Successful Perioperative Management of Titanium Cranioplasty in a Patient with Severe Hemophilia A
Abstract
1. Introduction
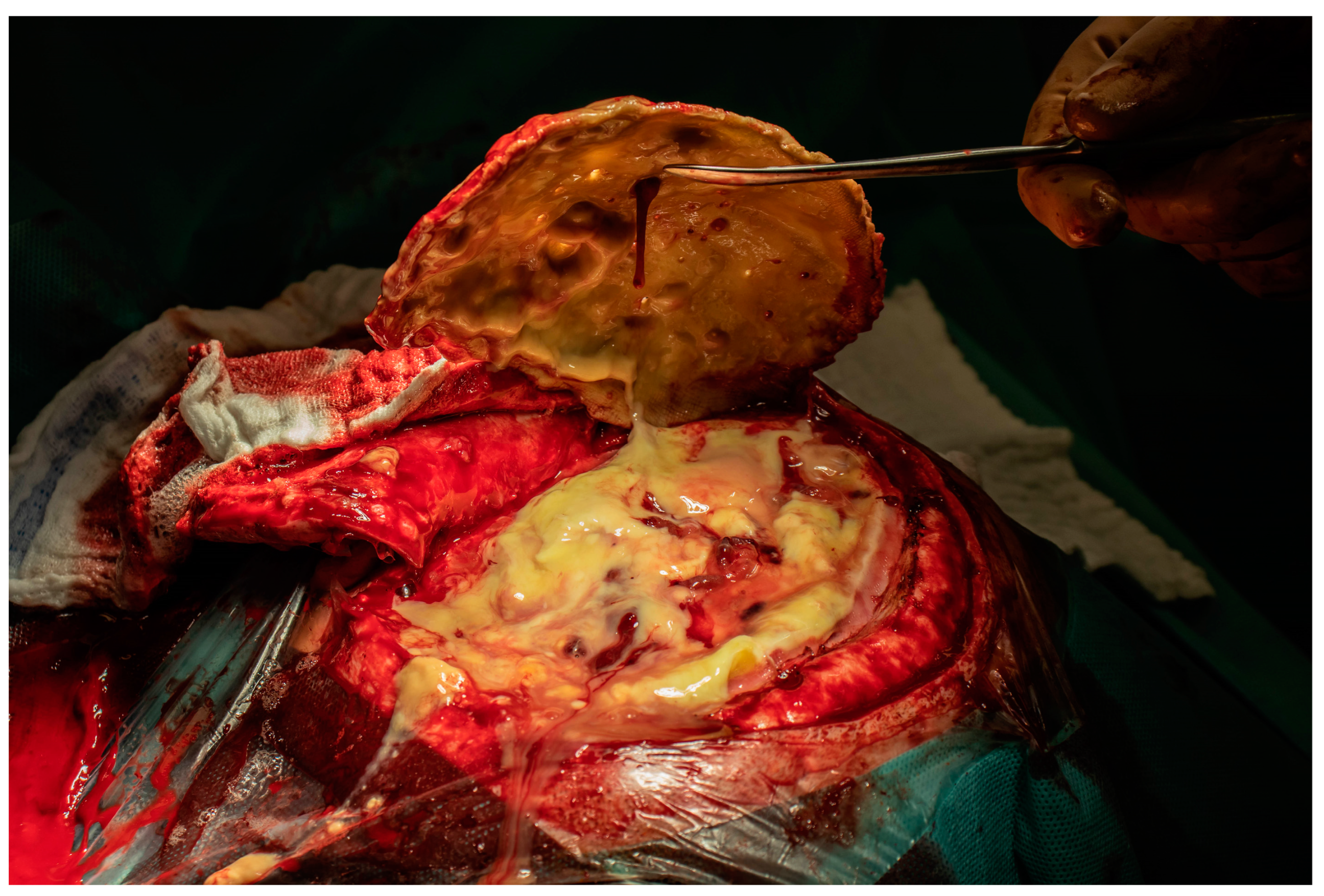
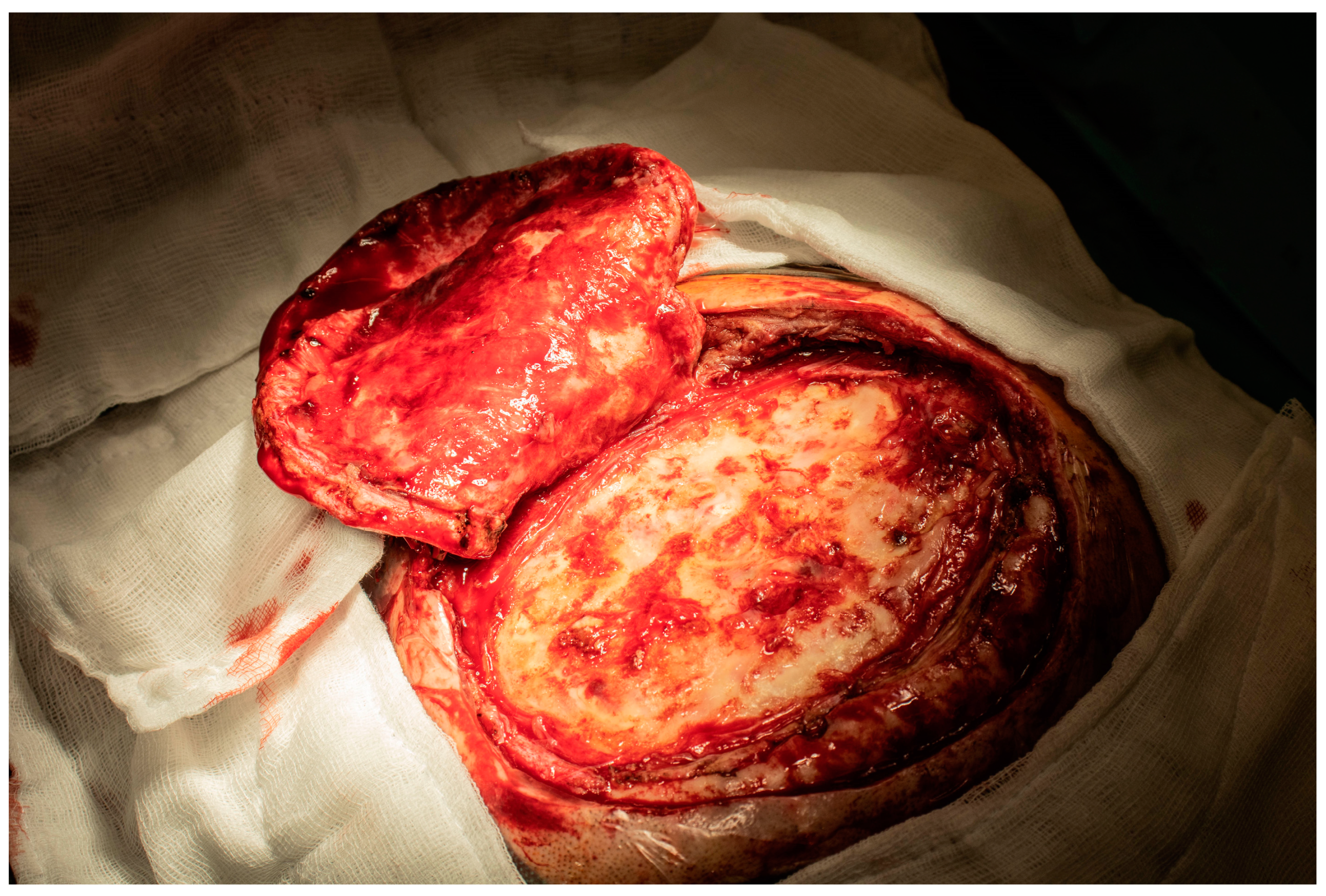
