CAR-T Therapy in Multiple Myeloma: Looking Beyond
Abstract
1. Introduction
2. CAR-T
2.1. Structure and Mechanism of Action
2.2. CAR-T Manufacturing
3. BCMA-Targeting CAR-T
3.1. BCMA
3.2. Idecabtagene Vicleucel
3.3. Ciltacabtagene Autoleucel
4. Non-BMCA CAR-T
4.1. GPRC5D
4.2. FcRL5
4.3. CS1
5. Limitations of CAR-T Therapy in MM: Toxicities and Resistance Mechanisms
5.1. CAR-T Toxicities
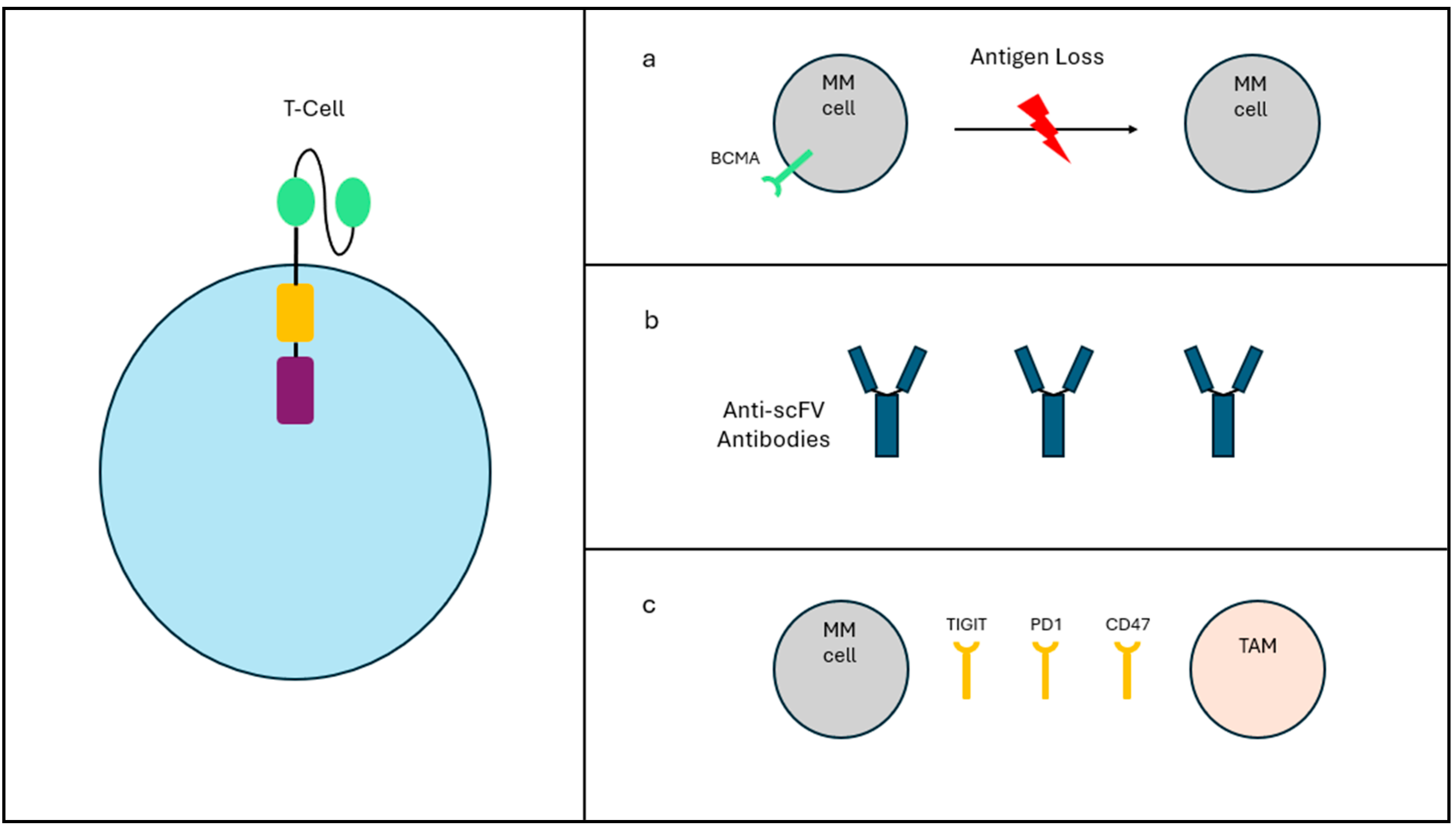
5.2. Resistance Mechanisms
6. Overcoming Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.D.; Baljevic, M.; Baz, R.; Campagnaro, E.; Castillo, J.J.; Costello, C.; D’Angelo, C.; et al. Multiple Myeloma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2023, 21, 1281–1301. [Google Scholar] [CrossRef]
- Ludwig, H.; Terpos, E.; van de Donk, N.; Mateos, M.-V.; Moreau, P.; Dimopoulos, M.-A.; Delforge, M.; Rodriguez-Otero, P.; San-Miguel, J.; Yong, K.; et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: A consensus report of the European Myeloma Network. Lancet Oncol. 2023, 24, e255–e269. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Iacobelli, S.; Pasquini, M.C.; Modi, R.; Giaccone, L.; Blade, J.; Schonland, S.; Evangelista, A.; Perez-Simon, J.A.; Hari, P.; et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transpl. 2020, 55, 1810–1816. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Paiva, B.; San-Miguel, J.F. Roadmap to cure multiple myeloma. Cancer Treat. Rev. 2021, 100, 102284. [Google Scholar] [CrossRef] [PubMed]
- Vormittag, P.; Gunn, R.; Ghorashian, S.; Veraitch, F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018, 53, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, Y.; Asakura, Y.; Utsunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987, 149, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef]
- Guo, B.; Chen, M.; Han, Q.; Hui, F.; Dai, H.; Zhang, W.; Zhang, Y.; Wang, Y.; Zhu, H.; Han, W. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J. Cell. Immunother. 2016, 2, 28–35. [Google Scholar] [CrossRef]
- Levine, B.L. Performance-enhancing drugs: Design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Ther. 2015, 22, 79–84. [Google Scholar] [CrossRef]
- Cavazza, A.; Moiani, A.; Mavilio, F. Mechanisms of retroviral integration and mutagenesis. Hum. Gene Ther. 2013, 24, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, G.; Fraietta, J.A.; Gerson, J.N.; Van Deerlin, V.M.; Morrissette JJ, D.; Caponetti, G.C.; Paruzzo, L.; Harris, J.C.; Chong, E.A.; Susanibar Adaniya, S.P.; et al. T-cell Lymphoma and Secondary Primary Malignancy Risk after Commercial CAR T-cell Therapy. Nat. Med. 2024, 30, 984–989. [Google Scholar] [CrossRef]
- Poorebrahim, M.; Sadeghi, S.; Fakhr, E.; Abazari, M.F.; Poortahmasebi, V.; Kheirollahi, A.; Askari, H.; Rajabzadeh, A.; Rastegarpanah, M.; Linē, A.; et al. Production of CAR T-cells by GMP-grade lentiviral vectors: Latest advances and future prospects. Crit. Rev. Clin. Lab. Sci. 2019, 56, 393–419. [Google Scholar] [CrossRef] [PubMed]
- Prommersberger, S.; Reiser, M.; Beckmann, J.; Danhof, S.; Amberger, M.; Quade-Lyssy, P.; Einsele, H.; Hudecek, M.; Bonig, H.; Ivics, Z. CARAMBA: A first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther. 2021, 28, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Motais, B.; Charvátová, S.; Walek, Z.; Hájek, R.; Bagó, J.R. NK92 Expressing Anti-BCMA CAR and Secreted TRAIL for the Treatment of Multiple Myeloma: Preliminary In Vitro Assessment. Cells 2023, 12, 2748. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; Kerre, T.; Havelange, V.; Poiré, X.; Lewalle, P.; Wang, E.S.; Brayer, J.B.; Davila, M.L.; Moors, I.; Machiels, J.-P.; et al. CYAD-01, an autologous NKG2D-based CAR T-cell therapy, in relapsed or refractory acute myeloid leukaemia and myelodysplastic syndromes or multiple myeloma (THINK): Haematological cohorts of the dose escalation segment of a phase 1 trial. Lancet Haematol. 2023, 10, e191–e202. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S.; Sperling, A.S.; Ansuinelli, M.; Nikiforow, S.; Quinn, D.; Bu, D.; Mataraza, J.; Pearson, D.; Rispoli, L.; Credi, M.A.; et al. T-ChargeTM Manufacturing of the Anti-BCMA CAR-T, Durcabtagene Autoleucel (PHE885), Promotes Expansion and Persistence of CAR-T Cells with High TCR Repertoire Diversity. Blood 2023, 142 (Suppl. S1), 3469. [Google Scholar] [CrossRef]
