Haemostaseological Changes of VWF and FVIII during Pregnancy and the Oestrus Cycle in a Porcine Model of Von Willebrand Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Primary and Secondary Haemostasis
2.3. Assessment of Pregnancy
2.4. VWF-Multimers
2.5. Immunohistochemistry (IHC)
2.6. Statistics and Software
3. Results
3.1. Primary and Secondary Haemostasis
3.1.1. Collagen and Epinephrine (C/EPI) (Figure 1A)

3.1.2. Collagen and Adenosine 5′-Diphosphate (C/ADP) (Figure 1B)
3.1.3. Prostaglandin E1, Adenosine 5′-Diphosphate, and Calcium (P2Y) (Figure 1C)
3.1.4. VWF Activity (Figure 2)
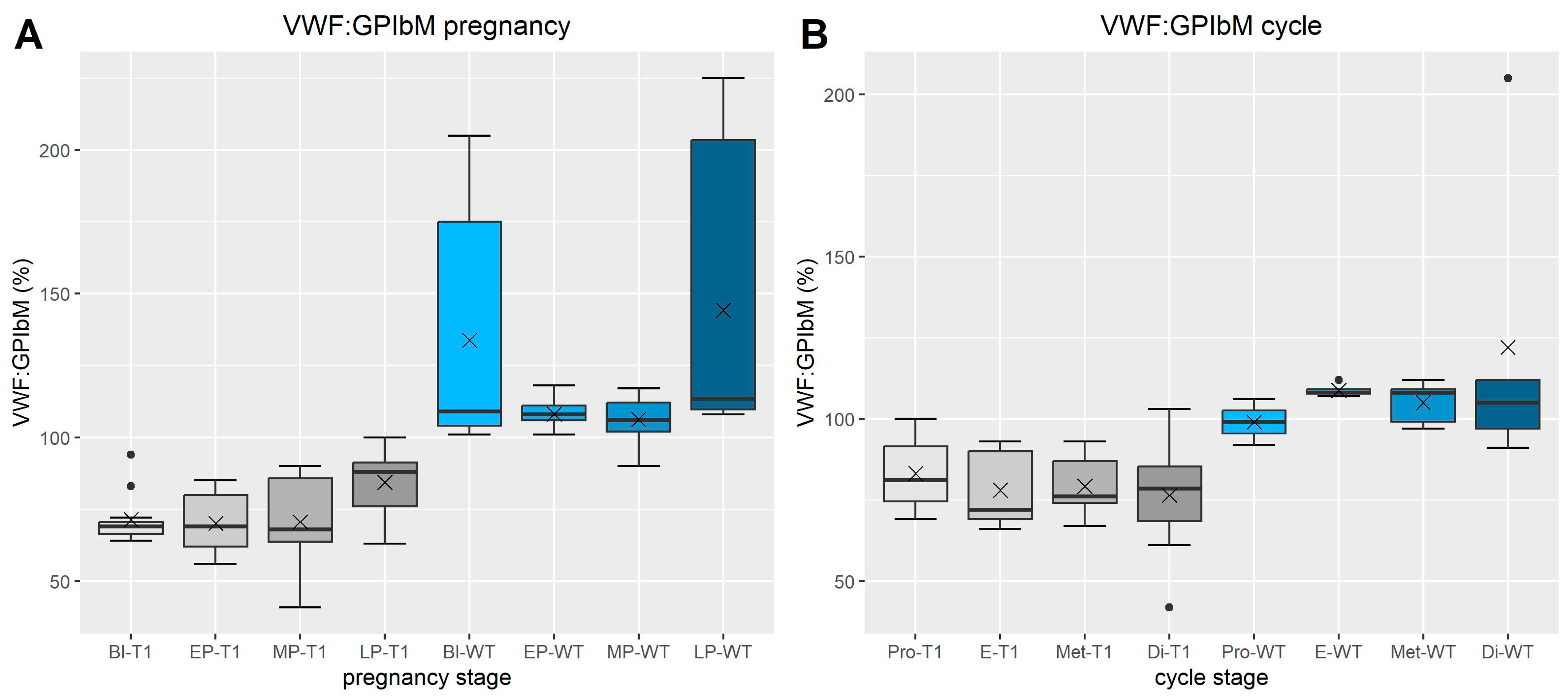
3.1.5. FVIII Chromogen (Figure 3)
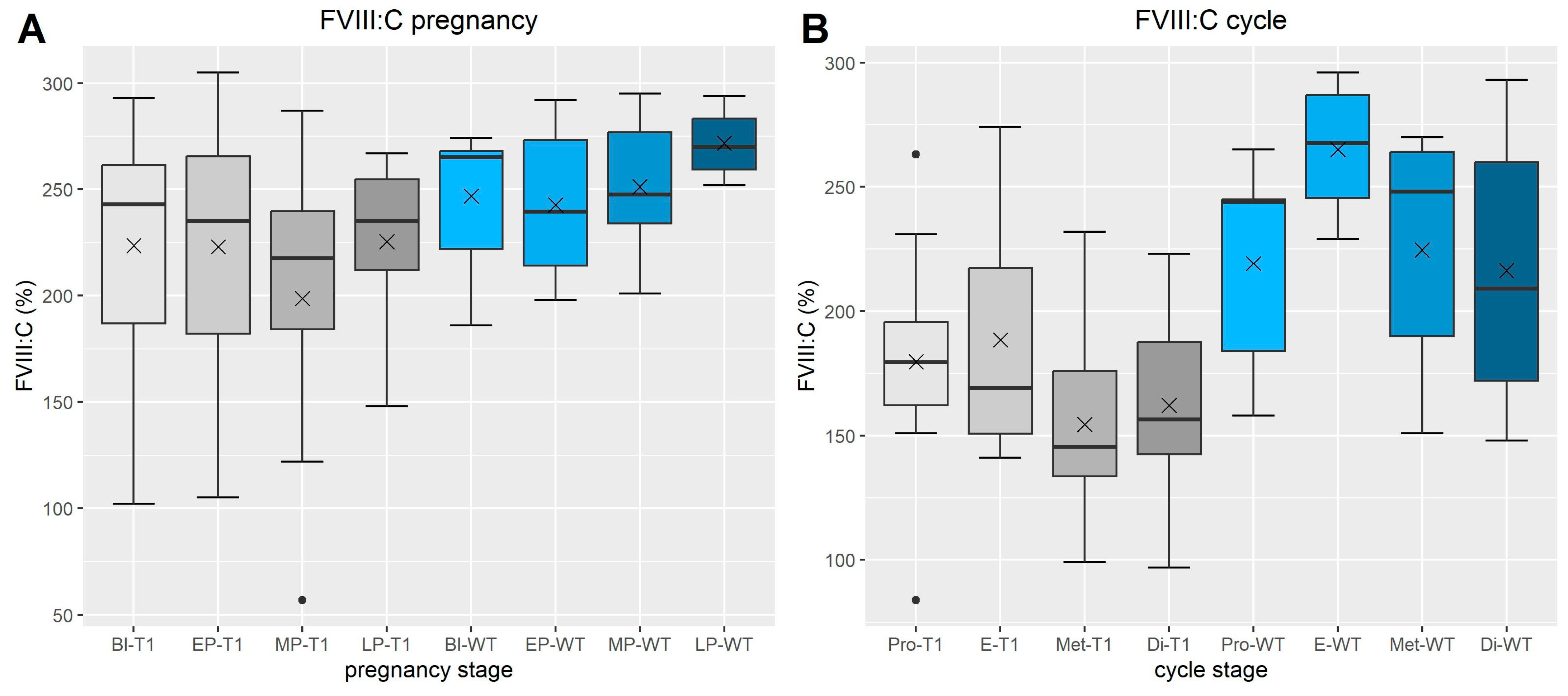
3.2. VWF-Multimers (Figure 4 and Figure 5)

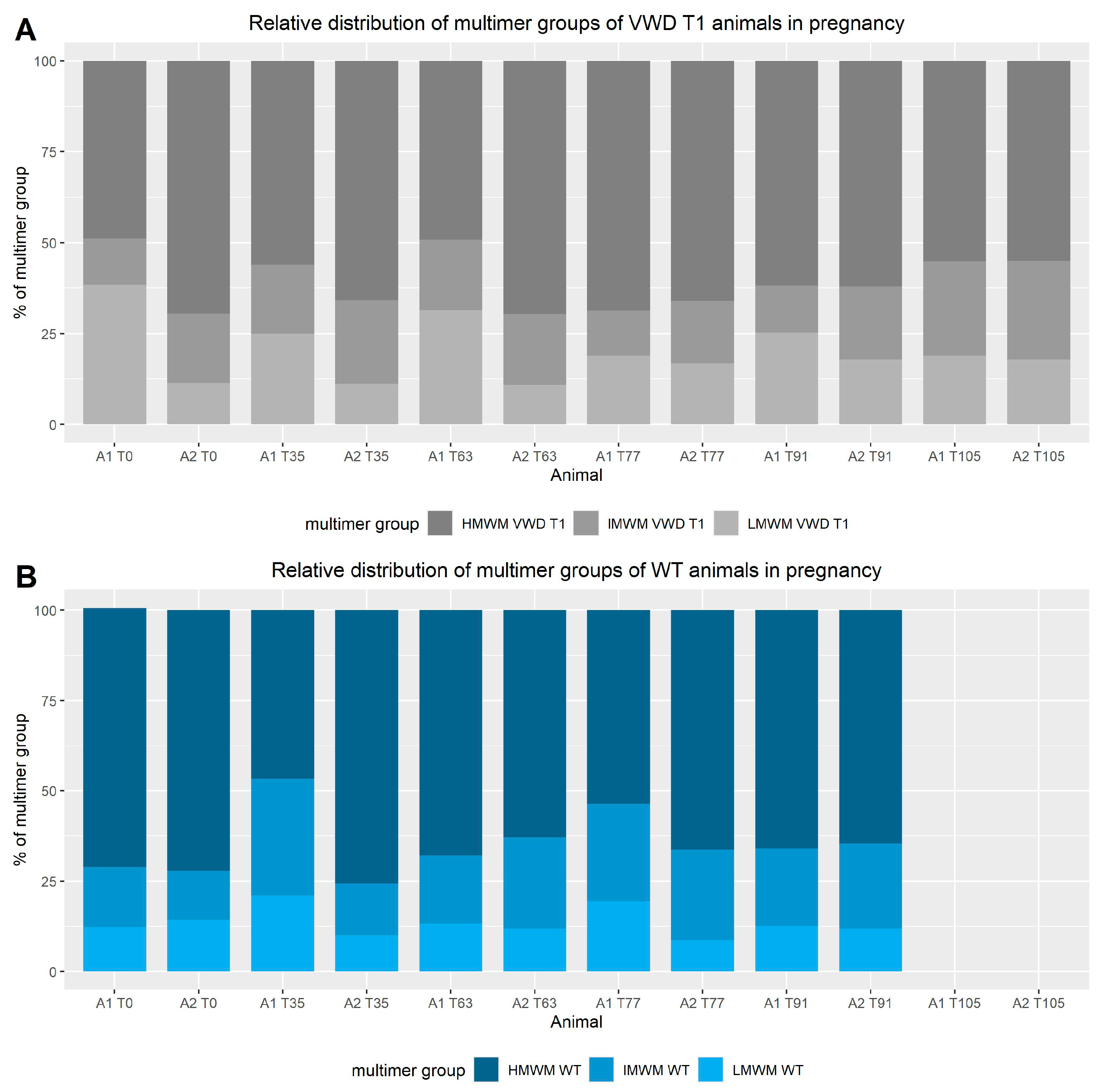
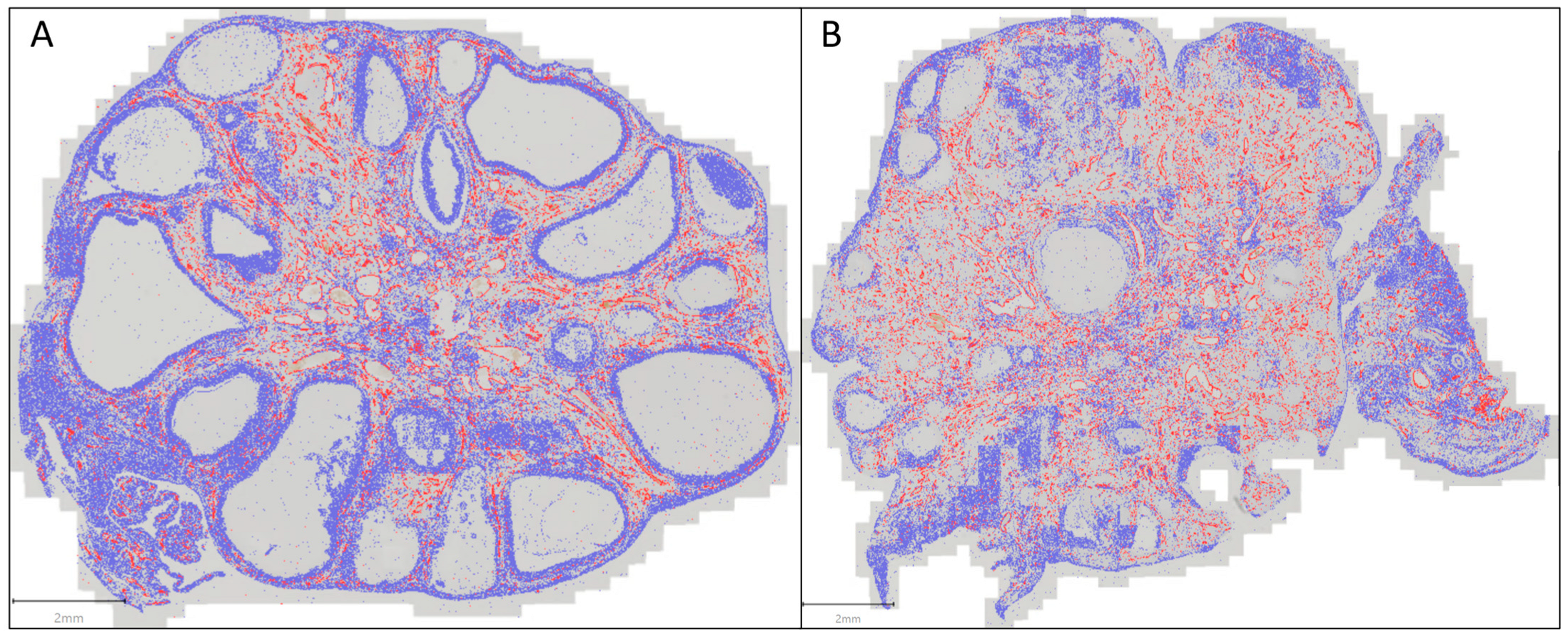
3.3. Evaluation of the Immunohistochemistry (IHC) (Figure 6 and Figure 7)
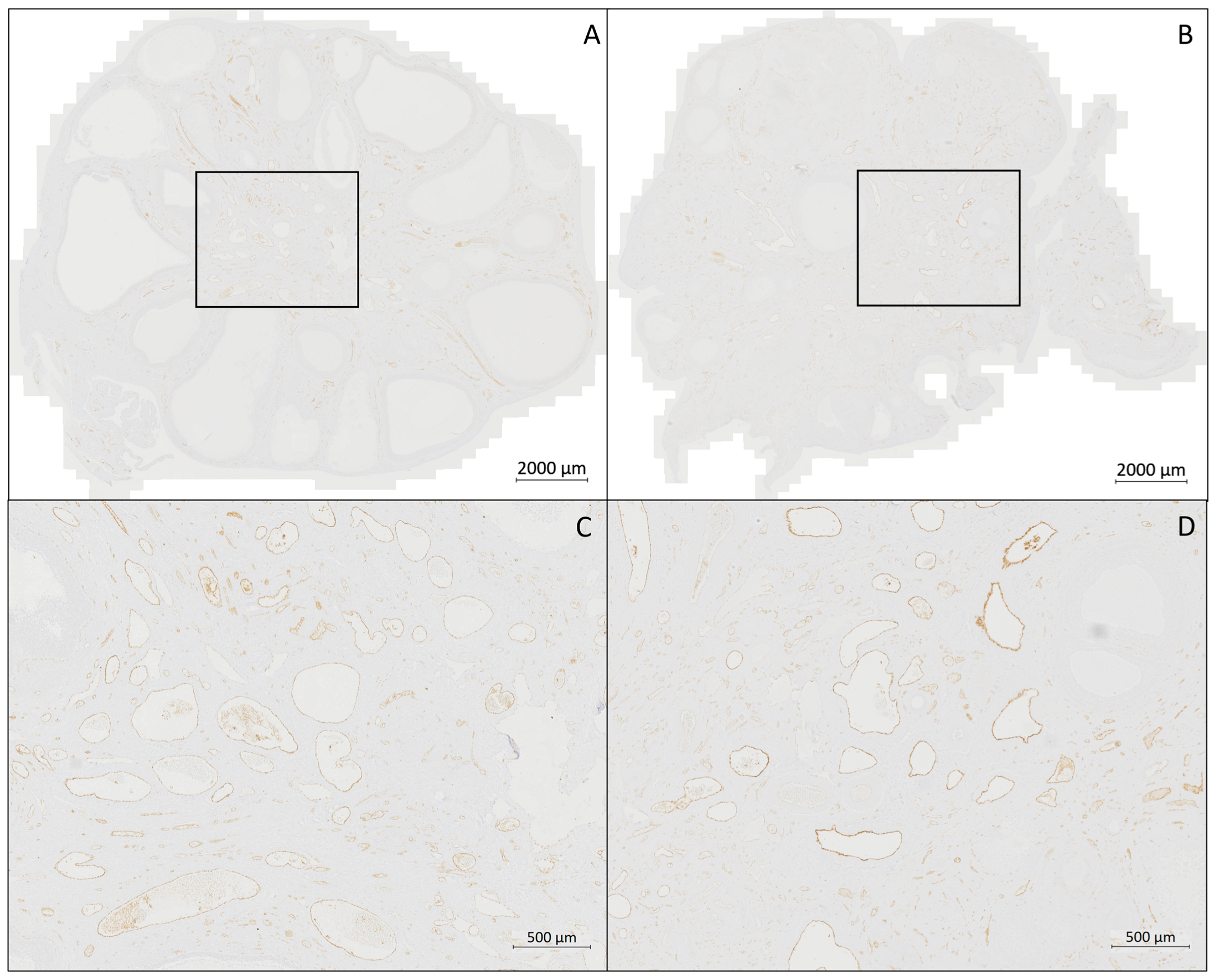
4. Discussion
4.1. Pregnancy and Haemostaseological Changes
4.2. Multimers
4.3. Oestrus Cycle and Haemostaseological Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, P.D.; Connell, N.T.; Ameer, B.; Di Paola, J.; Eikenboom, J.; Giraud, N.; Haberichter, S.; Jacobs-Pratt, V.; Konkle, B.; McLintock, C.; et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021, 5, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Ledford-Kraemer, M.R. Analysis of von Willebrand factor structure by multimer analysis. Am. J. Hematol. 2010, 85, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.E.; Kramer, G.; Mitterer, A.; Grillberger, L.; Reiter, M.; Mundt, W.; Dorner, F.; Eibl, J. Effect of multimerization of human and recombinant von Willebrand factor on platelet aggregation, binding to collagen and binding of coagulation factor