Abstract
Background: Sickle cell disease (SCD) is an autosomal recessive haemoglobin disorder, affecting about 7.74 million individuals worldwide, but it is more prevalent among Africans and Asians. SCD is characterised by many complications, and it is a major health issue in Nigeria, the country with the largest burden of the disease globally. This work aims to present the design and implementation of electronic registries (ER) for SCD in a tertiary hospital in Nigeria. Methods: Registry design was initiated during a staff exchange programme within the ARISE initiative (EU grant agreement no. 824021). Ethical approval was obtained, and paper records were retrieved and transferred into one adult and one paediatric database, developed with Microsoft Access. Results: Data from 2659 SCD patients were entered in the ERs, including 698 (26.3%) adults and 1961 (73.7%) children. There were 287 (41%) male adults, 404 (58%) female and 7 (1%) patients whose gender was missing. There were 1041 (53.1%) male children, 906 (46.2%) female and 14 (0.7%) whose gender was missing. Information on phenotype was available for 2385 subjects, and most of them (2082, 87.3%) were SS. The most prevalent SCD-related complication was painful events (26.6% in adults and 68.7% in children, considering valid cases). Conclusions: About 60% of SCD patients in the centre were included in the ERs providing useful, hands-on recommendations for future ER design in SCD. These ERs might be an appropriate tool for collecting and analysing SCD patients’ data.
1. Introduction
Sickle cell disease (SCD) is an autosomal recessive haemoglobin disorder, affecting about 7.74 million individuals worldwide [1]. The disease occurs worldwide but is more prevalent among Africans and Asians. SCD is characterised by many complications, including chronic pain, acute pain crises, acute chest syndrome, stroke, organ damage, an increased risk of severe infections, and reduced longevity [2]. SCD is a major health issue in Nigeria; the country has by far the largest burden of the disease globally. More than 40 million Nigerians are carriers of the sickle cell gene (Hb S), and an estimated 150,000 babies are born every year with sickle cell anaemia (Hb SS). Unfortunately, an estimated 66.7% of these babies hardly reach five years of age; they die mostly from a lack of information about the disease and lack of access to proper diagnosis and care [3].
Information technology tools such as electronic registries (ERs) in healthcare systems can improve the quality of healthcare services [4]. In addition to providing more accurate and timely information regarding patient care, it has been found to improve the efficiency of healthcare organisations, especially in terms of patient data management [5]. Several approaches are being applied across different healthcare systems around the world, including the use of patient registries, which have been identified as a method of improving quality and cost efficiency in health and healthcare [6,7]. High-quality patient registries provide valuable information to determine demographics, clinical features, prevalence and variation in clinical practices “in the real world” [8,9,10,11]. ER assists in the surveillance of important health conditions, provides a better understanding of patient health status, and patient’s needs, provides a platform to evaluate practice over time, and allows for estimation of resource requirements [11,12].
Sickle cell disease (SCD) ER could enable the collection of information about the SCD population, burden of SCD, care and research needs and to support patients’ management, monitoring, evaluation, and health education [12]. In Nigeria, there have been several efforts to collect data at facility levels, some community level and national level. However, there is a need to standardise and scale up data collection for national planning.
Well-designed, properly managed and carefully implemented SCD ER can be used for research, training and rendering effective clinical and diagnostic services. Therefore, we planned to undertake a pilot in a tertiary hospital in Nigeria.
The main objective of the study was to test the feasibility of data collection and implementation of a registry that can be used to collect reliable demographics, clinical, and other information about persons with SCD according to the registry protocol. Additionally, this experience has allowed for the following:
- The assessment of baseline logistics and infrastructure for SCD ER in Ahmadu Bello University Teaching Hospital Zaria (ABUTH);
- The determination of basic information required for a comprehensive SCD ER based on existing registries elsewhere;
- The design and implementation of two ERs for SCD, one for children and another for adults;
- The training of users (physicians, nurses, laboratory staff, health information/ICT staff) on the use of the ERs;
- The use of the data collected to conduct baseline analysis of the epidemiology and clinical characteristics of SCD patients.