- Mailankody, S.; Matous, J.V.; Chhabra, S.; Liedtke, M.; Sidana, S.; Oluwole, O.O.; Malik, S.; Nath, R.; Anwer, F.; Cruz, J.C.; et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: Phase 1 UNIVERSAL trial interim results. Nat. Med. 2023, 29, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K. Anti-BCMA Immunotoxins: Design, Production, and Preclinical Evaluation. Biomolecules 2020, 10, 1387. [Google Scholar] [CrossRef]
- Madry, C.; Laabi, Y.; Callebaut, I.; Roussel, J.; Hatzoglou, A.; Le Coniat, M.; Mornon, J.P.; Berger, R.; Tsapis, A. The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int. Immunol. 1998, 10, 1693–1702. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Acharya, C.; An, G.; Moschetta, M.; Zhong, M.Y.; Feng, X.; Cea, M.; Cagnetta, A.; Wen, K.; van Eenennaam, H.; et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016, 127, 3225–3236. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.L.; Emre, N.C.T.; Lamy, L.; Ngo, V.N.; Wright, G.; Xiao, W.; Powell, J.; Dave, S.; Yu, X.; Zhao, H.; et al. IRF4 addiction in multiple myeloma. Nature 2008, 454, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-T.; Anderson, K.C. B cell maturation antigen (BCMA)-based immunotherapy for multiple myeloma. Expert Opin. Biol. Ther. 2019, 19, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Ghermezi, M.; Li, M.; Vardanyan, S.; Harutyunyan, N.M.; Gottlieb, J.; Berenson, A.; Spektor, T.M.; Andreu-Vieyra, C.; Petraki, S.; Sanchez, E.; et al. Serum B-cell maturation antigen: A novel biomarker to predict outcomes for multiple myeloma patients. Haematologica 2017, 102, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.A.; Ortiz, R.J.; Lesokhin, A.M.; Richter, J. Soluble B-cell maturation antigen in multiple myeloma. Am. J. Hematol. 2024, 99, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Xu, N.; Ng, N.; Sanchez, E.; Soof, C.M.; Patil, S.; Udd, K.; Bujarski, S.; Cao, J.; et al. Serum B-cell maturation antigen (BCMA) reduces binding of anti-BCMA antibody to multiple myeloma cells. Leuk. Res. 2019, 81, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Durante, M.; Ahn, S.; Leblay, N.; Poorebrahim, M.; Maity, R.; Tilmont, R.; Barakat, E.; Jung, D.; Ziccheddu, B.; et al. The Impact of Soluble BCMA and BCMA Gain on Anti-BCMA Immunotherapies in Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 4688. [Google Scholar] [CrossRef]
- De Luca, F.; Allegra, A.; Di Chio, C.; Previti, S.; Zappalà, M.; Ettari, R. Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 3136. [Google Scholar] [CrossRef] [PubMed]
- Friedman, K.M.; Garrett, T.E.; Evans, J.W.; Horton, H.M.; Latimer, H.J.; Seidel, S.L.; Horvath, C.J.; Morgan, R.A. Effective Targeting of Multiple B-Cell Maturation Antigen–Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum. Gene Ther. 2018, 29, 585–601. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Lin, Y.; Raje, N.S.; Berdeja, J.G.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Massaro, M.; et al. Idecabtagene vicleucel for relapsed and refractory multiple myeloma: Post hoc 18-month follow-up of a phase 1 trial. Nat. Med. 2023, 29, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Liu, J.; Wang, B.-Y.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Martin, T.G.; Usmani, S.Z.; Berdeja, J.G.; Jakubowiak, A.J.; Agha, M.E.; Cohen, A.D.; Deol, A.; Htut, M.; Lesokhin, A.M.; et al. CARTITUDE-1 final results: Phase 1b/2 study of ciltacabtagene autoleucel in heavily pretreated patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2023, 41 (Suppl. S16), 8009. [Google Scholar] [CrossRef]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.-V.; Fernández de Larrea, C.; Martínez-López, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef]