VIII. Thromb. Res. 1996, 84, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sie, P.; Caron, C.; Azam, J.; Goudemand, J.; Grandjean, H.; Boneu, B.; Fournie, A. Reassessment of von Willebrand factor (VWF), VWF propeptide, factor VIII:C and plasminogen activator inhibitors 1 and 2 during normal pregnancy. Br. J. Haematol. 2003, 121, 897–903. [Google Scholar] [CrossRef]
- Lyall, F.; Greer, I.A. The vascular endothelium in normal pregnancy and pre-eclampsia. Rev. Reprod. 1996, 1, 107–116. [Google Scholar] [CrossRef]
- Drury-Stewart, D.N.; Lannert, K.W.; Chung, D.W.; Teramura, G.T.; Zimring, J.C.; Konkle, B.A.; Gammill, H.S.; Johnsen, J.M. Complex changes in von Willebrand factor-associated parameters are acquired during uncomplicated pregnancy. PLoS ONE 2014, 9, e112935. [Google Scholar] [CrossRef] [PubMed]
- Mattoso, C.R.; Takahira, R.K.; Beier, S.L.; Araujo, J.P., Jr.; Corrente, J.E. Evaluation of von Willebrand factor during pregnancy, lactation and oestrous cycle in bitches affected and unaffected by von Willebrand disease. Reprod. Domest. Anim. 2013, 48, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Lehner, S.; Ekhlasi-Hundrieser, M.; Detering, C.; Allerkamp, H.; Pfarrer, C.; von Depka Prondzinski, M. A 12.3-kb Duplication Within the VWF Gene in Pigs Affected by Von Willebrand Disease Type 3. G3 Genes|Genomes|Genet. 2018, 8, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Motto, D.G.; Di Paola, J. Diagnostic approach to von Willebrand disease. Blood 2015, 125, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.S.; Keeney, S.; Bolton-Maggs, P.H.; Hay, C.R.; Will, A.; Cumming, A.M. The mutation spectrum associated with type 3 von Willebrand disease in a cohort of patients from the north west of England. Haemophilia 2009, 15, 1048–1057. [Google Scholar] [CrossRef]