2. Materials and Methods
The design of the registries was initiated during a staff exchange programme funded by the African Research and Innovative Initiative for Sickle Cell Education: Improving Research Capacity for Service Improvement (European Union grant no. 824021—ARISE-H2020-MSCA-RISE-2018/H2020-MSCA-RISE-2018) and in collaboration among researchers from ABUTH and Fondazione per la Ricerca Farmacologica Gianni Benzi Onlus Italy (FGB). The EU grant took care of staff transport from Nigeria to Italy, accommodation and daily subsistence during the period of staff exchange programme between ABUTH and FGB. The ABUTH site lead paid the salary of the hired data clerks and health information officers, while the study PI and data manager paid the administrative fee for obtaining ethical approval, printing copies of proposals for ethical committee review and other logistics. This experience describes the transfer of information on SCD patients from paper-based records (PRs) to an ER.
2.1. Study Site
The study was conducted in the departments of Haematology and Blood Transfusion, Paediatrics and Health Information Management (HIM) of ABUTH Zaria North-West Nigeria. ABUTH is a federal government-owned tertiary-level hospital with a 750-bed capacity and 17 clinical departments. There are over 1400 adults and 3000 paediatric patients with SCD receiving care at the facility [13].
At ABUTH, there are two dedicated clinics, one for children and one for adults. Patients in the paediatric clinic are aged 6 months to 18 years normally, but some spill up to maximum of 24 years if they are not psychologically ready to migrate to adult clinic or the family has other younger children with SCD. So, for ease of transportation and to reduce the cost of logistics, older children may be delayed before moving to adult clinic.
Patients carry forward their hospital numbers and records when migrating to the adult clinic. So, patients were not captured twice in this manuscript. Data in this manuscript were cross-section at the time of the study, even though the yearly clinical visits were captured. No information about mortality was available in the PRs.
2.2. Study Period
PRs of SCD patients’ visits from the oldest available in the hospital’s record library at the time of study (dated 1998) to the most recent (2023) were transferred to the ERs over a 7-month period from 1st February 2023 to 28th September 2023. The researchers were unable to locate and retrieve all PRs, due to lack of dedicated staff, financial resources and time. However, we retrieved about 60% of PRs available in the PRs Library. We hope to capture the remaining 40% when funding is available so that we can have full electronic records of all our patients.
2.3. Ethical Approval
Ethical approval was obtained from the institution’s ethics committee on 27 January 2023 (ABUTHZ/HREC/F43/2023) and request for waiving consent on retrospective data collection was granted by the committee.
Permissions of Heads of Departments of Haematology and Blood Transfusion, Paediatrics and Health Information Management were obtained for data collection.
2.4. Database Design
Microsoft Access was used to develop the ERs. Four out of thirty-nine fields in adult records—visit details, major findings at visit, treatment and transfusions—have additional data reported through linked tables. Seven out of fifty fields in paediatric records—full blood counts (FBC), transcranial doppler (TCD), liver function test and creatinine (LFT and Cr), ECHO, visit records, research participation and year review—have linked tables for details. Queries and reports were designed for data extraction at the time of need.
2.5. Data Collection
A data manager was responsible for the full action. Two data clerks and two health information officers were trained by the data manager on the operation of the ERs. Paediatric clinical information of the last clinic visit collected as PR in patients’ case folders were transferred to the ER by the four trained health information officers and data clerks while that of adults was completed by the data manager.
2.6. Data Management
Every week, the data collected were entered, reviewed and stored in the master database by the data manager. The data collected were backed up and encrypted in an external hard disk to avoid unauthorised access to the data. The data obtained were analysed using MS Excel statistical tools.
2.7. Data Sharing
Raw data from the registry were not shared and will not be shared. Only aggregated data will be disseminated and presented as results of the study. Primary (raw) data will be available only to study principal investigator and authorised study team members, bound by professional secrecy. Only aggregated data (e.g., cumulative data and statistics) may be made available to third parties, if requested, for specific and verified purposes (e.g., summary of research activities, abstracts/presentations or other scientific articles).