- Bar, N.; Diels, J.; Van Sanden, S.; Mendes, J.; Hernando, T.; Cost, P.; Schecter, J.M.; Lendvai, N.; Patel, N.; Ishida, T.; et al. Comparative Efficacy of Ciltacabtagene Autoleucel Versus Idecabtagene Vicleucel in the Treatment of Patients with Relapsed or Refractory Multiple Myeloma Previously Treated with 2-4 Prior Lines of Therapy Using a Matching-Adjusted Indirect Comparison. Blood 2023, 142 (Suppl. S1), 2141. [Google Scholar] [CrossRef]
- Bräuner-Osborne, H.; Jensen, A.A.; Sheppard, P.O.; Brodin, B.; Krogsgaard-Larsen, P.; O’Hara, P. Cloning and characterization of a human orphan family C G-protein coupled receptor GPRC5D. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2001, 1518, 237–248. [Google Scholar] [CrossRef]
- Smith, E.L.; Harrington, K.; Staehr, M.; Masakayan, R.; Jones, J.; Long, T.J.; Ng, K.Y.; Ghoddusi, M.; Purdon, T.J.; Wang, X.; et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl. Med. 2019, 11, eaau7746. [Google Scholar] [CrossRef]
- Mailankody, S.; Devlin, S.M.; Landa, J.; Nath, K.; Diamonte, C.; Carstens, E.J.; Russo, D.; Auclair, R.; Fitzgerald, L.; Cadzin, B.; et al. GPRC5D-Targeted CAR T Cells for Myeloma. N. Engl. J. Med. 2022, 387, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.; Berdeja, J.; Htut, M.; Kocoglu, M.; Gregory, T.; Anderson, L.D.; Rossi, A.; Egan, D.; Costa, L.; Kelly, L.; et al. S193: BMS-986393 (CC-95266), A G protein–coupled receptor class C group 5 member D (GPRC5D)–Targeted CAR T-cell therapy for relapsed/refractory multiple myeloma (RRMM): Results from a phase 1 study. HemaSphere 2023, 7 (Suppl. S3), e9863287. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, G.; Zhou, L.; Zhou, J.; Chen, S.; Zhang, W.; Wang, D.; Luo, X.; Cui, J.; Huang, S.; et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): A first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol. 2023, 10, e107–e116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bachier, C.R.; Cavo, M.; Corradini, P.; Delforge, M.; Janowski, W.; Lesokhin, A.M.; Mina, R.; Paris, L.; Rosiñol, L.; et al. CAMMA 2: A phase I/II trial evaluating the efficacy and safety of cevostamab in patients with relapsed/refractory multiple myeloma (RRMM) who have triple-class refractory disease and have received a prior anti-B-cell maturation antigen (BCMA) agent. J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS8064. [Google Scholar] [CrossRef]
- Trudel, S.; Cohen, A.D.; Krishnan, A.Y.; Fonseca, R.; Spencer, A.; Berdeja, J.G.; Lesokhin, A.; Forsberg, P.A.; Laubach, J.P.; Costa, L.J.; et al. Cevostamab Monotherapy Continues to Show Clinically Meaningful Activity and Manageable Safety in Patients with Heavily Pre-Treated Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from an Ongoing Phase I Study. Blood 2021, 138 (Suppl. S1), 157. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, H.; Qin, H.; Tang, K.; Shi, X.; Zhu, T.; Gao, Y.; Zhang, Y.; Tian, X.; Fu, J.; et al. Chimeric antigen receptor T cells targeting FcRH5 provide robust tumour-specific responses in murine xenograft models of multiple myeloma. Nat. Commun. 2023, 14, 3642. [Google Scholar] [CrossRef] [PubMed]
- NCT06196255. Available online: https://clinicaltrials.gov/study/NCT06196255 (accessed on 1 March 2024).