- Allerkamp, H.; Lehner, S.; Ekhlasi-Hundrieser, M.; Detering, C.; Pfarrer, C.; Depka Prondzinski, M.V. Characterization of a Porcine Model for Von Willebrand Disease Type 1 and 3 Regarding Expression of Angiogenic Mediators in the Nonpregnant Female Reproductive Tract. Comp. Med. 2019, 69, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Brouland, J.P.; Egan, T.; Roussi, J.; Bonneau, M.; Pignaud, G.; Bal, C.; Vaiman, M.; André, P.; Hervé, P.; Mazmanian, G.M.; et al. In vivo regulation of von willebrand factor synthesis: Von Willebrand factor production in endothelial cells after lung transplantation between normal pigs and von Willebrand factor-deficient pigs. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 3055–3062. [Google Scholar] [CrossRef] [PubMed]
- Möller, R.; Kaiser, K.; Baulain, U.; Petersen, B.; Detering, C.; Ekhlasi-Hundrieser, M.; Pfarrer, C.; von Depka Prondzinski, M.; Lehner, S. Influence of Von Willebrand Disease (VWD) and pregnancy on the expression of angiogenic factors in the porcine female reproductive tract. Reprod. Biol. 2022, 22, 100700. [Google Scholar] [CrossRef]
- Allerkamp, H.; Lehner, S.; Ekhlasi-Hundrieser, M.; Detering, C.; von Depka Prondzinski, M.; Pfarrer, C. Expression of angiogenic factors in the uteroplacental unit is altered at time of placentation in a porcine model of von Willebrand disease type 1. Reprod. Biol. 2019, 19, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, H.C.; Hegardt, B.; Downie, H.G.; Mustard, J.F.; Murphy, E.A. Adrenaline and experimental thrombosis. Br. J. Haematol. 1966, 12, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lim, J.; Chang, K.; Kim, Y.; Kim, M.; Park, H.I.; Kim, J.; Shin, S. A comparison of INNOVANCE(R) PFA P2Y and VerifyNow P2Y12 assay for the assessment of clopidogrel resistance in patients undergoing percutaneous coronary intervention. J. Clin. Lab. Anal. 2012, 26, 262–266. [Google Scholar] [CrossRef]