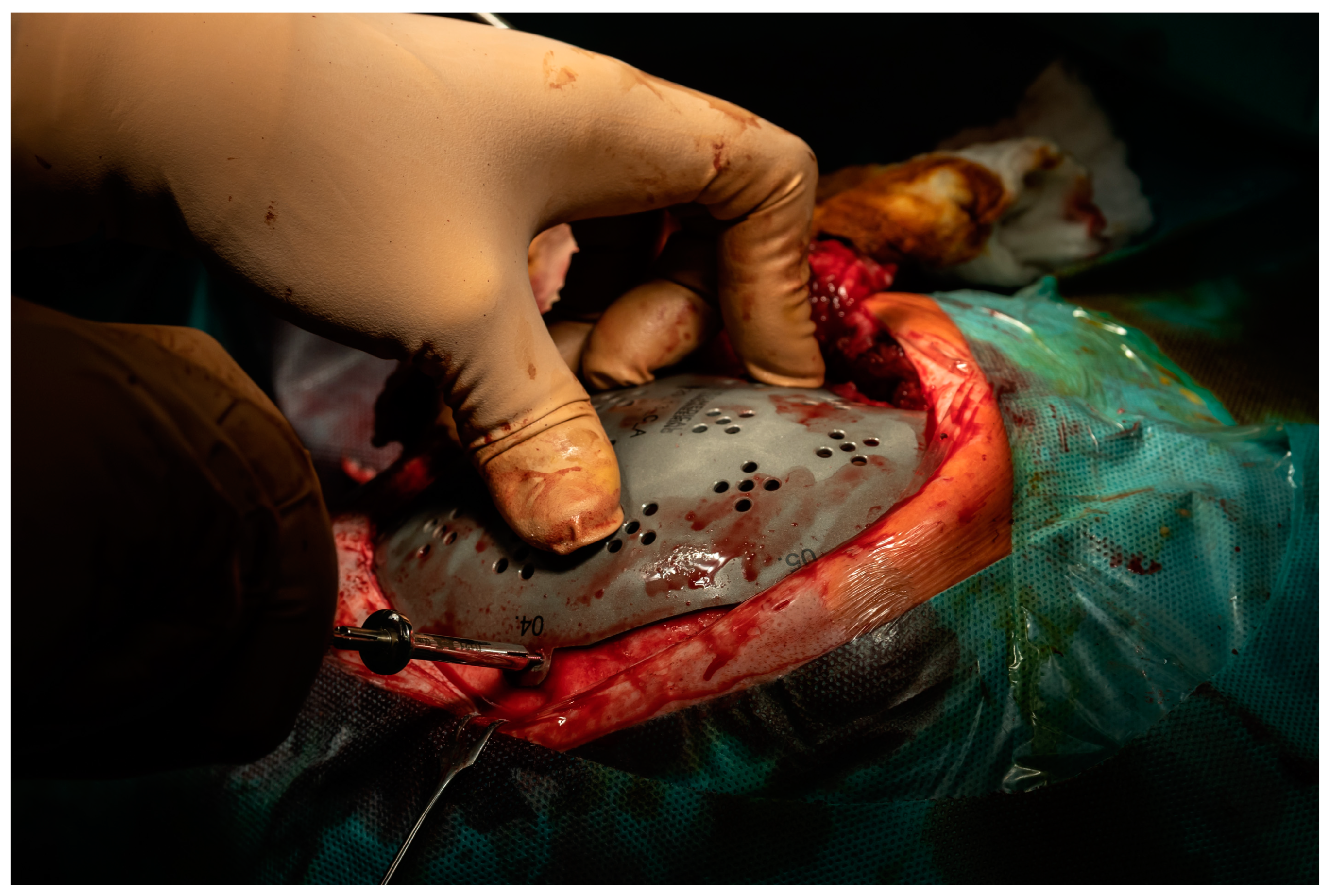
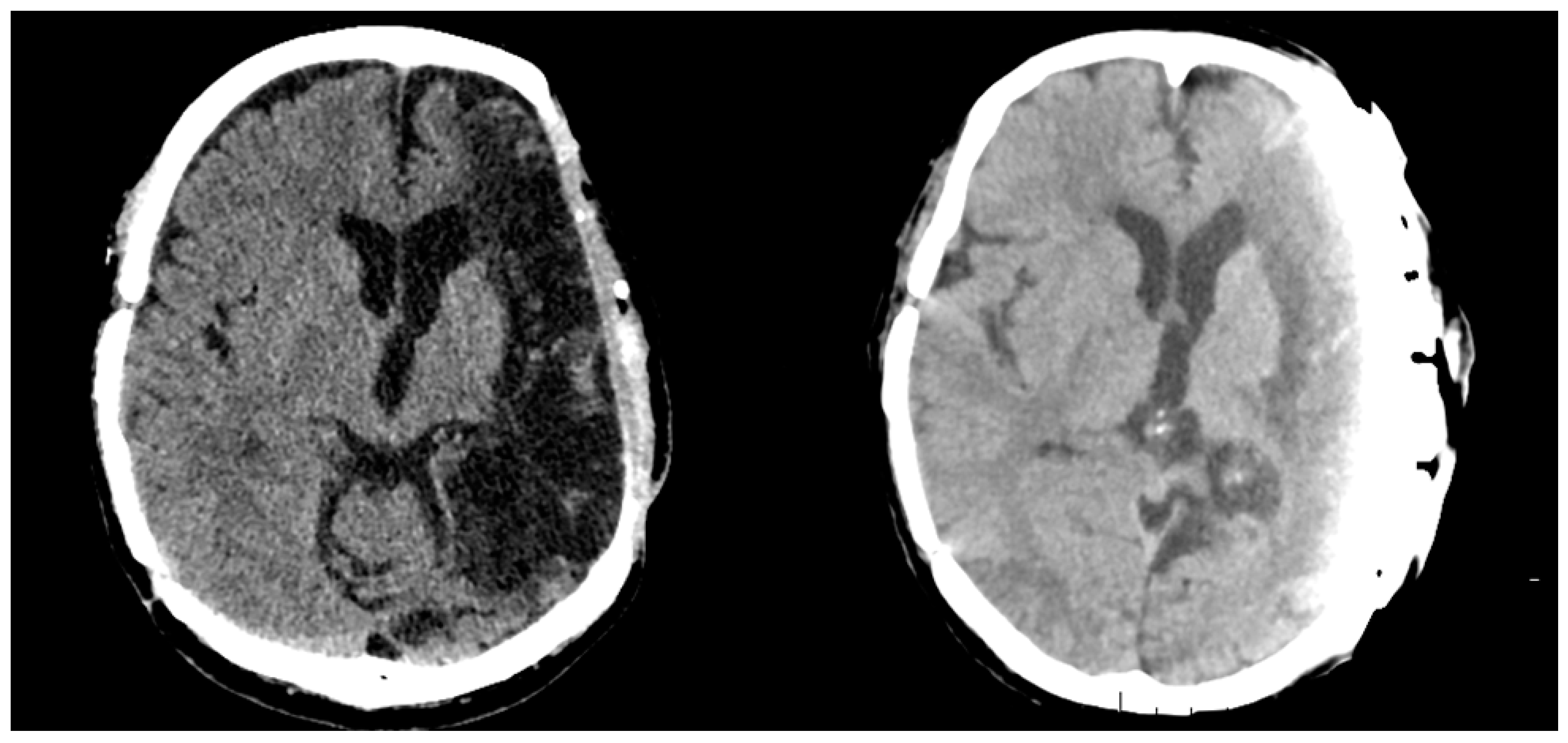
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chopra, P.; Singh, M.; Singh, A.; Masi, A.; Yurkofski, J.; Zaita, B.; Kaur, G. Perioperative Management of Spontaneous Intracranial Hemorrhage in a Patient with Hemophilia A in a Resource Limited Country. Cureus 2023, 15, e43485. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the management of hemophilia. Haemophilia 2013, 19, e1–e47. [Google Scholar] [CrossRef]
- Hemophilia, A. National Bleeding Disorders Foundation. Available online: https://www.bleeding.org/bleeding-disorders-a-z/types/hemophilia-a (accessed on 23 April 2025).