Figure 1 describes the steps of the registry implementation process.
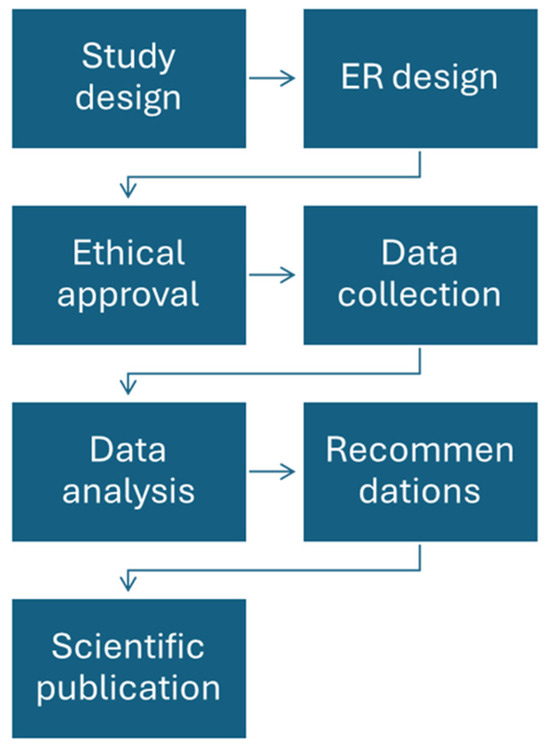
Figure 1.
Registry implementation process.
3. Results
3.1. Assessment of Logistics and Infrastructure
There was no baseline logistics available to support ER use at ABUTH at the time of this study (no dedicated ICT staff, no suitable PCs or tools). There was an electronic health records (EHR) system introduced in Q1 2023 in ABUTH and, at the time of the data collection, only prospective electronic data entry of general patients’ records (only demographic information) was available. Data for the study were collected independently of this EHR. A network infrastructure was available for the institution’s EHR, and there was dedicated intranet/internet access.
3.2. Determination of Basic Information Required for a Comprehensive SCD ER
We identified the required fields and the basic information needed for the two SCD ERs after due consultations. Specifically, adult and paediatric ABUTH Haemoglobinopathy cards were reviewed together with the Sickle Pan Africa Research Consortium (SPARCO) registry case report form (CRF). The CRF developed by the International Haemoglobinopathies Research Network Registry (INHERENT) was also reviewed [14].
Based on these sources, 38 fields for the adult ER (ref. Annex S1—data collection tables in the registry for adult patients, in Supplementary Material) and 48 (ref. Annex S2—data collection tables in the registry for paediatric patients, in Supplementary Material) for the paediatric one were identified as pertaining and the ERs were designed and implemented.
3.3. Design and Implementation of the ERs and Training of Users
The ERs were designed and tested by training haematologists, paediatricians, health information officers and data clerks to have a pilot data collection phase before the main data entry phase. Based on the feedback provided, the following gaps were identified and addressed:
- Paucity of resources: the study team agreed to maximise the use of the available resources to have the highest number of PRs transferred; this implied the need to minimise the budget for technical instruments, thus no new hardware nor software licenses were bought;
- Finance: the study PI and the ABUTH site lead voluntarily agreed to contribute with their own money;
- Staff: some of the needed manpower was temporarily employed and some members of the study team volunteered to be involved for free in study-related activities;
- Time: extra hours aside our daily schedules were added in order to meet up;
- Logistics: this has been taken care of by the study PI, ABUTH site lead and some members of the study team;
- Lack of embedded functionalities in MS Access: pictorial data presentations like dashboards used in advance registries were considered not essential at this stage of development.
It was suggested to incorporate the two ERs into the hospital EHR system and recommend a dedication of staff that can be trained to update the ERs regularly.