- Favaloro, E.J. Clinical utility of closure times using the platelet function analyzer-100/200. Am. J. Hematol. 2017, 92, 398–404. [Google Scholar] [CrossRef]
- Oliver, S.; Vanniasinkam, T.; Mohammed, S.; Vong, R.; Favaloro, E.J. Semi-automated von Willebrand factor multimer assay for von Willebrand disease: Further validation, benefits and limitations. Int. J. Lab. Hematol. 2019, 41, 762–771. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Miller, R.G. Simultaneous Statistical Inference; McGraw-Hill: New York, NY, USA, 1966. [Google Scholar]
- Castaman, G.; James, P.D. Pregnancy and delivery in women with von Willebrand disease. Eur. J. Haematol. 2019, 103, 73–79. [Google Scholar] [CrossRef]
- James, A.H. More than menorrhagia: A review of the obstetric and gynaecological manifestations of von Willebrand disease. Thromb. Res. 2007, 120 (Suppl. 1), S17–S20. [Google Scholar] [CrossRef]
- Burgess, H.J.; Woods, J.P.; Abrams-Ogg, A.C.; Wood, R.D. Use of a questionnaire to predict von Willebrand disease status and characterize hemorrhagic signs in a population of dogs and evaluation of a diagnostic profile to predict risk of bleeding. Can. J. Vet. Res. 2009, 73, 241. [Google Scholar] [PubMed]
- Ardillon, L.; Ternisien, C.; Fouassier, M.; Sigaud, M.; Lefrancois, A.; Pacault, M.; Ribeyrol, O.; Fressinaud, E.; Boisseau, P.; Trossaert, M. Platelet function analyser (PFA-100) results and von Willebrand factor deficiency: A 16-year ‘real-world’ experience. Haemophilia 2015, 21, 646–652. [Google Scholar] [CrossRef]
- Favaloro, E.J. The utility of the PFA-100 in the identification of von Willebrand disease: A concise review. Semin. Thromb. Hemost. 2006, 32, 537–545. [Google Scholar] [CrossRef]
