Abstract
Background: Transfusional iron overload causes significant morbidity and mortality in sickle cell disease (SCD). Nevertheless, red blood cell transfusions continue to be essential in its management. This study describes the transfusion patterns among SCD hospitalizations. Methods: Hospitalizations for SCD in the 2017–2018 Nationwide Readmissions Database were divided into two groups based on whether they received transfusions. Descriptive analysis was performed to compare their demographics and complications. Multivariable logistic regression was performed to determine the factors associated with transfusions. Results: Out of 109,783 hospitalizations, 28,300 were transfused, and 81,483 were not transfused. Females and older individuals were higher in the transfused category than the non-transfused category (59.49% vs. 53.52% and 28.86% vs. 21.27%, respectively; p < 0.001 for both). The wealthiest population was more likely to be in the transfused category (11.27% vs. 8.34%; p < 0.001). Admissions to teaching hospitals, large metropolitan hospitals, and highest-volume hospitals were higher in the non-transfused category vs. transfused category (79.89% vs. 72.17%; p < 0.001, 69.26% vs. 65.35%; p 0.003 and 74.71% vs. 63.51%; p < 0.001, respectively). Most admissions were transfused once, with three or more transfusions being given more in the non-teaching hospitals than the teaching hospitals (1.27% vs. 0.41%; p 0.01). Furthermore, a higher proportion of early transfusions occurred in the non-teaching hospitals (65.6% vs. 57.82% for admission days 1 and 2; p < 0.001). Admission to a teaching hospital was associated with lower blood transfusion odds than a non-teaching hospital. Conclusion: A quarter of admissions for SCD receive a blood transfusion. In addition to performing more frequent and early transfusions, the odds of being transfused are higher in non-teaching hospitals.
1. Introduction
Sickle cell disease (SCD) is the most prevalent inherited red blood cell (RBC) disorder worldwide [1]. It is characterized by a single amino acid switch in the beta-globin chain of hemoglobin, resulting in sickle hemoglobin (HbS). The deoxygenation of HbS leads to intraerythrocytic polymerization and a change in the morphology of RBCs, from which the condition derives its name. The resulting change in the shape and physical properties of RBCs leads to hemolytic anemia and vaso-occlusion, causing acute and chronic tissue ischemia. Both processes are responsible for most of the clinical manifestations of SCD [2].
RBC transfusion is an integral part of the management of patients with SCD. It can take the form of a simple transfusion of additional units of blood without the removal of sickle blood, or an exchange transfusion (automated or manual) with the removal of sickle blood that is replaced by normal RBCs [3]. In an acute setting, a simple transfusion is indicated to treat symptomatic anemia resulting from transient aplastic crisis, acute hepatic or splenic sequestration, hyper hemolytic crisis, mild acute chest syndrome (ACS), among others. Similarly, exchange transfusion is commonly used to manage ACS, acute stroke, or multiorgan failure [4,5]. Elective RBC transfusion is also utilized in preoperative settings to minimize perioperative complications [6]. The most common indication for chronic RBC transfusion is primary and secondary stroke prevention [7,8]. In some cases, it is also utilized for the management of patients with recurrent ACS and frequent painful crises that are not well controlled with disease-modifying agents [9].
With advancements in red cell phenotyping and the use of more sensitive microbiological testing for blood donors, complications from blood transfusions, such as the risk of alloimmunization or the transmission of infections, have been substantially reduced. However, alloimmunization and iron overload pose persistent challenges among patients with sickle cell disease who require frequent RBC transfusions [3]. Additionally, transfusions carry a risk of transfusion reactions and hyper-hemolysis. Patients undergoing extended simple transfusion typically require iron chelation therapy after approximately one year of transfusions. Noncompliance with iron chelation can lead to iron overload in vital organs such as the liver, endocrine organs, and heart [10]. In contrast, exchange transfusion, when combined with iron chelation, proves to be efficacious in mitigating transfusional iron overload. Furthermore, exchange transfusion does not elevate the rate of red blood cell alloimmunization nor does it hinder the ability to maintain hemoglobin S levels within the desired target range of 30%, even in the presence of increased reticulocytosis [11]. Despite the noteworthy adverse effects and limited indications for simple transfusion, there is an observed upward trend in its utilization. In a single-center study from the U.K., the rate of blood transfusions in adult sickle cell patients was found to have increased significantly between 2000 and 2009 [12]. Similarly, a study conducted in the U.S. also demonstrated an increase in blood transfusions among children with SCD [13].