3.4. Demographic and Clinical Information of SCD Patient Population at ABUTH
Data from 2659 out of the estimated 4400 (60%) SCD patients were collected from PRs using the two designed ERs. Their demographic characteristics are presented in Table 1.

Table 1.
Demographic characteristics of SCD patients.
3.5. Complications
SCD-related complications and non-SCD-related comorbidity documented at clinic visits are presented in Table 2.

Table 2.
SCD-related complications and non-SCD-related comorbidity.
The assessment of the relationship between SCD and complications was made by the reference physician at the time of the visit and was part of the PRs.
There is a similarity in missing information in both adult and paediatric patients and a disparity in documented SCD complications and non-SCD-related comorbidity. The most common SCD complication in adults and children is SCD painful events (128 adults, 26.6% out of the total valid cases, and 833 children, 68.7% out of the total valid cases).
4. Discussion
Registry design requires resources such as human, financial and infrastructure. ARISE staff exchange programme provided us with human resources development through training and mentorship. Due to a lack of funding, individual funding was used to finance the study. Computers could not be purchased, hiring and training of enough data clerks could not be possible, and patients could not be recruited for prospective data collection to present the most recent information about the target population. Payment of administrative fees for obtaining ethical approval, printing of copies of proposals for ethical committee review, and payment of data clerks, and research assistants, as well as taking care of other logistics were also completed with personal resources. The following aspects have been documented as major challenges while running this pilot experience.
Transferring all the existing PRs into the electronic registries was not possible within the period of the study because only the data manager captured the adult records, and four health information officers/data clerks were hired to capture paediatric data. For this reason, about 58.2% and 65.4% of the total population of adult and paediatric SCD patients attending ABUTH were captured in the ERs. Because of these reasons, full retrospective data collection could not be completed due to lack of resources.
The obtainment of ethical approval, the head of the departments’ permission and inputs of all stakeholders took time because of administrative procedures that had to be followed. As an example, we had to write officially to all the heads of departments and obtain their approvals before starting the work. The stakeholders who are adult and paediatric haematologists had to provide their professional advice and recommendations, which took about four weeks.
Accessing patients’ PRs was challenging because the folders were not in one single location. Paediatric folders are separate from that of adults in different locations. Some were in other clinics because the patients were referred to other specialists and some were in the wards when the patients were on admission.
Capturing information was really difficult because some handwriting was not clearly legible, some terminologies were not understood by the data clerks, and some abbreviations were not known to them. The clerks often contacted the health information officers they were working with to obtain some clarification on what they did not understand, and if what they were asking was beyond the health information officers, they would usually refer them to one of the medically trained members of the study team. And a short training on some terminologies and abbreviations was given to them, which actually eased their difficulties. This particular challenge highlights the advantage of an electronic record system over a paper-based because a particular format and template has to be followed by everyone.
This study was conducted through retrospective abstraction of PR data of adults and paediatric SCD patients into newly developed ERs.
Even if data have to be cautionary interpreted, due to underreporting and missingness, information on demographic, clinical and complications are in line with similar experiences published in the literature. There was an estimated equal male:female SCD patient distribution (49.9% versus 49.3%) among SCD patients, which was at variance with Paintsil et al. who reported a male predominance reported in SPARCO ER in Ghana [15]. Similar to reports from Brazil and England [16,17], but different from studies by Isa et al. and Asare et al. in Nigeria and Ghana, respectively, that show female predominance [18,19].
SCD-SS was the most prevalent phenotype in the study sample, and a high frequency of SCD-SS phenotype was also reported by Paintsil et al. and others in different studies [15,16,17,18,19,20,21,22,23,24]. This may be because SCD-SS has a severe clinical manifestation and thus requires care in the hospital as compared to those with SCD-SC [17]. We reported 5.0% SCD-SS+F in the adult registry 10.8% in the paediatric one, 7.3% SCD-SC in adults, 1.8% in children 0.3% SCD-CC in adults and no one among children. It was documented that Hb C is known to be unique to West Africa and more commonly in Mali, Burkina Faso, and Ghana [23].