- Suzuki, S.; Morishita, S. Platelet hemostatic capacity (PHC) and fibrinolytic inhibitors during pregnancy. Semin. Thromb. Hemost. 1998, 24, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Magon, N. Hormones in pregnancy. Niger. Med. J. 2012, 53, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.P.; Niewiarowski, S.; Ream, V.J. Release of adenine nucleotides and platelet factor 4 from platelets of man and four other species. J. Lab. Clin. Med. 1970, 75, 607–618. [Google Scholar] [PubMed]
- Addonizio, V.P., Jr.; Edmunds, L.H., Jr.; Colman, R.W. The function of monkey (M. mulatta) platelets compared to platelets of pig, sheep, and man. J. Lab. Clin. Med. 1978, 91, 989–997. [Google Scholar] [PubMed]
- Gewirtz, H.; Steiner, M.; Sasken, H.; Most, A.S. Measurement by electrical impedance aggregometry of porcine platelets response to selected physiological agonists. Proc. Soc. Exp. Biol. Med. 1985, 179, 324–330. [Google Scholar] [CrossRef]
- Royo, T.; Vidal, M.; Badimon, L. Porcine platelet von Willebrand antigen II (vW AgII): Inhibitory effect on collagen-induced aggregation and comparative distribution with human platelets. Thromb. Haemost. 1998, 80, 677–685. [Google Scholar] [PubMed]
- Jin, J.; Kunapuli, S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. USA 1998, 95, 8070–8074. [Google Scholar] [CrossRef]
- van Gestel, M.A.; Heemskerk, J.W.; Slaaf, D.W.; Heijnen, V.V.; Reneman, R.S.; oude Egbrink, M.G. In vivo blockade of platelet ADP receptor P2Y12 reduces embolus and thrombus formation but not thrombus stability. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Koessler, J.; Ehrenschwender, M.; Kobsar, A.; Brunner, K. Evaluation of the new INNOVANCE(R) PFA P2Y cartridge in patients with impaired primary haemostasis. Platelets 2012, 23, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Foidart, J.M.; Hustin, J.; Dubois, M.; Schaaps, J.P. The human placenta becomes haemochorial at the 13th week of pregnancy. Int. J. Dev. Biol. 1992, 36, 451–453. [Google Scholar]
- Almeida, F.; Dias, A. Pregnancy in pigs: The journey of an early life. Domest. Anim. Endocrinol. 2022, 78, 106656. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.A.; Lee, C.A.; Sabin, C.A.; Pollard, D.; Economides, D.L. Pregnancy in women with von Willebrand’s disease or factor XI deficiency. Br. J. Obstet. Gynaecol. 1998, 105, 314–321. [Google Scholar]
- Nowak-Göttl, U.; Limperger, V.; Kenet, G.; Degenhardt, F.; Arlt, R.; Domschikowski, J.; Clausnizer, H.; Liebsch, J.; Junker, R.; Steppat, D. Developmental hemostasis: A lifespan from neonates and pregnancy to the young and elderly adult in a European white population. Blood Cells Mol. Dis. 2017, 67, 2–13. [Google Scholar] [CrossRef]
- Delbrück, C.; Miesbach, W. The Course of von Willebrand Factor and Factor VIII Activity in Patients with von Willebrand Disease during Pregnancy. Acta Haematol. 2019, 142, 71–78. [Google Scholar] [CrossRef]
- Knol, H.M.; Kemperman, R.F.; Kluin-Nelemans, H.C.; Mulder, A.B.; Meijer, K. Haemostatic variables during normal menstrual cycle. A systematic review. Thromb. Haemost. 2012, 107, 22–29. [Google Scholar] [CrossRef]
- Govorov, I.; Bremme, K.; Lindahl, T.L.; Holmstrom, M.; Komlichenko, E.; Chaireti, R.; Mints, M. Thrombin generation during a regular menstrual cycle in women with von Willebrand disease. Sci. Rep. 2018, 8, 17467. [Google Scholar] [CrossRef]