- Touré, S.A.; Seck, M.; Sy, D.; Bousso, E.S.; Faye, B.F.; Diop, S. Life-threatening bleeding in patients with hemophilia (PWH): A 10-year cohort study in Dakar, Senegal. Hematology 2022, 27, 379–383. [Google Scholar] [CrossRef]
- Simurda, T.; Drotarova, M.; Dobrotova, M.; Brunclikova, M.; Necas, L.; Cibula, L.; Kubisz, P.; Stasko, J. Perioperative Monitoring with Rotational Thromboelastometry in a Severe Hemophilia A Patient Undergoing Elective Ankle Surgery. Semin. Thromb. Hemost. 2024, 50, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Mazzeffi, M.; Boggio, L.N.; Simpson, M.L.; Tanaka, K.A. Hemophilia: A Review of Perioperative Management for Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Ar, M.C.; Balkan, C.; Kavaklı, K. Extended Half-Life Coagulation Factors: A New Era in the Management of Hemophilia Patients. Turk. J. Hematol. 2019, 36, 141–154. [Google Scholar] [CrossRef]
- Balkaransingh, P.; Young, G. Novel therapies and current clinical progress in hemophilia A. Ther. Adv. Hematol. 2018, 9, 49–61. [Google Scholar] [CrossRef]
- Ar, M.C.; Vaide, I.; Berntorp, E.; Björkman, S. Methods for individualising factor VIII dosing in prophylaxis. Eur. J. Haematol. Suppl. 2014, 76, 16–20. [Google Scholar] [CrossRef]
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef]
- Löfqvist, T.; Nilsson, I.M.; Berntorp, E.; Pettersson, H. Haemophilia prophylaxis in young patients—A long-term follow-up. J. Intern. Med. 1997, 241, 395–400. [Google Scholar] [CrossRef]
- Hidalgo, O.B.; Garcia, M.F.M.; Cabestre, A.B.; Gimenez, J.C.J.; Mesa, M.G.; Albareda, F.B. Neurosurgery in a patient with severe hemophilia B: An experience using eftrenonacog alfa as perioperative management. Clin. Case Rep. 2022, 10, e05848. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kucukyuruk, B.; Abuzayed, B.; Aydin, S.; Sanus, G.Z. Cranioplasty: Review of materials and techniques. J. Neurosci. Rural. Pract. 2011, 2, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Cabraja, M.; Klein, M.; Lehmann, T.N. Long-term results following titanium cranioplasty of large skull defects. Neurosurg. Focus 2009, 26, E10. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Fong, A.J.; Buchanan, E.P.; Monson, L.; Khechoyan, D.; Lam, S. History of synthetic materials in alloplastic cranioplasty. Neurosurg. Focus 2014, 36, E20. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, M.; Zhang, J.; Ning, W.; Yue, A.; Zhao, R.; Sun, Y.; Yu, C. Skull bone rumor: A review of clinicopathological and neuroimaging characteristics of 426 cases at a single center. Cancer Conunun. 2019, 39, 8. [Google Scholar]
- Shida, T.; Koseki, H.; Yoda, I.; Horiuchi, H.; Sakoda, H.; Osaki, M. Adherence ability of Staphylococcus epidermidis on prosthetic biomaterials: An in vitro study. Int. J. Nanomed. 2013, 8, 3955–3961. [Google Scholar]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26, 1–158. [Google Scholar] [CrossRef]
