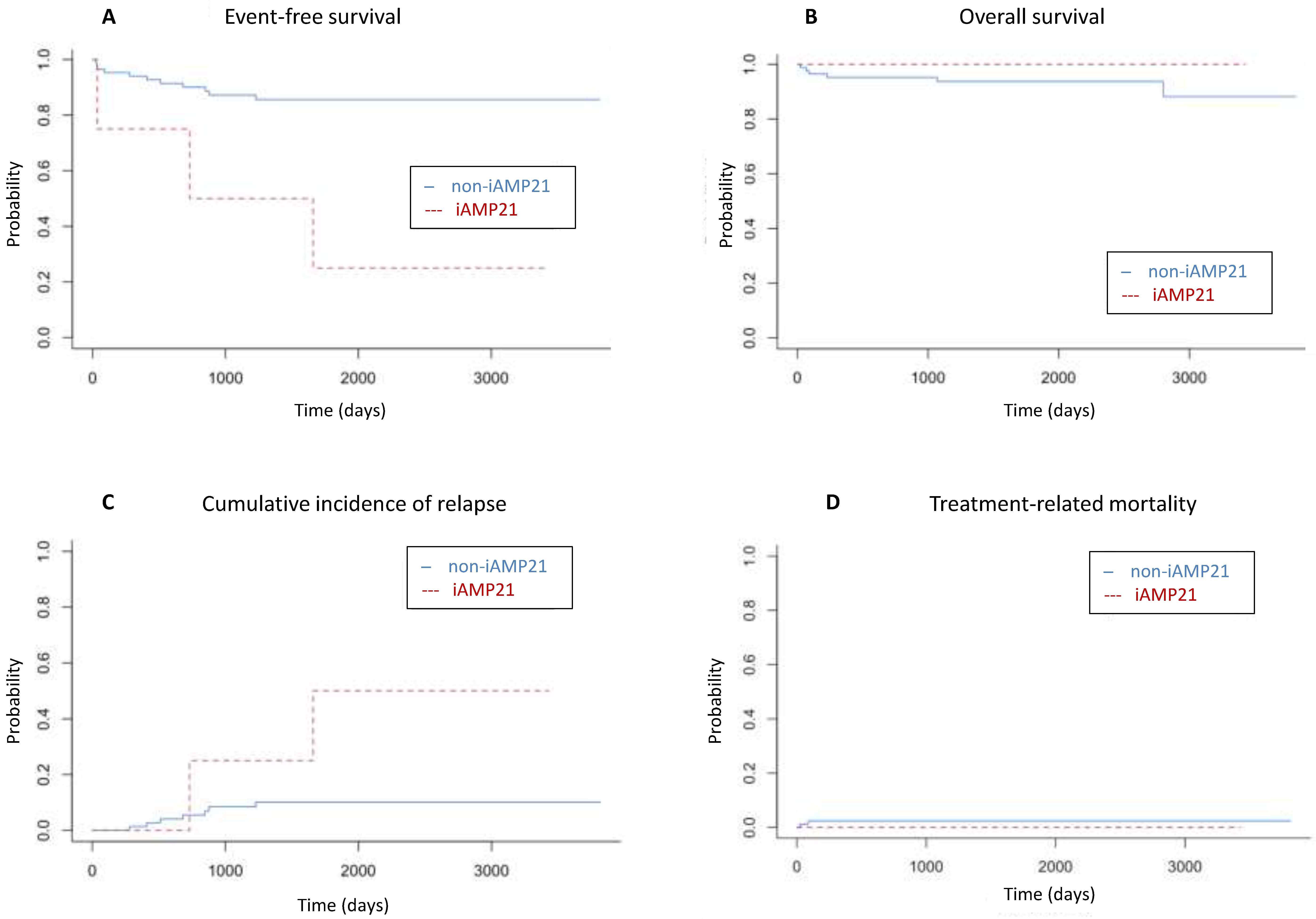
| iAMP21 | Positive | 25 | 4.5–100 | 0.001 |
| Negative | 85.6 | 78–93.9 | ||
| Sex | Male | 85.3 | 74.7–97.4 | 0.3 |
| Female | 77.1 | 64.3–92.5 | ||
| Age | <10 years | 83.2 | 74.2–93.3 | 0.8 |
| ≥10 years | 78 | 60–100 | ||
| Ethnic group | Caucasian | 86.2 | 77.6–95.8 | 0.006 |
| Hispanic | 53.9 | 32.3–90.1 | ||
| Other | 100 | - | ||
| WBC (×109/L) | <20 | 81.8 | <20 | 0.9 |
| ≥20 | 82.3 | ≥20 | ||
| WBC (×109/L) | <50 | 83.4 | <50 | 0.4 |
| ≥50 | 75.9 | ≥50 | ||
| NCI risk score | Standard | 83.2 | 73.1–94.6 | 0.9 |
| High | 79.7 | 65.6–96.8 | ||
| CNS involvement 1 | CNS1 | 82.8 | 74.2–92.4 | 0.6 |
| CNS2 | 66.7 | 30–100 | ||
| CNS3 | 75 | 42.6–100 | ||
| Initial risk-group 2 | Standard | 90.9 | 79.7–100 | 0.6 |
| Intermediate | 78.1 | 67.2–90.9 | ||
| High | 80 | 51.6–100 | ||
| Day +8 blast count on PB (×109/L) | <1 | 83.2 | 74.7–92.6 | 0.1 |
| ≥1 | 62.5 | 32–100 | ||
| Day +15 BM blasts | <25% | 83.9 | 75–93.8 | 0.1 |
| ≥25% | 68.6 | 44.5–100 | ||
| Day +15 BM blasts | <5% | 85.4 | 76.3–95.5 | 0.02 |
| ≥5% | 65.5 | 44.9–95.4 | ||
| Day +15 MRD | <10% | 83.6 | 74.6–93.7 | 0.2 |
| ≥10% | 74.5 | 55.7–99.6 | ||
| Day +15 MRD | <0.1% | 84.1 | 73.9–95.9 | 0.3 |
| ≥0.1% | 79.1 | 66.4–94.2 | ||
| Day +33 BM blasts | <5% | 86.7 | 78.8–95.4 | <0.001 |
| ≥5% | 0 | - | ||
| Day +33 MRD | <1% | 86.4 | 78.4–95.3 | <0.001 |
| ≥1% | 42.9 | 18.2–100 | ||
| Day +78 MRD | <0.1% | 87.6 | 79.8–96.5 | 0.2 |
| ≥0.1% | 66.7 | 30–100 | ||
| Final risk-group 2 | Standard | 100 | - | 0.1 |
| Intermediate | 79.4 | 68–92.8 | ||