Concerning pharmacological treatment with HU, we found that the percentage of patients using it is low (17.5% of valid cases), despite the fact Nigeria has the highest burden of SCD worldwide and yet, the use of HU among SCD patients in Nigeria is very low [24,25,26,27,28,29,30]. Only eight of eighteen SCD specialist health institutions studied by Galadanci et al. and colleagues in Nigeria in 2014 prescribed HU to their patients, and within those institutions, only 5–33% of their patients were effectively on HU [26]. Utilisation of HU among health care providers, caregivers and patients has remained low. Some reported reasons for this low level of utilisation include side effects, cost, availability, poor awareness among patients poor adherence, or poor availability of guidelines on the administration and monitoring of HU [31,32,33].
With reference to SCD complications, sickle cell painful events were the most prevalent complications in our study. The top reported diagnosis among SCD-SS patients in SPARCO Ghana ER was sickle cell painful events [15]. The sickle cell painful event was also the most reported event in studies carried out at different centres in the USA and a centre in the UK [18,34,35,36]. Sickle cell pain events and VOC appear to be higher in children than in adults from our study; however, this could also be due to underreporting of the complication in the adult population. Some SCD-related complications/ comorbidities (e.g., osteomyelitis in adults) were zero percent (0.0%), most likely due to poor documentation in paper records.
Study Limitations
The aim of the study was to capture all the available paper-based records. However, due to some limitations, only 58.2% and 65.4% of adult and paediatric SCD data were transferred from PRs to ER. These limitations include the paucity of resources, both in terms of funding, time and personnel, the non-availability of some folders due to referrals of patients to specialist clinics, non-legible handwriting, missing data and non-uniformity in data entry between adult and paediatric haematologists.
This has limited our pilot study to some extent, but we believe the information provided by this pilot has a meaningful representation of SCD patients attending the Ahmadu Bello University Teaching Hospital.
5. Conclusions
Registries hold great promise and provide valuable information about the SCD population—who they are and what their care and research needs are [12]. The authors designed and implemented the SCD ERs described here as a pilot study in order to inform full implementation as part of the ARISE objective of promoting e-health technologies for SCD management in Africa. The ability to conduct meaningful analysis on the data that were collected, addressing specific research questions, demonstrated that the ERs designed for this study might be an appropriate tool for collecting, storing, and analysing data of persons living with SCD if implemented fully and managed appropriately. This can be used to support patients’ management, monitoring, evaluation, research, health education and promotion, ultimately leading to improved health outcomes for the patients.
Plans for the future include (a) capturing the remaining 40% of the PRs so that we can have full electronic records of all our patients (b) incorporating the two ERs into the hospital EHR system, (c) as an alternative or bridging solution to the previous point (b), the deployment of a major release of the ERs aimed at moving to a different technical solution (e.g., RedCap) that could facilitate online and simultaneous access to the registries allowing both a larger team to work on data entry and healthcare professionals dealing with adult patients and children to use stored data and (d) using the registry for prospective data capture for old and newly enrolled SCD patients attending our institution. However, this can only be achieved if human resources, funding and infrastructure are made available by the ABUTH management and/or from external sources, including grant opportunities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/hemato5030025/s1, Annex S1—data collection tables in the registry for adult patients and Annex S2—data collection tables in the registry for paediatric patients.
Author Contributions
Conceptualisation, M.A.I. and F.B.; methodology, M.A.I., F.B. and L.R.; data curation, M.A.I.; writing—original draft preparation, M.A.I. and L.R.; writing—review and editing, H.R.A., A.H., I.N.I., F.J.A., S.A., U.N. and B.P.D.I.; supervision, F.B. and B.P.D.I.; project administration and funding acquisition, F.B. and B.P.D.I. All authors have read and agreed to the published version of the manuscript.
Funding
The ARISE project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 824021.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ABUTH Ethics Committee on 27 January 2023 (ABUTHZ/HREC/F43/2023).
Informed Consent Statement
A request for waiving individual consent on retrospective data collection was granted by the committee.
