A Rare Case of Primary Bone Follicular Lymphoma with Multiple Osteolytic Lesions: A Case Report and Review of the Literature
Abstract
1. Introduction
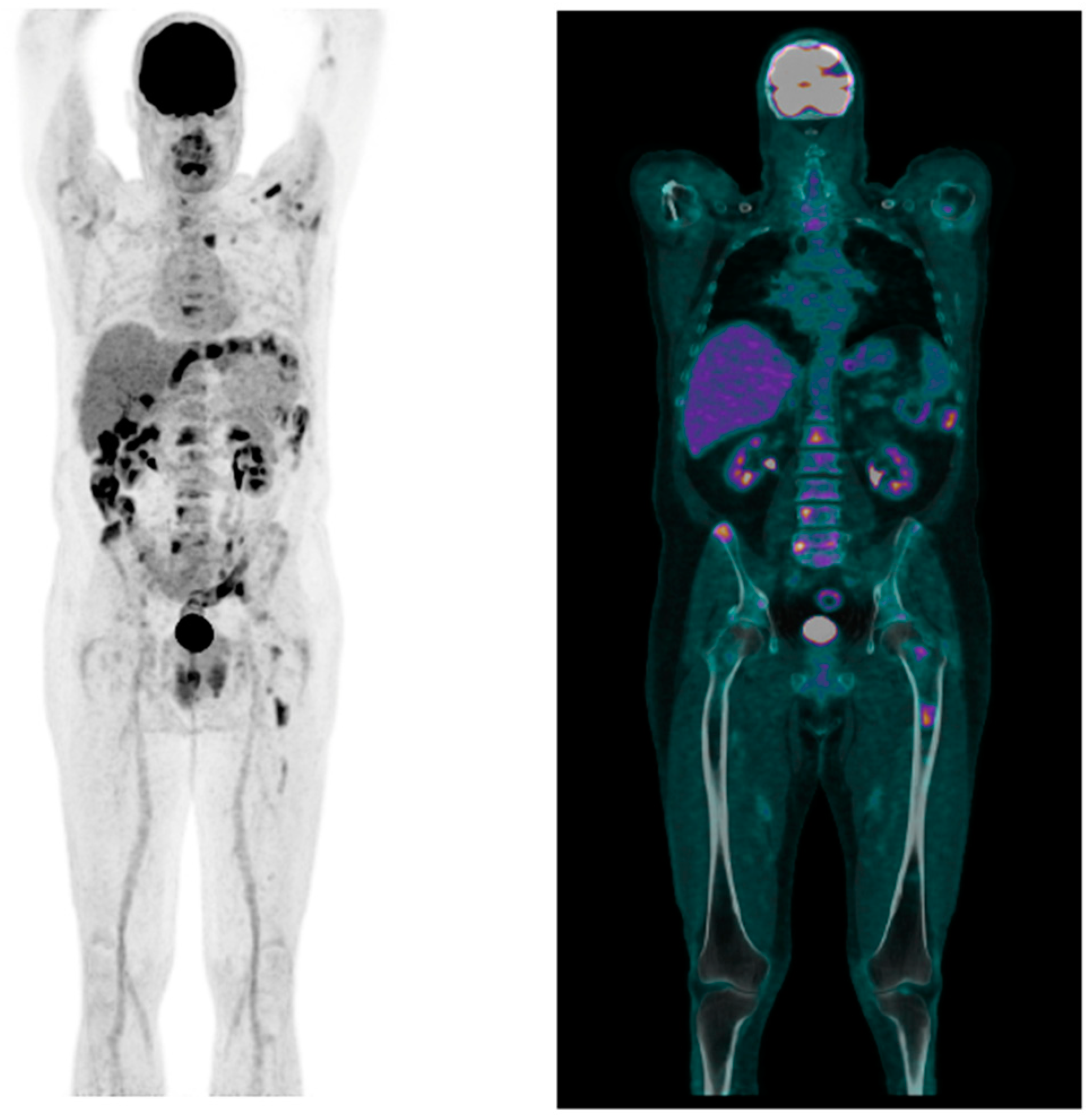
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, A.; Alam, K.; Maheshwari, V.; Khan, R.; Nobin, H.; Narula, V. Primary bone lymphomas-Clinical cases and review of literature. J. Bone Oncol. 2013, 2, 132–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hogendoorn, P.C.W.; Bovée, J.V.M.G.; Nielsen GPFletcher, C.D.; Bridges, A.J.; Hogendoorn, C.W.; Mertens, F. Chondrosarcoma (grade I-III), including primary and secondary variants and periosteal chondrosarcoma. In WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC Press: Lyon, France, 2013; pp. 264–268. [Google Scholar]
- Fletcher, C.D.M.; Unni, K.K.; Mertens, F. (Eds.) World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2002. [Google Scholar]
- Messina, C.; Ferreri, A.J.; Govi, S.; Bruno-Ventre, M.; Gracia Medina, E.A.; Porter, D.; Radford, J.; Heo, D.S.; Park, H.Y.; Pro, B.; et al. Clinical features, management and prognosis of multifocal primary bone lymphoma: A retrospective study of the international extranodal lymphoma study group (the IELSG 14 study). Br. J. Haematol. 2014, 164, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.M.; Blay, J.Y.; Bovée, J.V.M.G.; Bredella, A.; Cool, P.; Nielsen, G.P.; Yoshida, A. Bone tumours: Introduction. In WHO Classification of Tumours Soft Tissue and Bone Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2020; pp. 340–344. [Google Scholar]
- Maruyama, D.; Watanabe, T.; Beppu, Y.; Kobayashi, Y.; Kim, S.W.; Tanimoto, K.; Makimoto, A.; Kagami, Y.; Terauchi, T.; Matsuno, Y.; et al. Primary bone lymphoma: A new and detailed characterization of 28 patients in a single-institution study. Jpn. J. Clin. Oncol. 2007, 37, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Beal, K.; Allen, L.; Yahalom, J. Primary bone lymphoma: Treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer 2006, 106, 2652–2656. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Jambhekar, N.A.; Soman, C.S.; Advani, S.H. Primary lymphoma of bone: A clinicopathologic study of 25 cases reported over 10 years. J. Surg. Oncol. 1991, 46, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Govi, S.; Christie, D.; Mappa, S.; Marturano, E.; Bruno-Ventre, M.; Messina, C.; Medina, E.A.; Porter, D.; Radford, J.; Heo, D.S.; et al. The clinical features, management and prognosis of primary and secondary indolent lymphoma of the bone: A retrospective study of the International Extranodal Lymphoma Study Group (IELSG #14 study). Leuk. Lymphoma 2014, 55, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Vannata, B.; Zucca, E. Primary extranodal B-cell lymphoma: Current concepts and treatment strategies. Chin. Clin. Oncol. 2015, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Sarro, R.; Bisig, B.; Guey, B.; Missiaglia, E.; Cairoli, A.; Omoumi, P.; Letovanec, I.; Judith, A.F.; Robert, P.H.; de Leval, L. Follicular Lymphoma Presenting with Symptomatic Bone Involvement: A Clinicopathologic and Molecular Analysis of 16 Cases. Mod. Pathol. 2024, 37, 100440, ISSN 0893-3952. [Google Scholar] [CrossRef]
- López, C.; Mozas, P.; López-Guillermo, A.; Beà, S. Molecular Pathogenesis of Follicular Lymphoma: From Genetics to Clinical Practice. Hemato 2022, 3, 595–614. [Google Scholar] [CrossRef]