- Chu, E.; Wu, J.; Kang, S.S.; Kang, Y. SLAMF7 as a Promising Immunotherapeutic Target in Multiple Myeloma Treatments. Curr. Oncol. 2023, 30, 7891–7903. [Google Scholar] [CrossRef]
- O’Neal, J.; Ritchey, J.K.; Cooper, M.L.; Niswonger, J.; Sofía González, L.; Street, E.; Rettig, M.P.; Gladney, S.W.; Gehrs, L.; Abboud, R.; et al. CS1 CAR-T targeting the distal domain of CS1 (SLAMF7) shows efficacy in high tumor burden myeloma model despite fratricide of CD8+CS1 expressing CAR-T cells. Leukemia 2022, 36, 1625–1634. [Google Scholar] [CrossRef]
- NCT03710421. (n.d.). Available online: https://clinicaltrials.gov/study/NCT03710421 (accessed on 5 March 2024).
- NCT06185751. (n.d.). Available online: https://clinicaltrials.gov/study/NCT06185751 (accessed on 5 March 2024).
- Li, C.; Xu, J.; Luo, W.; Liao, D.; Xie, W.; Wei, Q.; Zhang, Y.; Wang, X.; Wu, Z.; Kang, Y.; et al. Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma. Leukemia 2024, 38, 149–159. [Google Scholar] [CrossRef]
- NCT05950113. (n.d.). Available online: https://clinicaltrials.gov/study/NCT05950113 (accessed on 7 March 2024).
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Hansen, D.K.; Sidana, S.; Peres, L.C.; Colin Leitzinger, C.; Shune, L.; Shrewsbury, A.; Gonzalez, R.; Sborov, D.W.; Wagner, C.; Dima, D.; et al. Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma: Real-World Experience from the Myeloma CAR T Consortium. J. Clin. Oncol. 2023, 41, 2087–2097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, A.D.; Mateos, M.V.; Cohen, Y.C.; Rodriguez-Otero, P.; Paiva, B.; van de Donk, N.W.C.J.; Martin, T.; Suvannasankha, A.; De Braganca, K.C.; Corsale, C.; et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood 2023, 141, 219–230. [Google Scholar] [CrossRef]
- Anderson, L.D., Jr.; Dhakal, B.; Jain, T.; Oluwole, O.O.; Shah, G.L.; Sidana, S.; Perales, M.A.; Pasquini, M.C. Chimeric Antigen Receptor T Cell Therapy for Myeloma: Where Are We Now and What Is Needed to Move Chimeric Antigen Receptor T Cells Forward to Earlier Lines of Therapy? Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transplant. Cell. Ther. 2024, 30, 17–37. [Google Scholar] [CrossRef]
- Sidana, S.; Ahmed, N.; Akhtar, O.S.; Heim, M.; Brazauskas, R.; Hansen, D.K.; Ferreri, C.; Freeman, C.L.L.; Afrough, A.; Anderson, L.D., Jr.; et al. Real World Outcomes with Idecabtagene Vicleucel (Ide-Cel) CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 1027. [Google Scholar] [CrossRef]
- Miller, K.C.; Johnson, P.C.; Abramson, J.S.; Soumerai, J.D.; Yee, A.J.; Branagan, A.R.; O’Donnell, E.K.; Saucier, A.; Jacobson, C.A.; Frigault, M.J.; et al. Effect of granulocyte colony-stimulating factor on toxicities after CAR T cell therapy for lymphoma and myeloma. Blood Cancer J. 2022, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Drillet, G.; Lhomme, F.; De Guibert, S.; Manson, G.; Houot, R. Prolonged thrombocytopenia after CAR T-cell therapy: The role of thrombopoietin receptor agonists. Blood Adv. 2023, 7, 537–540. [Google Scholar] [CrossRef]
- Rejeski, K.; Hansen, D.K.; Bansal, R.; Sesques, P.; Ailawadhi, S.; Logue, J.M.; Bräunlein, E.; Cordas Dos Santos, D.M.; Freeman, C.L.; Alsina, M.; et al. The CAR-HEMATOTOX score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. J. Hematol. Oncol. 2023, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.; Sheng, Y.; Huang, C.Y.; Bylsma, S.; Lo, M.; Kennedy, V.; Natsuhara, K.; Martin, T.; Wolf, J.; Shah, N.; et al. Infectious complications in patients with relapsed refractory multiple myeloma after BCMA CAR T-cell therapy. Blood Adv. 2022, 6, 2045–2054. [Google Scholar] [CrossRef]
- Little, J.S.; Tandon, M.; Hong, J.S.; Nadeem, O.; Sperling, A.S.; Raje, N.; Munshi, N.; Frigault, M.; Barmettler, S.; Hammond, S.P. Respiratory infections predominate after day 100 following B-cell maturation antigen-directed CAR T-cell therapy. Blood Adv. 2023, 7, 5485–5495. [Google Scholar] [CrossRef]