Data Availability Statement
Raw data from the registry were not shared and will not be shared. Only aggregated data will be disseminated and presented as results of the study. Primary (raw) data will be available only to study principal investigator and authorised study team members, bound by professional secrecy. Only aggregated data (e.g., cumulative data and statistics) may be made available to third parties, if requested, for specific and verified purposes (e.g., summary of research activities, abstracts/presentations or other scientific articles).
Acknowledgments
We thank the management of Ahmadu Bello University (ABU) Zaria and ABUTH for permitting a staff exchange programme between ABU, ABUTH and FGB. We thank ABUTH doctors, nurses, medical laboratory scientists, health information officers, research assistants and all the staff. We thank the people living with sickle cell disease, parents/guardians and all the caregivers, this work would not have been possible without them.
Conflicts of Interest
Authors declare no conflicts of interest.
References
- Thomson, A.M.; A McHugh, T.; Oron, A.P.; Teply, C.; Lonberg, N.; Tella, V.V.; Wilner, L.B.; Fuller, K.; Hagins, H.; Aboagye, R.G.; et al. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2020–2021: A systematic analysis from the Global Burden of Disease study 2021. Lancet 2023, 10, e585–e599. [Google Scholar] [CrossRef]
- Kanter, J.; Meier, E.R.; Hankins, J.S. Improving outcomes for patients with sickle cell disease in the United States. Making the case for more resources, surveillance, and longitudinal data. JAMA Health Forum 2021, 2, e213467. [Google Scholar] [CrossRef] [PubMed]
- Sickle Cell Foundation Nigeria. Sickle Cell Disorder Registry Nigeria (SCDRN), an Initiative of Sickle Cell Foundation Nigeria and Point Care Health Initiative; Rhieos–Ventures: London, UK, 2022. [Google Scholar]
- Shahraki, A.D.; Safdari, R.; Shahmoradi, L.; Malak, J.S.; Pourghaz, B.; Ghabaee, M. Acute stroke registry planning experiences. J. Regist. Manag. 2018, 45, 37–42. [Google Scholar]
- Antoniou, Z.C.; Schiza, E.C.; Neokleous, K.C.; Angastiniotis, M.; Pattichis, C.S.; Schizas, C.N. E-health services for the European Reference Network on Rare Anaemias (eENERCA). Stud. Health Technol. Inform. 2015, 213, 153–156. [Google Scholar] [PubMed]
- Lundström, M.; Barry, P.; Brocato, L.; Fitzpatrick, C.; Henry, Y.; Rosen, P.; Stenevi, U. European registry for quality improvement in cataract surgery. Int. J. Health Care Qual. Assur. 2014, 27, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Ahern, S.; Evans, S.M.; Hopper, I.; Earnest, A. Clinical quality registries for clinician-level reporting: Strengths and limitations. Med. J. Aust. 2018, 208, 323. [Google Scholar] [CrossRef]
- Inusa, B.P.D.; Colombatti, R. European migration crises: The role of national hemoglobinopathy registries in improving patient access to care. Pediatr. Blood Cancer 2017, 64, 21–32. [Google Scholar] [CrossRef]
- Kosaryan, M.; Karami, H.; Abbas Alipour, M.D.; Akbarzadeh, R. Designing an electronic registry for patients with beta-thalassemia major for Mazandaran province. Int. J. Caring Sci. 2017, 10, 575–582. [Google Scholar]
- Davoodi, S.; Haghighi, K.; Rostam, S.; Kalhori, N.; Hosseini, N.; Mohammadzadeh, Z.; Saftdari, R. Occupational disease registries characteristics and experiences. Acta Inform. Med. 2017, 25, 136–140. [Google Scholar] [CrossRef]
- Modell, B.; Khan, M.; Darlison, M. National register for surveillance of inherited disorders: Beta-thalassaemia in the United Kingdom. Bull. World Health Organ. 2001, 79, 1006–1013. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action; Martinez, R.M.; Osei-Anto, H.A.; McCormick, M. (Eds.) Screening, Registries, and Surveillance. In Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action; National Academies Press (US): Washington, DC, USA, 2020; Volume 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK566461/ (accessed on 21 December 2023).