- Jaffe, E.S.; Carbone, A. Evolution in the Definition of Follicular Lymphoma and Diffuse Large B-Cell Lymphoma: A Model for the Future of Personalized Medicine. Hemato 2022, 3, 466–474. [Google Scholar] [CrossRef]
- Jacobs, A.J.; Michels, R.; Stein, J.; Levin, A.S. Socioeconomic and demographic factors contributing to outcomes in patients with primary lymphoma of bone. J. Bone Oncol. 2014, 4, 32–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huan, Y.; Qi, Y.; Zhang, W.; Chu, J. Primary bone lymphoma of radius and tibia: A case report and review of literature. Medicine 2017, 96, e6603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coppes, M.J.; Patte, C.; Couanet, D.; Caillaud, J.M.; Salloum, E.; Brugières, L.; Hartmann, O.; Kalifa, C.; Bernard, A.; Lemerle, J. Childhood malignant lymphoma of bone. Med. Pediatr. Oncol. 1991, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Kraus, M.D.; Dorfman, D.M. Lymphoma presenting as a solitary bone lesion. Am. J. Clin. Pathol. 1999, 111, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.; Alçada, M.; Mestre, A.; Duarte, M.B.; Roque, F. Primary Bone Lymphoma: A Rare Cause of Chronic Back Pain. Cureus 2022, 14, e21147. [Google Scholar] [CrossRef]
- Govi, S.; Christie, D.; Messina, C.; Bruno Ventre, M.; Gracia Medina, E.A.; Porter, D.; Radford, J.; Seog Heo, D.; Park, Y.; Martinelli, G.; et al. The clinical features, management and prognostic effects of pathological fractures in a multicenter series of 373 patients with diffuse large B-cell lymphoma of the bone. Ann. Oncol. 2014, 25, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Gianelli, U.; Patriarca, C.; Moro, A.; Ponzoni, M.; Giardini, R.; Massimino, M.; Alfano, R.M.; Armiraglio, E.; Nuciforo, P.; Bosari, S.; et al. Lymphomas of the bone: A pathological and clinical study of 54 cases. Int. J. Surg. Pathol. 2002, 10, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Bruno Ventre, M.; Ferreri, A.J.; Gospodarowicz, M.; Govi, S.; Messina, C.; Porter, D.; Radford, J.; Heo, D.S.; Park, Y.; Martinelli, G.; et al. Clinical features, management, and prognosis of an international series of 161 patients with limited-stage diffuse large B-cell lymphoma of the bone (the IELSG-14 study). Oncologist 2014, 19, 291–298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krishnan, A.; Shirkhoda, A.; Tehranzadeh, J.; Armin, A.R.; Irwin, R.; Les, K. Primary bone lymphoma: Radiographic-MR imaging correlation. Radiographics 2003, 23, 1371–1383, discussion 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Dürr, H.R.; Müller, P.E.; Hiller, E.; Maier, M.; Baur, A.; Jansson, V.; Refior, H.J. Malignant lymphoma of bone. Arch. Orthop. Trauma. Surg. 2002, 122, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Heyning, F.H.; Kroon, H.M.; Hogendoorn, P.C.; Taminiau, A.H.; van der Woude, H.J. MR imaging characteristics in primary lymphoma of bone with emphasis on non-aggressive appearance. Skelet. Radiol. 2007, 36, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Stiglbauer, R.; Augustin, I.; Kramer, J.; Schurawitzki, H.; Imhof, H.; Radaszkiewicz, T. MRI in the diagnosis of primary lymphoma of bone: Correlation with histopathology. J. Comput. Assist. Tomogr. 1992, 16, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Paes, F.M.; Kalkanis, D.G.; Sideras, P.A.; Serafini, A.N. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics 2010, 30, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Seam, P.; Juweid, M.E.; Cheson, B.D. The role of FDG-PET scans in patients with lymphoma. Blood 2007, 110, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Wu, H.B.; Wang, M.; Han, Y.J.; Li, H.S.; Zhou, W.L.; Wang, Q.S. Utility of F-18 FDG PET/CT on the evaluation of primary bone lymphoma. Eur. J. Radiol. 2015, 84, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. The role of 18F-FDG PET/CT in staging and restaging primary bone lymphoma. Nucl. Med. Commun. 2017, 38, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Misgeld, E.; Wehmeier, A.; Krömeke, O.; Gattermann, N. Primary non-Hodgkin’s lymphoma of bone: Three cases and a short review of the literature. Ann. Hematol. 2003, 82, 440–443. [Google Scholar] [CrossRef]
- Scoccianti, G.; Rigacci, L.; Puccini, B.; Campanacci, D.A.; Simontacchi, G.; Bosi, A.; Capanna, R. Primary lymphoma of bone: Outcome and role of surgery. Int. Orthop. 2013, 37, 2437–2442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramadan, K.M.; Shenkier, T.; Sehn, L.H.; Gascoyne, R.D.; Connors, J.M. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: A population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann. Oncol. 2007, 18, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, N.P.; Jones, J.J.; Kramer, B.S.; Hudson, T.M.; Carter, R.L.; Enneking, W.F.; Marcus, R.B., Jr.; Million, R.R. The management of primary lymphoma of bone. Radiother. Oncol. 1987, 9, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Held, G.; Zeynalova, S.; Murawski, N.; Ziepert, M.; Kempf, B.; Viardot, A.; Dreyling, M.; Hallek, M.; Witzens-Harig, M.; Fleckenstein, J.; et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J. Clin. Oncol. 2013, 31, 4115–4122. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togni, C.; La Verde, G.; Pelliccia, S.; Bianchi, M.P.; Di Napoli, A.; Lanzolla, T.; Zerunian, M.; Laghi, A.; Maiorana, G.; Taglietti, A.; et al. A Rare Case of Primary Bone Follicular Lymphoma with Multiple Osteolytic Lesions: A Case Report and Review of the Literature. Hemato 2024, 5, 388-395. https://doi.org/10.3390/hemato5040028
Togni C, La Verde G, Pelliccia S, Bianchi MP, Di Napoli A, Lanzolla T, Zerunian M, Laghi A, Maiorana G, Taglietti A, et al. A Rare Case of Primary Bone Follicular Lymphoma with Multiple Osteolytic Lesions: A Case Report and Review of the Literature. Hemato. 2024; 5(4):388-395. https://doi.org/10.3390/hemato5040028
Chicago/Turabian StyleTogni, Chiara, Giacinto La Verde, Sabrina Pelliccia, Maria Paola Bianchi, Arianna Di Napoli, Tiziana Lanzolla, Marta Zerunian, Andrea Laghi, Gianluca Maiorana, Ambra Taglietti, and et al. 2024. "A Rare Case of Primary Bone Follicular Lymphoma with Multiple Osteolytic Lesions: A Case Report and Review of the Literature" Hemato 5, no. 4: 388-395. https://doi.org/10.3390/hemato5040028
APA StyleTogni, C., La Verde, G., Pelliccia, S., Bianchi, M. P., Di Napoli, A., Lanzolla, T., Zerunian, M., Laghi, A., Maiorana, G., Taglietti, A., & Tafuri, A. (2024). A Rare Case of Primary Bone Follicular Lymphoma with Multiple Osteolytic Lesions: A Case Report and Review of the Literature. Hemato, 5(4), 388-395. https://doi.org/10.3390/hemato5040028