- Logue, J.M.; Peres, L.C.; Hashmi, H.; Colin-Leitzinger, C.M.; Shrewsbury, A.M.; Hosoya, H.; Gonzalez, R.M.; Copponex, C.; Kottra, K.H.; Hovanky, V.; et al. Early cytopenias and infections after standard of care idecabtagene vicleucel in relapsed or refractory multiple myeloma. Blood Adv. 2022, 6, 6109–6119. [Google Scholar] [CrossRef]
- Afrough, A.; Abraham, P.R.; Turer, L.; Kaur, G.; Sannareddy, A.; Hansen, D.K.; Anderson, L.D., Jr. Toxicity of CAR T-Cell Therapy for Multiple Myeloma. Acta Haematol. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Kortüm, K.M.; Raab, M.S.; Weinhold, N. The Impact of Tumor Heterogeneity on Diagnostics and Novel Therapeutic Strategies in Multiple Myeloma. Int. J. Mol. Sci. 2019, 20, 1248. [Google Scholar] [CrossRef]
- Samur, M.K.; Fulciniti, M.; Aktas Samur, A.; Bazarbachi, A.H.; Tai, Y.-T.; Prabhala, R.; Alonso, A.; Sperling, A.S.; Campbell, T.; Petrocca, F.; et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 2021, 12, 868. [Google Scholar] [CrossRef]
- John, L.; Poos, A.M.; Brobeil, A.; Schinke, C.; Huhn, S.; Prokoph, N.; Lutz, R.; Wagner, B.; Zangari, M.; Tirier, S.M.; et al. Resolving the spatial architecture of myeloma and its microenvironment at the single-cell level. Nat. Commun. 2023, 14, 5011. [Google Scholar] [CrossRef]
- Mi, X.; Penson, A.; Abdel-Wahab, O.; Mailankody, S. Genetic Basis of Relapse after GPRC5D-Targeted CAR T Cells. Blood 2023, 142 (Suppl. S1), 336. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.-J.; Yang, S.-S.; Sun, Y.; Wu, W.; Liu, Y.-F.; Xu, J.; Zhuang, Y.; Zhang, W.; Weng, X.-Q.; et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc. Natl. Acad. Sci. USA 2019, 116, 9543–9551. [Google Scholar] [CrossRef]
- Gauthier, J.; Bezerra, E.D.; Hirayama, A.V.; Fiorenza, S.; Sheih, A.; Chou, C.K.; Kimble, E.L.; Pender, B.S.; Hawkins, R.M.; Vakil, A.; et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood 2021, 137, 323–335. [Google Scholar] [CrossRef]
- Leblay, N.; Maity, R.; Hasan, F.; Neri, P. Deregulation of Adaptive T Cell Immunity in Multiple Myeloma: Insights Into Mechanisms and Therapeutic Opportunities. Front. Oncol. 2020, 10, 636. [Google Scholar] [CrossRef]
- Hashmi, H.; Hansen, D.K.; Peres, L.C.; Puglianini, O.C.; Freeman, C.; De Avila, G.; Sidana, S.; Shune, L.; Sborov, D.W.; Davis, J.; et al. Factors associated with refractoriness or early progression after idecabtagene vicleucel in patients with relapsed/refractory multiple myeloma: U.S. Myeloma Immunotherapy Consortium real world experience. Haematologica 2023, 109, 1514–1524. [Google Scholar] [CrossRef]
- Mouhieddine, T.H.; Van Oekelen, O.; Melnekoff, D.T.; Li, J.; Ghodke-Puranik, Y.; Lancman, G.; Thibaud, S.; Pan, D.; Rajeeve, S.; Agte, S.; et al. Sequencing T-cell redirection therapies leads to deep and durable responses in patients with relapsed/refractory myeloma. Blood Adv. 2023, 7, 1056–1064. [Google Scholar] [CrossRef]
- Cencini, E.; Sicuranza, A.; Ciofini, S.; Fabbri, A.; Bocchia, M.; Gozzetti, A. Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications. Curr. Oncol. 2023, 30, 6111–6133. [Google Scholar] [CrossRef]
- Mishra, A.K.; Schmidt, T.M.; Martell, E.B.; Chen, A.S.; Dogru, R.E.; Hematti, P.; Callander, N.S. PD1+ TIGIT+ 2B4+ KLRG1+ Cells Might Underlie T Cell Dysfunction in Patients Treated with BCMA-Directed Chimeric Antigen Receptor T Cell Therapy. Transplant. Cell. Ther. 2024, 30, 191–202. [Google Scholar] [CrossRef]
- Davis, J.A.; Dima, D.; Ahmed, N.; DeJarnette, S.; McGuirk, J.; Jia, X.; Raza, S.; Khouri, J.; Valent, J.; Anwer, F.; et al. Impact of Frailty on Outcomes after Chimeric Antigen Receptor T Cell Therapy for Patients with Relapsed/Refractory Multiple Myeloma. Transplant. Cell. Ther. 2024, 30, 298–305. [Google Scholar] [CrossRef]
- Du, J.; Qiang, W.; Lu, J.; Jia, Y.; He, H.; Liu, J.; Guo, P.; Yang, Y.; Feng, Z.; Jin, L.; et al. Updated Results of a Phase I Open-Label Single-Arm Study of Dual Targeting BCMA and CD19 Fastcar-T Cells (GC012F) As First-Line Therapy for Transplant-Eligible Newly Diagnosed High-Risk Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 1022. [Google Scholar] [CrossRef]