- Nichols, T.C.; Bellinger, D.A.; Merricks, E.P.; Raymer, R.A.; Kloos, M.T.; Defriess, N.; Ragni, M.V.; Griggs, T.R. Porcine and canine von Willebrand factor and von Willebrand disease: Hemostasis, thrombosis, and atherosclerosis studies. Thrombosis 2010, 2010, 461238. [Google Scholar] [CrossRef] [PubMed]
- Au, C.L.; Rogers, P.A. Immunohistochemical staining of von Willebrand factor in human endometrium during normal menstrual cycle. Hum. Reprod. 1993, 8, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, M.A.; Thomson, R.L.; Moran, L.J.; Wycherley, T.P. The Impact of Menstrual Cycle Phase on Athletes’ Performance: A Narrative Review. Int. J. Environ. Res. Public. Health 2021, 18, 1667. [Google Scholar] [CrossRef] [PubMed]
- Soede, N.M.; Langendijk, P.; Kemp, B. Reproductive cycles in pigs. Anim. Reprod. Sci. 2011, 124, 251–258. [Google Scholar] [CrossRef]
- Shilo, M.; Mayo, A.; Alon, U. A Mechanism for Ovulation Number Control. Front. Endocrinol. 2022, 13, 816967. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Sales, K.J. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol. Metab. 2004, 15, 398–404. [Google Scholar] [CrossRef]
- Mandalaki, T.; Louizou, C.; Dimitriadou, C.; Symeonidis, P. Variations in factor VIII during the menstrual cycle in normal women. N. Engl. J. Med. 1980, 302, 1093–1094. [Google Scholar] [CrossRef]
| Equipment, Manufacturer | Factor | Test Set | Additional Reagents | Human Reference Range |
|---|---|---|---|---|
| ACL TOP 750 BASE, Werfen GmbH, Kirchheim near Munich | VWF Act | Siemens INNOVANCE VWF Ac | HemosIL factor diluent (Werfen GmbH, Kirchheim, Germany) | 48–173% |
| F8 cSL | HemosIL ELECTRACHROME Factor VIII | HemosIL factor diluent (Werfen GmbH, Kirchheim, Germany) | 70–210% | |
| PFA-200™, Siemens Healthcare, Erlangen, Germany | C/EPI | <165 s | ||
| C/ADP | <121 s | |||
| P2Y | <107 s | |||
| KABE LABORTECHNIK GmbH | Blood tubes for primary coagulation | Primavette® S PHC/PFA | ||