- Hassan, A. and Ahmad, H.R. Data from the African Research and Innovative Initiative for Sickle Cell Education Task 2.2; Ahmadu Bello University Teaching Hospital SPARCO: Shika-Zaria, Nigeria, 2020. [Google Scholar]
- Kountouris, P.; Stephanou, C.; Archer, N.; Bonifazi, F.; Giannuzzi, V.; Kuo, K.H.M.; Maggio, A.; Makani, J.; Mañú-Pereira, M.d.M.; Michailidou, K.; et al. The International Hemoglobinopathy Research Network (INHERENT): An international initiative to study the role of genetic modifiers in hemoglobinopathies. Am. J. Hematol. 2021, 96, E416–E420. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, V.; Amuzu, E.X.; Nyanor, I.; Asafo-Adjei, E.; Mohammed, A.R.; Yawnumah, S.A.; Oppong-Mensah, Y.G.; Nguah, S.B.; Obeng, P.; Dogbe, E.E.; et al. Establishing a Sickle Cell Disease Registry in Africa: Experience from the Sickle Pan-African Research Consortium, Kumasi-Ghana. Front. Genet. 2022, 13, 802355. [Google Scholar] [CrossRef]
- Telfer, P.; Coen, P.; Chakravorty, S.; Wilkey, O.; Evans, J.; Newell, H.; Smalling, B.; Amos, R.; Stephens, A.; Rogers, D.; et al. Clinical Outcomes in Children with Sickle Cell Disease Living in England: A Neonatal Cohort in East London. Haematologica 2007, 92, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.A.d.M.; de Medeiros, T.M.D.; Alves, J.J.P.; Bezerra, C.M.; Fernandes, J.V.; Serafim, S.S.; Fernandes, M.Z.; Sonati, M.d.F. Socioeconomic and Demographic Characteristics of Sickle Cell Disease Patients from a Low-Income Region of Northeastern Brazil. Rev. A Bras. Hematol. 2015, 37, 172–177. [Google Scholar] [CrossRef]
- Isa, H.; Adegoke, S.; Madu, A.; Hassan, A.-A.; Ohiaeri, C.; Chianumba, R.; Brown, B.; Okocha, E.; Ugwu, N.; Diaku-Akinwumi, I.; et al. Sickle Cell Disease Clinical Phenotypes in Nigeria: A Preliminary Analysis of the Sickle Pan Africa Research Consortium Nigeria Database. Blood Cell Mol. Dis. 2020, 84, 102438. [Google Scholar] [CrossRef]
- Asare, E.V.; Wilson, I.; Benneh-Akwasi Kuma, A.A. Burden of Sickle Cell Disease in Ghana: The Korle-Bu Experience. Adv. Hematol. 2018, 2018, 6161270. [Google Scholar] [CrossRef] [PubMed]
- Bardakdjian-Michau, J.; Bahuau, M.; Hurtrel, D.; Godart, C.; Riou, J.; Mathis, M.; Goossens, M. Neonatal Screening for Sickle Cell Disease in France. J. Clin. Pathol. 2009, 62, 31–33. [Google Scholar] [CrossRef] [PubMed]
- De Castro Lobo, C.L.; Ballas, S.K.; Domingos, A.C.B.; Moura, P.G.; Nascimento, E.M.D.; Cardoso, G.P.; de Carvalho, S.M.F. Newborn screening program for hemoglobinopathies in Rio de Janeiro, Brazil. Pediatr. Blood Cancer 2014, 61, 34–39. [Google Scholar] [CrossRef]
- Grosse, S.D.; Odame, I.; Atrash, H.K.; Amendah, D.D.; Piel, F.B.; Williams, T.N. Sickle Cell Disease in Africa: A Neglected Cause of Early Childhood Mortality. Am. J. Prev. Med. 2011, 41 (Suppl. S4), S398–S405. [Google Scholar] [CrossRef]
- Piel, F.B.; Howes, R.E.; Patil, A.P.; Nyangiri, O.A.; Gething, P.W.; Bhatt, S.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. The Distribution of Haemoglobin C and its Prevalence in Newborns in Africa. Sci. Rep. 2013, 3, 1671. [Google Scholar] [CrossRef]