- Oliver-Caldes, A.; Gonzalez-Calle, V.; Cabañas, V.; Lopez-Muñoz, N.; Rodriguez Otero, P.; Reguera, J.L.; Español-Rego, M.; Inoges, S.; Zabaleta, A.; Lopez Corral, L.; et al. ARI0002h (Cesnicabtagene Autoleucel), an Academic Point-of-Care B-Cell Maturation Antigen (BCMA)-Directed Chimeric Antigen Receptor (CAR) T-Cell Strategy: Activity and Safety after Fractionated Initial Therapy and Booster Dose in 60 Patients with Relapsed/Refractory Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 1026. [Google Scholar] [CrossRef]
- Van De Donk, N.W.C.J.; Anguille, S.; Caers, J.; Liefaard, M.C.; Jacques, C.; Van Muyden, A.D.D. Rationale for and Design of Papilio-1: A Phase 1/2, Multicenter, Open-Label Study to Evaluate the Feasibility, Safety, and Efficacy of Point-of-Care-Manufactured Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells (GLPG5301) in Relapsed/Refractory Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 4859. [Google Scholar] [CrossRef]
- Larson, R.C.; Kann, M.C.; Graham, C.; Mount, C.W.; Castano, A.P.; Lee, W.-H.; Bouffard, A.A.; Takei, H.N.; Almazan, A.J.; Scarfó, I.; et al. Anti-TACI single and dual-targeting CAR T cells overcome BCMA antigen loss in multiple myeloma. Nat. Commun. 2023, 14, 7509. [Google Scholar] [CrossRef]
- Madan, S.; Costello, C.L.; Lipe, B.; Cowan, A.J.; Medvedova, E.; Hillengass, J.; Bergsagel, P.L.; Leleu, X.; Touzeau, C.; Morillo, D.; et al. Results from the Completed Dose Escalation Portion of the Phase 1 Study of HPN217, a Half-Life Extended Tri-Specific T Cell Activating Construct (TriTAC®) Targeting B Cell Maturation Antigen (BCMA) for Relapsed/Refractory Multiple Myeloma (MM). Blood 2023, 142 (Suppl. S1), 1012. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Alabanza, L.M.; Xiong, Y.; Vu, B.; Webster, B.; Wu, D.; Hu, P.; Zhu, Z.; Dropulic, B.; Dash, P.; Schneider, D. Armored BCMA CAR T Cells Eliminate Multiple Myeloma and Are Resistant to the Suppressive Effects of TGF-β. Front. Immunol. 2022, 13, 832645. [Google Scholar] [CrossRef]
- Ren, Q.; Zu, Y.; Su, H.; Lu, Q.; Xiang, B.; Luo, Y.; Zhang, J.; Song, Y. Single VHH-directed BCMA CAR-NK cells for multiple myeloma. Exp. Hematol. Oncol. 2023, 12, 98. [Google Scholar] [CrossRef]
- Park, E.; Mun, H.-J.; Seo, E.; Hwang, S.; Lee, J.H.; Song, S.; Sung, H.; Kim, H.-Y.; Kwon, M.-J. CAR NK92 Cells Targeting BCMA Can Effectively Kill Multiple Myeloma Cells Both In Vitro and In Vivo. Biomedicines 2024, 12, 248. [Google Scholar] [CrossRef]
- Dholaria, B.; Kocoglu, M.H.; Kin, A.; Asch, A.S.; Ramakrishnan, A.; Bachier, C.; Rodriguez, T.E.; Shune, L.; McArthur, K.; McCaigue, J.; et al. Early Safety Results of P-BCMA-ALLO1, a Fully Allogeneic Chimeric Antigen Receptor T-Cell (CAR-T), in Patients with Relapsed / Refractory Multiple Myeloma (RRMM). Blood 2023, 142 (Suppl. S1), 3479. [Google Scholar] [CrossRef]
- Jia, X.; Yan, B.; Tian, X.; Liu, Q.; Jin, J.; Shi, J.; Hou, Y. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int. J. Biol. Sci. 2021, 17, 3281–3287. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, D.; Li, H.; Zhu, Z.; Zhang, Z.; Chen, Y.; Yang, N.; Li, J.; Wang, Z.; Niu, T.; et al. Delivery of CD47-SIRPα checkpoint blocker by BCMA-directed UCAR-T cells enhances antitumor efficacy in multiple myeloma. Cancer Lett. 2024, 585, 216660. [Google Scholar] [CrossRef]
- NCT05257083. (n.d.). Available online: https://clinicaltrials.gov/study/NCT05257083 (accessed on 15 March 2024).
- NCT06045806. (n.d.). Available online: https://clinicaltrials.gov/study/NCT06045806 (accessed on 15 March 2024).
- NCT06048250. (n.d.). Available online: https://clinicaltrials.gov/study/NCT06048250 (accessed on 11 February 2024).
- NCT06179888. (n.d.). Available online: https://clinicaltrials.gov/study/NCT06179888 (accessed on 20 February 2024).