| Blood tubes for secondary coagulation factors and multimers | Primavette® S Coagulation | |||
| Hydrasys 2 Scan Focusing, SEBIA GmbH, Mainz, Germany | Hydragel 11 Von WILLEBRAND MULTIMERS Kit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, R.; Kaiser, K.; Baulain, U.; Petersen, B.; Detering, C.; Ekhlasi-Hundrieser, M.; Zimmermann, R.; Mühlfeld, C.; von Depka Prondzinski, M.; Pfarrer, C.; et al. Haemostaseological Changes of VWF and FVIII during Pregnancy and the Oestrus Cycle in a Porcine Model of Von Willebrand Disease. Hemato 2024, 5, 48-65. https://doi.org/10.3390/hemato5010006
Möller R, Kaiser K, Baulain U, Petersen B, Detering C, Ekhlasi-Hundrieser M, Zimmermann R, Mühlfeld C, von Depka Prondzinski M, Pfarrer C, et al. Haemostaseological Changes of VWF and FVIII during Pregnancy and the Oestrus Cycle in a Porcine Model of Von Willebrand Disease. Hemato. 2024; 5(1):48-65. https://doi.org/10.3390/hemato5010006
Chicago/Turabian StyleMöller, Rabea, Katharina Kaiser, Ulrich Baulain, Björn Petersen, Carsten Detering, Mahnaz Ekhlasi-Hundrieser, Richard Zimmermann, Christian Mühlfeld, Mario von Depka Prondzinski, Christiane Pfarrer, and et al. 2024. "Haemostaseological Changes of VWF and FVIII during Pregnancy and the Oestrus Cycle in a Porcine Model of Von Willebrand Disease" Hemato 5, no. 1: 48-65. https://doi.org/10.3390/hemato5010006
APA StyleMöller, R., Kaiser, K., Baulain, U., Petersen, B., Detering, C., Ekhlasi-Hundrieser, M., Zimmermann, R., Mühlfeld, C., von Depka Prondzinski, M., Pfarrer, C., & Lehner, S. (2024). Haemostaseological Changes of VWF and FVIII during Pregnancy and the Oestrus Cycle in a Porcine Model of Von Willebrand Disease. Hemato, 5(1), 48-65. https://doi.org/10.3390/hemato5010006






