Abstract
Multiple myeloma (MM) presents unique challenges in the elderly population due to increased frailty and comorbidities. Balancing treatment efficacy, safety, and quality of life is essential in managing elderly patients. While two-drug regimens were often favored for elderly patients, recent studies show promising outcomes with anti-CD38 antibody-based therapies, particularly daratumumab and lenalidomide with minimal dexamethasone. Continuous low-intensity treatments have shown improved progression-free survival and overall survival, with significant benefits observed in elderly patients. The DRd combination has now emerged as the standard of care for elderly MM patients, offering a favorable balance of efficacy, safety, and convenience. Ongoing trials are evaluating the addition of bortezomib in an induction phase for fit patients. New-generation immunotherapies hold promise for further refining treatment approaches, potentially leading to treatment discontinuation in select patient populations with sustained minimal residual disease negativity.
1. Introduction
Multiple myeloma (MM), an incurable hematological malignancy, is predominantly affecting older adults with a median age of 69 years and approximately one third of patients over the age of 75 years at diagnosis [1]. The increased incidence of multiple myeloma (MM) with age, combined with the aging population, is anticipated to generate an important increase in the number of elderly MM patients by 2030 [2]. The ultimate objective of treatments should be to achieve a cure for every patient, including older individuals. However, it is widely accepted that there are certain differences in treatment goals between younger and older patients. When it comes to treating elderly patients, it is crucial to evaluate a subtle balance between effectiveness, safety, and maintaining their quality of life [3]. Moreover, elderly patients represent a heterogeneous population, as depicted by frailty assessments.
2. Adapting Treatments for Elderly Patients
In the past two decades, several tools have been developed to assess frailty in different populations, including older adults in general [4,5], patients with cancer [6], and more recently, specifically patients with myeloma [7,8]. The initial work in the field of MM frailty assessment was generated less than 10 years ago by the International Myeloma Working Group Frailty Score [9]. It demonstrated that frail patients have shorter survival but also more frequent non-hematological side effects or treatment discontinuation. Treatment intensity is often questioned in elderly patients. A commonly expressed opinion is that for older or frail patients, two-drug regimens may be preferable over three-drug regimens. However, this perspective requires careful evaluation and discussion with patients, as anti-CD38 antibody-based therapies have demonstrated significant efficacy benefits, including improvements in quality of life and greater and faster improvement in bone pain, while maintaining an acceptable tolerability profile for each patient [10]. The use of short-term dexamethasone should, however, be considered in regards to the important toxicity of long-term dexamethasone [11,12]. The GIMEMA group has led a dexamethasone sparing regimen in elderly patients, who were randomly allocated to either lenalidomide and low-dose dexamethasone continuously or lenalidomide and nine cycles of low-dose dexamethasone followed by lenalidomide alone. Both treatment approaches were equally effective, but discontinuing dexamethasone was associated with a better safety profile [13]. Furthermore, the ongoing IFM2017-03 trial is evaluating the possibility of earlier discontinuation after only two cycles in the context of the daratumumab and lenalidomide combination. With the advent of new effective agents, it is expected that the use of dexamethasone will be significantly reduced in the future for older patients.
3. Continuous Treatments Improve Progression-Free Survival
Such low-intensity treatments, given continuously, have proven to be greatly effective. In the FIRST trial, three treatment arms were compared: MPT (melphalan, prednisone, thalidomide), Rd (lenalidomide, dexamethasone) for 18 months, and continuous Rd (until progression) [14]. This study involved comparing the same treatment for a fixed duration vs. continuous treatment. There was a PFS advantage in favor of the continuous Rd arm, with a median of 25.5 months compared to 20.7 months for the Rd 18-month arm (p < 0.001). It is worth noting that the overall survival at 4 years was not statistically different between the two groups, with 59% for the continuous Rd arm and 56% for the Rd 18-month arm (p = 0.31), requiring further evaluation in the long term. The PFS2 (sum of PFS for first-line and second-line therapies) remained in favor of the continuous Rd arm, emphasizing the importance of the months of benefit gained during the first-line therapy with continuous treatment. The toxicity profile was similar in both groups, although there was a moderate increase in the risk of infections in the continuous Rd arm (29% grade 3 or 4 vs. 22%), possibly due to a higher number of cycles. However, the median number of cycles in the continuous Rd arm was 24, and the median PFS was 26 months, suggesting that continuous treatment had to be discontinued for some patients, and maintaining treatment until progression may be challenging depending on the profile of tolerance (Figure 1). These results led to the marketing authorization and reimbursement of continuous Rd as a first-line treatment for patients ineligible for autologous stem cell transplantation, continuing until disease progression.