- Anderson, G.S.F.; Walker, I.; Roy, J.P.; Chapman, M.A. And-Gate CAR T-Cells to Improve Tumour Specificity and Targeting of Low-Expression Antigens in Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 751. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, G.; Li, G.; Wang, Y. Chimeric antigen receptor T-cell therapy-induced nervous system toxicity: A real-world study based on the FDA Adverse Event Reporting System database. BMC Cancer 2024, 24, 10. [Google Scholar] [CrossRef]
- Patel, U.; Oluwole, O.O.; Kassim, A.; Jayani, R.; Belliveau, P.; Savani, B.; Dholaria, B.R. Sequencing bispecific antibodies and CAR T cell therapy in multiple myeloma with prior exposure to BCMA-targeted therapies. J. Clin. Oncol. 2023, 41 (Suppl. S16), e20049. [Google Scholar] [CrossRef]

| ClinicalTrials.gov Identifier | Phase | Title | Target | Sponsor |
|---|---|---|---|---|
| NCT03601078 | II | An Efficacy and Safety Study of bb2121 in Subjects With Relapsed and Refractory Multiple Myeloma and in Subjects With High-Risk Multiple Myeloma (KARMMA-2) | BCMA | Celgene/BMS, Lawrence, NJ, USA |
| NCT03710421 | I | CS1-CAR T Therapy Following Chemotherapy in Treating Patients With Relapsed or Refractory CS1 Positive Multiple Myeloma | CS1 | City of Hope Medical Center, Duarte, CA, USA |
| NCT03758417 | II | A Study of LCAR-B38M CAR-T Cells, a Chimeric Antigen Receptor T-cell (CAR-T) Therapy Directed Against B-cell Maturation Antigen (BCMA) in Chinese Participants With Relapsed or Refractory Multiple Myeloma | BCMA | Nanjing Legend Biotech Co., Nanjing, China |
| NCT04727008 | I | CXCR4 Modified Anti-BCMA CAR T Cells for Multiple Myeloma | BCMA | Sichuan University, Chengdu, China |
| NCT04816526 | II | Descartes-08 Consolidation Treatment in Patients With High-Risk Multiple Myeloma Who Have Residual Disease After Induction Therapy | BCMA | Cartesian Therapeutics, Gaithersburg, MD, USA |
| NCT04923893 | III | A Study of Bortezomib, Lenalidomide and Dexamethasone (VRd) Followed by Cilta-cel, a CAR-T Therapy Directed Against BCMA Versus VRd Followed by Lenalidomide and Dexamethasone (Rd) Therapy in Participants With Newly Diagnosed Multiple Myeloma for Whom ASCT is Not Planned as Initial Therapy (CARTITUDE-5) | BCMA | Janssen Research and Development, LLC, Beerse, Belgium |
| NCT04935580 | I/II | Study of FasT CAR-T GC012F Injection in High Risk TE NDMM Patients | BCMA/ CD19 | Shanghai Changzheng Hospital, Shanghai, China |
| NCT04960579 | I | P-BCMA-ALLO1 Allogeneic CAR-T Cells in the Treatment of Subjects With Multiple Myeloma | BCMA | Poseida Therapeutics, Inc., San Diego, CA, USA |
| NCT05020444 | I | TriPRIL CAR T Cells in Multiple Myeloma | APRIL | Marcela V. Maus, M.D., Ph.D., Boston, MA, USA |
| NCT05113342 | I/II | Descartes-25 in Relapsed/Refractory Multiple Myeloma | BCMA | Cartesian Therapeutics, Gaithersburg, MD, USA |
| NCT05117008 | II | Maintenance Belantamab Mafodotin (Blenrep®) After B-cell Maturation Antigen-Directed Chimeric Antigen Receptor T-cell Therapy in Patients With Relapsed and/or Refractory Multiple Myeloma (EMBRACE) | BCMA | Medical College of Wisconsin, Milwaukee, WI, USA |
| NCT05181501 | I | A Study of Fully Human BCMA CAR-T (CT103A) in Patients With Newly Diagnosed High-risk Multiple Myeloma (FUMANBA-2) | BCMA | Nanjing IASO Biotechnology Co., Ltd., Nanjing, China |
| NCT05257083 | III | A Study of Daratumumab, Bortezomib, Lenalidomide and Dexamethasone (DVRd) Followed by Ciltacabtagene Autoleucel Versus Daratumumab, Bortezomib, Lenalidomide and Dexamethasone (DVRd) Followed by Autologous Stem Cell Transplant (ASCT) in Participants With Newly Diagnosed Multiple Myeloma (CARTITUDE-6) | BCMA | Stichting European Myeloma Network, Amsterdam, Netherlands |
| NCT05325801 | I | A Study of CAR-T Cells Targeting Both BCMA and GPRC5D in Treatment of Relapsed or Refractory Multiple Myeloma | BCMA/ GPRC5D | Zhejiang University, Hangzhou, China |
| NCT05411497 | I | Genetically Engineered Cells (MUC1-Activated T-Cells) for the Treatment of MUC1 Positive Recurrent or Refractory Multiple Myeloma | MUC1 | Mayo Clinic, Scottsdale, AZ, USA |
| NCT05412329 | I | Study of Dual Targeted CD19/BCMA FASTCART GC012F in Relapsed/Refractory Multiple Myeloma | CD19/ BCMA | Shanghai Changzheng Hospital, Shanghai, China |
| NCT05431608 | I | A Study of MCARH109 and MCARH125 in People With Multiple Myeloma | BCMA/ GPRC5D | Memorial Sloan Kettering Cancer Center, New York, NY, USA |
| NCT05498545 | I | Universal BCMA-targeted LUCAR-B68 Cells in Patients With Relapsed/Refractory Multiple Myeloma | BCMA | Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China |
| NCT05509530 | II | Safety and Efficacy of Anti-BCMA/GPRC5D CAR-T Cell Therapy in Treating Relapsed and Refractory Multiple Myeloma(rr/MM) | BCMA/ GPRC5D | Xuzhou Medical University, Xuzhou, China |
| NCT05632380 | I/II | ASCT in Combination With C-CAR088 for Treating Patients With Ultra High-risk Multiple Myeloma (MM) | BCMA | Institute of Hematology and Blood Diseases Hospital, China, Tianjin, China |