The aim of this study is to describe the RBC transfusion patterns among hospitalized patients with SCD and to characterize the epidemiological and clinical factors associated with RBC transfusion, utilizing a national database. Additionally, we aim to study the effect of RBC transfusion on the duration of hospitalization and the 30-day readmission rate post hospital discharge. The findings have previously been described in an American Society of Hematology meeting abstract [14].
2. Materials and Methods
2.1. Data Source and Population
This retrospective cohort study utilized the National Readmissions Database (NRD) for 2017 and 2018 to identify hospitalizations with the primary diagnosis of SCD using appropriate ICD-10-CM (International Classification of Diseases, Tenth Revision, Clinical Modification) codes (D5700, D5701, D5702, D57211, D57212, D57219, D57411, D57412, D57419, D57811, D57812, D57819, D571, D5720, D5740, and D5780). NRD is an administrative database developed by the Agency for Healthcare Research and Quality (AHRQ) for the Healthcare Cost and Utilization Project (HCUP). It is a large, publicly available database consisting of approximately 35 million hospital discharges after applying weights. NRD contains both patient- and hospital-level information. Data elements comprise, but are not confined to, diagnoses, procedures, patient demographics (such as sex and age), primary payer of hospitalization, discharge month, quarter, and year, total charges, length of stay, and essential data elements pertinent to readmission analyses. It is crucial to note that the data elements that could directly or indirectly disclose the identity of individuals are omitted. Since admissions in NRD are captured based on the calendar year without a link to the previous or the following year, we excluded index hospitalizations from December. As the database contains publicly available, depersonalized information, the study was exempt from review by Institutional Review Board [15].
2.2. Baseline Variables and Comparison Groups
Admissions with primary diagnosis of SCD were categorized into those who received RBC transfusions and those without RBC transfusions. RBC transfusions were identified using respective ICD-10-PCS (Procedure Coding System) codes (30230N1, 30230P1, 30233N1, 30233P1, 30240N1, 30240P1, 30243N1, and 30243P1). The focus of our investigation is limited to the administration of simple packed RBC units. Therefore, in the context of this study, the terms “RBC transfusion” or “transfusions” pertain to simple packed RBC transfusion only. Baseline variables included various patient demographics (including age, sex, primary payer, and median household income of residents in the patient’s ZIP code), hospital characteristics such as hospital size, teaching status, ownership, urban–rural designation, and hospital volume), complications during hospitalization, comorbidities, and outcome variables including in-hospital mortality, disposition of the patient, length of stay, and total hospital charges. Hospital volume was estimated after dividing the weighted SCD admissions into quintiles. Comorbidities were identified using ICD-10-CM codes and the Charlson comorbidity index. ICD-10 codes used in the study are listed in the Supplementary Materials (Table S1).
2.3. Statistical Analysis and Study Outcomes
Weights were applied to produce national estimates and analysis was performed with Stata software version 15.1 (Stata Corporation, College Station, TX, USA) using methodology provided by the HCUP. Descriptive analysis was performed to compare demographics, hospital characteristics, various comorbid conditions, and complications between the two groups. All-cause 30-day readmission rate was calculated after excluding index admissions from December, deaths during index admissions, and elective readmissions. We used Pearson’s chi-square test to obtain percentages for categorical variables and adjusted the Wald test to compare continuous values. We also estimated the number of RBC transfusions received during the hospitalization and timing of RBC transfusions to compare the transfusion pattern between teaching and non-teaching hospitals and in different age groups. To elucidate factors associated with RBC transfusion during admission, we first performed a univariable Cox regression analysis on baseline variables including age, sex, primary expected payer, median household income, hospital teaching status, hospital size, hospital urban–rural designation, hospital volume, Charlson comorbidity index score, venous thromboembolism, acute respiratory failure, acute chest syndrome, cerebrovascular accident, chronic renal disease, acute kidney injury, fluid and electrolyte imbalance, infection or sepsis, and major bleeding. Significant variables (with a cutoff p-value of 0.2) were screened for collinearity and fitted into a multivariable logistic regression model using the backward selection method. The results were reported as the adjusted odds ratio (aOR), 95% confidence intervals (CI), and p values.