- Jimoh, A.O.; Adebisi, I.M.; Ndakotsu, M.A. Drug use pattern in sickle cell disease in a hematology unit of a teaching hospital in North-Western Nigeria. Ann. Niger. Med. 2014, 8, 32–36. [Google Scholar]
- Okocha, E.C.; Gyamfi, J.; Ryan, N. Barriers to Therapeutic Use of Hydroxyurea for Sickle Cell Disease in Nigeria: A Cross-Sectional Survey. Front. Genet. 2022, 12, 765958. [Google Scholar] [CrossRef]
- Galadanci, N.; Wudil, B.J.; Balogun, T.M.; Ogunrinde, G.O.; Akinsulie, A.; Hasan-Hanga, F.; Mohammed, A.S.; Kehinde, M.O.; Olaniyi, J.A.; Diaku-Akinwumi, I.N.; et al. Current Sickle Cell Disease Management Practices in Nigeria. Int. Health 2014, 6, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, S.A.; Adeodu, O.O.; Adekile, A.D. Sickle Cell Disease Clinical Phenotypes in Children from South-Western, Nigeria. Niger. J. Clin. Pract. 2015, 18, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Adewoyin, A.S.; Oghuvwu, O.S.; Awodu, O.A. Hydroxyurea Therapy in Adult Nigerian Sickle Cell Disease: A Monocentric Survey on Pattern of Use, Clinical Effects and Patient’s Compliance. Afr. Health Sci. 2017, 17, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, T.A.; Diaku-Akinwunmi, I.N.; Ojewunmi, O.O. Barriers to the Use of Hydroxyurea in the Management of Sickle Cell Disease in Nigeria. Hemoglobin 2019, 43, 188–192. [Google Scholar] [CrossRef]
- Ofakunrin, A.O.; Okpe, E.S.; O Afolaranmi, T.; Olaosebikan, R.R.; Kanhu, P.U.; Adekola, K.; Dami, N.; Sagay, A.S. Level of Utilization and Provider-Related Barriers to the Use of Hydroxyurea in the Treatment of Sickle Cell Disease Patients in Jos, North-Central Nigeria. Afr. Health Sci. 2021, 21, 765–774. [Google Scholar] [CrossRef]
- Powars, D.R.; Chan, L.S.; Hiti, A.; Ramicone, E.M.; Johnson, C. Outcome of Sickle Cell Anemia. Medicine 2005, 84, 363–376. [Google Scholar] [CrossRef]
- Kanter, J.; Kruse-Jarres, R. Management of Sickle Cell Disease from Childhood through Adulthood. Blood Rev. 2013, 27, 279–287. [Google Scholar] [CrossRef]
- Shah, N.; Bhor, M.; Xie, L.; Paulose, J.; Yuce, H. Sickle Cell Disease Complications: Prevalence and Resource Utilization. PLoS ONE 2019, 14, e0214355. [Google Scholar] [CrossRef]
- Stuart, M.J.; Nagel, R.L. Sickle-cell disease. Lancet 2004, 364, 1343–1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lanzkron, S.; Carroll, C.P.; Haywood, C. The burden of emergency department use for sickle-cell disease: An analysis of the national emergency department sampled at a base. Am. J. Hematol. 2010, 85, 797–799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action; Martinez, R.M.; Osei-Anto, H.A.; McCormick, M. (Eds.) Complications of Sickle Cell Disease and Current Management Approaches. In Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action; National Academies Press (US): Washington, DC, USA; Agency for Health Care Research and Quality: Rockville, MD, USA, 2020; Volume 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK566466/ (accessed on 21 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).