| NCT05698303 | I | A Study of Fully Human BCMA Chimeric Antigen Receptor Autologous T Cell Injection (CT103A) in the Treatment of Patients With Relapsed/Refractory Multiple Myeloma | BCMA | Nanjing IASO Biotechnology Co., Ltd., Nanjing, China |
| NCT05722418 | I | CRISPR-Edited Allogeneic Anti-BCMA CAR-T Cell Therapy in Patients With Relapsed/Refractory Multiple Myeloma (CaMMouflage) | BCMA | Caribou Biosciences, Inc., Berkeley, CA, USAs |
| NCT05739188 | I/II | Safety and Efficacy of Anti-GPRC5D CAR-T Cells Therapy in the Treatment of r/r MM | GPRC5D | 920th Hospital of Joint Logistics Support Force of People’s Liberation Army of China, Kunming, China |
| NCT05767359 | II | CAR-PRISM (Precision Intervention Smoldering Myeloma) | BCMA | Dana-Farber Cancer Institute, Boston, MA, USA |
| NCT05801939 | II | Cevostamab Following CAR T Cell Therapy for RRMM | FcRH5 | University of Pennsylvania, Philadelphia, PA, USA |
| NCT05846737 | II | BCMA CAR-T Cell Therapy in High-risk NDMM Patients With Positive MRD After First-line ASCT | BCMA | Institute of Hematology and Blood Diseases Hospital, Tianjin, China |
| NCT05850286 | II | A Study of X-VRD Combined With CART-ASCT-CART2 Treatment in NDMM Patients With P53 Abnormalities | XPO-1/ BCMA | Institute of Hematology and Blood Diseases Hospital, Tianjin, China |
| NCT05950113 | I | CART-BCMA/CS1 in Treating Patients With Relapsed or Refractory Multiple Myeloma | BCMA/ CS1 | Jonsson Comprehensive Cancer Center, Los Angeles, CA, USA |
| NCT05976555 | I | Phase I Trial of BCMA-TGF-BETA CAR-T Cells in Relapsed, Refractory Myeloma | BCMA | Medical College of Wisconsin, Milwaukee, WI, USA |
| NCT05998928 | II | A Clinical Study to Evaluate the Safety and Efficacy of BCMA-GPRC5D CAR-T in Patients With Relapsed/Refractory Multiple Myeloma Who Received Three or More Lines of Therapy | BCMA/ GPRC5D | Wuhan Union Hospital, Wuhan, China |
| NCT06045806 | III | A Study to Compare the Efficacy and Safety of Idecabtagene Vicleucel With Lenalidomide Maintenance Therapy Versus Lenalidomide Maintenance Therapy Alone in Adult Participants With Newly Diagnosed Multiple Myeloma Who Have Suboptimal Response After Autologous Stem Cell Transplantation (KarMMa-9) | BCMA | Bristol-Myers Squibb, Lawrence, NJ, USA |
| NCT06048250 | I | Mezigdomide (CC-92480) Post Idecabtagene Vicleucel in Treating Patients With Relapsed Multiple Myeloma | BCMA | City of Hope Medical Center, Duarte, CA, USA |
| NCT06066346 | II | A Study of Talquetamab for People With Multiple Myeloma Who Have Received BCMA CAR T-Cell Therapy | BCMA | Memorial Sloan Kettering Cancer Center, New York, NY, USA |
| NCT06066359 | I/II | Phase I/II Randomized Trial of Cord Blood-derived NK Cells Genetically Engineered With NY-ESO-1 TCR/IL-15 Cell Receptor for Relapsed/Refractory Multiple Myeloma | NY- ESO-1 | M.D. Anderson Cancer Center, Houston, TX, USA |
| NCT06132711 | I/II | Safety and Efficacy of APRIL-BAFF-Bicephali CAR-T in Relapsed, Refractory Multiple Myeloma | APRIL/ BAFF | Xuzhou Medical University, Xuzhou, China |
| NCT06179888 | II | Iberdomide versus Observation Off Therapy after Idecabtagene Vicleucel CAR-T for Multiple Myeloma | BCMA | National Cancer Institute (NCI), Bethesda, MD, USA |
| NCT06182696 | I/II | OriCAR-017 Chimeric Antigen Receptor (CAR) Modified T Cells for the Treatment of R/RMM | GPRC5D | OriCell Therapeutics Co., Ltd., Shanghai, China |
| NCT06185751 | I | Safety and Efficacy of CS1 CAR-T (WS-CART-CS1) in Subjects With Multiple Myeloma | CS1 | Washington University School of Medicine, Saint Louis, MO, USA |
| NCT06196255 | I/II | Safety and Efficacy of Anti-FcRL5 CAR-T Cell Therapy in Treating Relapsed and Refractory Multiple Myeloma (R/R MM) | FcRL5 | Xuzhou Medical University, Xuzhou, China |
| NCT06223646 | I/II | A Study of KQ-2003 CAR-T Cell Therapy for Patients With Relapsed or Refractory Multiple Myeloma | BCMA/ CD19 | Novatim Immune Therapeutics (Zhejiang) Co., Ltd., Beijing, China |
| NCT06242249 | I/II | Anti-BCMA CAR-NK Therapy in Relapsed or Refractory Multiple Myeloma | BCMA | Shahid Beheshti University of Medical Sciences, Tehran, Iran |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiorana, G.; Antolino, G.; La Verde, G.; Tafuri, A. CAR-T Therapy in Multiple Myeloma: Looking Beyond. Hemato 2024, 5, 180-198. https://doi.org/10.3390/hemato5020015
Maiorana G, Antolino G, La Verde G, Tafuri A. CAR-T Therapy in Multiple Myeloma: Looking Beyond. Hemato. 2024; 5(2):180-198. https://doi.org/10.3390/hemato5020015
Chicago/Turabian StyleMaiorana, Gianluca, Giusy Antolino, Giacinto La Verde, and Agostino Tafuri. 2024. "CAR-T Therapy in Multiple Myeloma: Looking Beyond" Hemato 5, no. 2: 180-198. https://doi.org/10.3390/hemato5020015
APA StyleMaiorana, G., Antolino, G., La Verde, G., & Tafuri, A. (2024). CAR-T Therapy in Multiple Myeloma: Looking Beyond. Hemato, 5(2), 180-198. https://doi.org/10.3390/hemato5020015