3. Results
Out of 109,783 weighted index hospitalizations with the primary diagnosis of SCD, 28,300 were transfused while 81,483 were not transfused. The proportion of females and older individuals (40 years and older) was higher in the transfused category than the not transfused group (59.49% vs. 53.52% and 28.86% vs. 21.27%, respectively; p < 0.001 for both). The transfused group (vs. non-transfused) had a higher percentage of individuals from the wealthiest communities (11.27% vs. 8.34%; p < 0.001). The proportion of admissions to teaching hospitals, large metropolitan hospitals, and highest-volume hospitals was higher in the non-transfused category compared to the transfused group (79.89% vs. 72.17%; p < 0.001, 69.26% vs. 65.35%; p 0.003 and 74.71% vs. 63.51%; p < 0.001, respectively). The comorbidity burden was higher in the transfused cohort compared to the SCD admissions that were not transfused (Charlson comorbidity index ≥ 3 in 8.5% vs. 4.71%; p < 0.001). ACS was present in 11.73% of the SCD hospitalizations that were transfused compared to 6.44% of the non-transfused group (p < 0.001). Similarly, the incidence of acute respiratory failure, acute kidney injury, fluid and electrolyte imbalance, and infection or sepsis was higher in the transfused compared to the non-transfused cohort (5.82% vs. 2.77%, 9.75% vs. 4.94%, 30.32% vs. 21.54%, and 26.83% vs. 18.93%, respectively; p < 0.001 for each). The mean length of stay was longer and the total hospitalization charges were higher in the transfused cohort than the non-transfused cohort (6.3 days vs. 4.6 days; p < 0.001 and $43,874 vs. $31,732; p < 0.001, respectively). Although the all-cause in-hospital mortality was higher in the SCD cohort that received a transfusion (0.43% vs. 0.28%; p 0.007), their 30-day readmission rate was lower compared to the SCD cohort that was not transfused (24.39% vs. 31.85%; p < 0.001) (Table 1).

Table 1.
Baseline characters and outcomes of sickle cell disease admissions.
During most admissions, the patients were transfused once, with multiple transfusions (three or more) being given more in the non-teaching hospitals than in the teaching hospitals (1.27% vs. 0.41%; p 0.01). Furthermore, more than half of all the transfusions happened within the first two days of admission, with a higher proportion of early transfusions in the non-teaching hospitals compared to the teaching hospitals (65.6% vs. 57.82% for admission days 1 and 2; p < 0.001) (Table 2). The proportion of transfusions in the first two days of admission was higher and transfusions on day 5 and later were lower for the age group ≥40 years compared to the 25–39-year-olds and the 18–24-year-olds (69.01% vs. 59.14% vs. 49.2% and 12.32% vs. 17.57% vs. 20.66%; p < 0.001) (Table 3).

Table 2.
Red blood cell transfusion patterns in sickle cell disease admissions across teaching and non-teaching hospitals.

Table 3.
Red blood cell transfusion patterns in sickle cell disease admissions across different age groups.
After adjusting for confounders, older age groups (age ≥ 40 years and 25–39 years) compared to 18–24-year-olds (aOR 1.48; 95% CI 1.36–1.62 and aOR 1.21; 95% CI 1.12–1.31, respectively), female sex (aOR 1.23; 95% CI 1.17–1.31), highest community-level income (aOR 1.52; 95% CI 1.31–1.75), admission to rural hospital vs. large metropolitan hospital (aOR 1.82; 95% CI 1.26–2.6), length of stay 4–7 vs. ≤3 days (aOR 1.56; 95% CI 1.46–1.66) and length of stay ≥ 8 vs. ≤3 days (aOR 2.28; 95% CI 2.05–2.53) were found to be associated with higher odds of RBC transfusion. Admission to a teaching hospital was associated with lower RBC transfusion odds (aOR 0.70; 95% CI 0.56–0.87) (Figure 1).
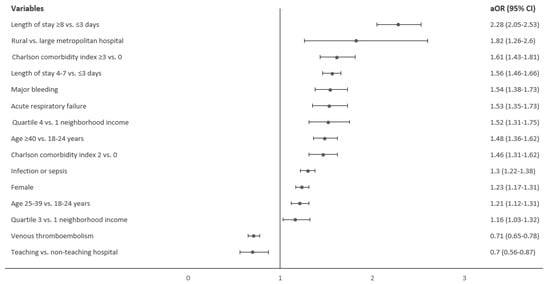
Figure 1.
Factors associated with red blood cell transfusion in sickle cell disease hospitalizations.
4. Discussion
This study elaborates on the patterns of RBC transfusions among SCD hospitalizations at the national level, utilizing the NRD. During the study period of 2017–2018, we found that 25.78% of total SCD hospital admissions included RBC transfusions. Similar results were demonstrated in a previous nationwide database study of sickle cell-related admissions in children [13].