- Belekar, V.; Jain, V.; Deshmukh, P.; Iratwar, S.W. Perioperative management of a patient with hemophilia A with acute subdural hematoma. J. Appl. Hematol. 2013, 4, 156. [Google Scholar] [CrossRef]
- Gurer, B.; Kertmen, H.; Yilmaz, E.R.; Dolgun, H.; Hasturk, A.E.; Sekerci, Z. The surgical outcome of traumatic extraaxial hematomas causing brain herniation. Turk. Neurosurg. 2017, 27, 37–52. [Google Scholar] [CrossRef]
- Hermans, C.; Apte, S.; Santagostino, E. Invasive procedures in patients with haemophilia: Review of low-dose protocols and experience with extended half-life FVIII and FIX concentrates and non-replacement therapies. Haemophilia 2021, 27, 46–52. [Google Scholar] [CrossRef]
- Belletrutti, M.; Bhatt, M.; Samji, N. Management of children with hemophilia A on emicizumab who need surgery. Front. Pediatr. 2023, 11, 1155853. [Google Scholar] [CrossRef] [PubMed]

| Laboratory Parameter | Result | Reference Range |
|---|---|---|
| Hemoglobin | 14.0 g/dL | 14–17 g/dL |
| RBC Count | 4.75 × 106/μL | 4.5–6.0 × 106/μL |
| Packed Cell Volume | 43.0% | 39–54% |
| Mean Corpuscular Volume | 89.7 fl | 82–98 fl |
| Mean Corpuscular Hemoglobin | 29.4 pg | 26–34 pg |
| MCHC | 33 g/dL | 31–36 g/dL |
| RDW | 13.8% | 11.6–14.5% |
| Platelet | 184 × 109/μL | 180–400 × 109/μL |
| MPV | 8.5 fl | 7.5–12.0 |
| Total Leukocyte Count | 5.3 × 103/μL | 3.9–10.0 × 103/μL |
| Neutrophils | 45.5% | 45–72% |
| Lymphocytes | 42.2% | 25–46% |
| Monocytes | 8.6% | 2–10% |
| Eosinophils | 3.1% | 1–5% |
| Fbg | 3.32 g/L | 1.80–4.20 g/L |
| PT | 109% | 75–120% |
| INR | 0.95 s | 0.8–1.2 s |
| TT | 12.4 s | 14–18 s |
| APTT | 102.9 s | 25–36 s |
| Control | 35.4 s | 0.85–1.25 s |
| FVIII | 0.008 IU/mL | 0.600–1.500 IU/mL |
| ATIII | 102.6% | 80–130% |
| Fe | 15.0 μmol/L | 12.5–32.2 μmol/L |
| VB12 | 750 pg/mL | 250–950 pg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micurova, G.; Belakova, K.M.; Simurda, T.; Drotarova, M.; Stasko, J.; Kolarovszki, B. Successful Perioperative Management of Titanium Cranioplasty in a Patient with Severe Hemophilia A. Hemato 2025, 6, 11. https://doi.org/10.3390/hemato6020011
Micurova G, Belakova KM, Simurda T, Drotarova M, Stasko J, Kolarovszki B. Successful Perioperative Management of Titanium Cranioplasty in a Patient with Severe Hemophilia A. Hemato. 2025; 6(2):11. https://doi.org/10.3390/hemato6020011
Chicago/Turabian StyleMicurova, Gabriela, Kristina Maria Belakova, Tomas Simurda, Miroslava Drotarova, Jan Stasko, and Branislav Kolarovszki. 2025. "Successful Perioperative Management of Titanium Cranioplasty in a Patient with Severe Hemophilia A" Hemato 6, no. 2: 11. https://doi.org/10.3390/hemato6020011
APA StyleMicurova, G., Belakova, K. M., Simurda, T., Drotarova, M., Stasko, J., & Kolarovszki, B. (2025). Successful Perioperative Management of Titanium Cranioplasty in a Patient with Severe Hemophilia A. Hemato, 6(2), 11. https://doi.org/10.3390/hemato6020011