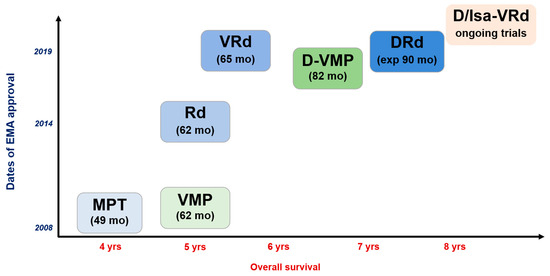
Figure 1.
Treatment landscape for newly diagnosed multiple myeloma patients who are not transplant eligible. MPT, melphalan-prednisone-thalidomide; VMP, bortezomib(Velcade)-melphalan-prednisone; Rd, lenalidomide(Revlimid)-dexamethasone; VRd, bortezomib(Velcade)-lenalidomide (Revlimid)-dexamethasone; D-VMP, daratumumab-bortezomib (Velcade)-melphalan-prednisone; DRd, daratumumab-lenalidomide(Revlimid)-dexamethasone; D/Isa-VRd, daratumumab or isatuximab-bortezomib(Velcade)-lenalidomide (Revlimid)-dexamethasone.
4. Best Overall Survival So Far Achieved with Continuous Treatments
More recently, the combination of daratumumab, lenalidomide, and dexamethasone (DRd) became a strong standard of care treatment for elderly patients. The MAIA study enrolled 737 newly diagnosed patients with MM who were not eligible for autologous stem cell transplantation (ASCT) [15,16]. Patients were randomly assigned to receive either DRd or Rd until disease progression. At a median follow-up of 64.5 months, median PFS was 61.9 months versus 34.4 months (HR of 0.55) in the DRd arm vs. Rd, respectively. Median OS was not reached for DRd (5-year OS of 66.6%) versus 65.5 months with Rd (HR of 0.66). These PFS results are remarkable in this patient population and were accompanied by a high overall response rate (ORR) of 93% with DRd, indicating that almost all patients achieved at least a partial response with this treatment regimen. The rate of minimal residual disease (MRD) negativity was 32% with DRd, compared to 11% with Rd [17]. Another important observation from the MAIA study is that only a small number of patients achieve very deep responses with negative minimal residual disease (MRD) status (only 15% with 1-year sustained MRD negativity). However, these patients have significantly prolonged median progression-free survival (PFS). This suggests that maintaining a continuous low-intensity treatment helps control residual clones and improves patient outcomes. The DRd regimen was generally well-tolerated, with the main differences in adverse events (grade 3 or higher) between the two groups observed in neutropenia (54% with DRd versus 37% with Rd) and infection (41% with DRd versus 29% with Rd; 19% versus 11% for pneumonia). Regarding quality of life, European Organization for Research and Treatment of Cancer (EORTC) Quality of Life (QoL) Questionnaire Core 30-item global health status scores improved from baseline in both groups and were consistently greater with DRd at all time points [10]. A global health status benefit was achieved with DRd, regardless of age (<75 and ≥75 years), baseline performance status score, or depth of response. DRd treatment resulted in a significantly greater reduction in pain scores as early as cycle 3 (p = 0.0007 vs. Rd); the magnitude of change was sustained through cycle 12. Reductions in pain with DRd were clinically meaningful in patients regardless of age, ECOG status, or depth of response. Similarly, PRO improvements were observed with DRd and Rd on the EuroQol five-dimensional descriptive system visual analog scale score. These results are unprecedented in MM, especially for elderly patients, with the favorable efficacy, safety, and quality of life balance of continuous DRd (Figure 1). Ongoing trials are evaluating the value of adding bortezomib initially to improve the rates of MRD negativity (IMROZ, CEPHEUS, and BENEFIT) in elderly and fit patients.