| High | 73 | 55.3–96.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, M.; Ramos-Elbal, E.; Galián, J.A.; Martínez-Banaclocha, H.; Plaza, M.; Martínez-Sánchez, V.; Galera, A.M.; Jiménez, I.; Llinares, M.E.; Bermúdez, M.; et al. Intrachromosomal Amplification of Chromosome 21 (iAMP21) Impacts Event-Free Survival but Not Overall Survival Among Pediatric Patients with Acute Lymphoblastic Leukemia: A Single-Center Experience Using an Asparaginase-Intensified Spanish Regimen. Hemato 2025, 6, 19. https://doi.org/10.3390/hemato6030019
Hidalgo M, Ramos-Elbal E, Galián JA, Martínez-Banaclocha H, Plaza M, Martínez-Sánchez V, Galera AM, Jiménez I, Llinares ME, Bermúdez M, et al. Intrachromosomal Amplification of Chromosome 21 (iAMP21) Impacts Event-Free Survival but Not Overall Survival Among Pediatric Patients with Acute Lymphoblastic Leukemia: A Single-Center Experience Using an Asparaginase-Intensified Spanish Regimen. Hemato. 2025; 6(3):19. https://doi.org/10.3390/hemato6030019
Chicago/Turabian StyleHidalgo, María, Eduardo Ramos-Elbal, José Antonio Galián, Helios Martínez-Banaclocha, Mercedes Plaza, Victoria Martínez-Sánchez, Ana María Galera, Irene Jiménez, María Esther Llinares, Mar Bermúdez, and et al. 2025. "Intrachromosomal Amplification of Chromosome 21 (iAMP21) Impacts Event-Free Survival but Not Overall Survival Among Pediatric Patients with Acute Lymphoblastic Leukemia: A Single-Center Experience Using an Asparaginase-Intensified Spanish Regimen" Hemato 6, no. 3: 19. https://doi.org/10.3390/hemato6030019
APA StyleHidalgo, M., Ramos-Elbal, E., Galián, J. A., Martínez-Banaclocha, H., Plaza, M., Martínez-Sánchez, V., Galera, A. M., Jiménez, I., Llinares, M. E., Bermúdez, M., Minguela, A., & Fuster, J. L. (2025). Intrachromosomal Amplification of Chromosome 21 (iAMP21) Impacts Event-Free Survival but Not Overall Survival Among Pediatric Patients with Acute Lymphoblastic Leukemia: A Single-Center Experience Using an Asparaginase-Intensified Spanish Regimen. Hemato, 6(3), 19. https://doi.org/10.3390/hemato6030019