5. Conclusions
Considering efficacy, safety, and convenience, it is likely fair to say that, today, the combination of lenalidomide and daratumumab with a minimal amount of dexamethasone is the current standard of care for newly diagnosed older patients with MM. The favorable tolerability profile supports the continuous administration of the treatment while maintaining a preserved quality of life. The advancement of immunotherapies such as CAR-T cells and bispecific antibodies may potentially modify the treatment approach for elderly patients in the near future. If increased rates of sustained MRD negativity are achieved with those immunotherapies, it may offer the possibility of discontinuing treatment in selected patient populations.
Author Contributions
Conceptualization, S.M. and T.F.; writing—review and editing, S.M. and T.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no financial conflict of interest.
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2018; based on November 2020 SEER data submission, posted to the SEER web site; National Cancer Institute: Bethesda, MD, USA. Available online: https://seer.cancer.gov/csr/1975_2018/ (accessed on 15 April 2021).
- Wildes, T.M.; Rosko, A.; Tuchman, S.A. Multiple myeloma in the older adult: Better prospects, more challenges. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Muhlbacher, A.C.; Nubling, M. Analysis of physicians’ perspectives versus patients’ preferences: Direct assessment and discrete choice experiments in the therapy of multiple myeloma. Eur. J. Health Econ. 2011, 12, 193–203. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tapia, C.; Canoui-Poitrine, F.; Bastuji-Garin, S.; Soubeyran, P.; Mathoulin-Pelissier, S.; Tournigand, C.; Paillaud, E.; Laurent, M.; Audureau, E.; ELCAPA Study Group. Optimizing the G8 Screening Tool for Older Patients With Cancer: Diagnostic Performance and Validation of a Six-Item Version. Oncologist 2016, 21, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Larocca, A.; Facon, T.; Zweegman, S.; Engelhardt, M. Defining the vulnerable patient with myeloma-a frailty position paper of the European Myeloma Network. Leukemia 2020, 34, 2285–2294. [Google Scholar] [CrossRef]
- Isaacs, A.; Fiala, M.; Tuchman, S.; Wildes, T.M. A comparison of three different approaches to defining frailty in older patients with multiple myeloma. J. Geriatr. Oncol. 2020, 11, 311–315. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Facon, T.; Plesner, T.; Usmani, S.Z.; Kumar, S.; Bahlis, N.J.; Hulin, C.; Orlowski, R.Z.; Nahi, H.; Mollee, P.; et al. Health-Related Quality of Life in Transplant-Ineligible Patients With Newly Diagnosed Multiple Myeloma: Findings From the Phase III MAIA Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Blood, E.; Vesole, D.; Fonseca, R.; Greipp, P.R. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Mary, J.-Y.; Pégourie, B.; Attal, M.; Renaud, M.; Sadoun, A.; Voillat, L.; Dorvaux, V.; Hulin, C.; Lepeu, G.; et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood 2006, 107, 1292–1298. [Google Scholar] [CrossRef]
- Larocca, A.; Bonello, F.; Gaidano, G.; D’agostino, M.; Offidani, M.; Cascavilla, N.; Capra, A.; Benevolo, G.; Tosi, P.; Galli, M.; et al. Dose/schedule-adjusted Rd-R vs continuous Rd for elderly, intermediate-fit patients with newly diagnosed multiple myeloma. Blood 2021, 137, 3027–3036. [Google Scholar] [CrossRef]
- Hulin, C.; Belch, A.; Shustik, C.; Petrucci, M.T.; Dührsen, U.; Lu, J.; Song, K.; Rodon, P.; Pégourié, B.; Garderet, L.; et al. Updated Outcomes and Impact of Age With Lenalidomide and Low-Dose Dexamethasone or Melphalan, Prednisone, and Thalidomide in the Randomized, Phase III FIRST Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef]
- San-Miguel, J.; Avet-Loiseau, H.; Paiva, B.; Kumar, S.K.; Dimopoulos, M.A.; Facon, T.; Mateos, M.-V.; Touzeau, C.; Jakubowiak, A.J.; Usmani, S.Z.; et al. Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. Blood 2022, 139, 492–501. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).