On the multivariable analysis, female gender and older age were found to be associated with higher odds of RBC transfusion. While not explored in this study, potential reasons for increased transfusions in females include lower baseline hemoglobin, menorrhagia, and liberal transfusion strategies during pregnancy [16]. Older patients were both more likely to receive a RBC transfusion overall and to receive early transfusions compared to younger patients. The severity of anemia worsens with age in SCD patients due to inappropriately lower erythropoietin production and a lower erythropoietic response caused by changes in the bone marrow niche and microenvironment with age, which may be an explanation for this finding [17,18,19,20].
In this study, most admissions were at teaching hospitals, and our analysis found that admission to a teaching hospital and a large metropolitan hospital was associated with a lower likelihood of receiving a transfusion. Previous studies have reported similar results [13]. The teaching hospitals had a lower proportion of patients who received two or more transfusions. Furthermore, more than half of all the transfusions occurred within the first two days of admission, with a higher proportion of early transfusions in the non-teaching hospitals compared to the teaching hospitals (65.6% vs. 57.82% for admission days 1 and 2; p < 0.001). The American Society of Hematology’s Choosing Wisely guidelines advise against the routine transfusion of SCD patients for chronic anemia or uncomplicated pain crisis unless there is an appropriate clinical indication [21]. It is possible that these guidelines are better adhered to in the academic setting. However, it should be noted that the optimal transfusion threshold for patients with SCD is a topic of active debate.
Around 60% of all transfusions occurred within the first two days of hospital admission, with the teaching hospitals having a higher proportion of hospitalizations with the first RBC transfusion after day five of admission (18%) than the non-teaching hospitals (13%) (p < 0.001). While the current study does not provide information on the number of patients on voxelotor, it raises an interesting question of whether individuals with low baseline hemoglobin levels at admission should be initiated on voxelotor to reduce the likelihood of requiring RBC transfusion. In the phase 3 HOPE study, voxelotor use resulted in a 1.1 g/dL increase in hemoglobin levels after 24 weeks, with the maximum increase noted as early as two weeks [22]. The timeline for observing the effects of voxelotor and its potential to reduce the need for RBC transfusions in acute situations is currently unclear, as the initial hemoglobin assessment in the HOPE study was conducted two weeks after voxelotor administration.
Patients belonging to the wealthiest communities were found to have 1.52 times higher odds of receiving RBC transfusions compared to those residing in the lowest income quartile neighborhoods. The reason for this trend is uncertain from a medical standpoint, although one potential explanation could be that individuals from wealthier areas tend to receive medical care in small-volume, non-teaching hospitals. This finding highlights the need for further exploration in future studies. As mentioned earlier, the observed disparity in RBC transfusion rates may be linked to a more consistent adherence to evidence-based transfusional practice guidelines in teaching hospitals and academic settings, potentially resulting in the superior achievement of quality measures. Nevertheless, it is crucial to acknowledge that our current study was not specifically designed to investigate the impact of the teaching status on outcomes in SCD. Further research is warranted to elucidate the complex interplay of socioeconomic factors and healthcare delivery, contributing to disparate transfusion patterns observed between teaching and non-teaching hospitals.
We found that a diagnosis of venous thromboembolism was associated with lower odds of receiving RBC transfusions (aOR 0.71). However, due to limitations in the database, we were unable to determine if the thromboembolism event was diagnosed during the index hospital stay or if the patient had a history of it. Previous studies have reported a higher incidence of venous thromboembolism in patients with hemoglobin SC disease and sickle beta plus thalassemia when compared to those with hemoglobin SS and sickle beta zero thalassemia. The proposed hypothesis in these studies was increased viscosity from elevated baseline hemoglobin level [23,24,25], which may also explain the reduced association with transfusion.
As sicker patients had a higher likelihood of receiving RBC transfusions, the RBC transfusion cohort had a longer length of stay and a higher all-cause in-hospital mortality rate. However, the RBC transfusion cohort had a 23% lower 30-day readmission rate (24.39% vs. 31.85%; p < 0.001). Although the cost-effectiveness of RBC transfusion was beyond the scope of this study, these findings raise interesting questions about its potential benefits.
Since this study utilizes an administrative database, the results should be interpreted with caution. First, as hospital billing codes were used for diagnosis, there is an increased risk of misclassification and ascertainment bias. Second, the distinction between the comorbidities present on admission and complications during hospitalization was an approximation due to the nature of this database. Finally, it was not possible to obtain the indication of RBC transfusion and we could not study the associated complications. While it would have been valuable to explore our findings in the context of treatment modalities for the long-term control of SCD and hemoglobin values at the time of transfusion, this information was not available in the NRD and therefore could not be studied.
This study provides insights into RBC transfusion patterns in SCD admissions, with intriguing findings such as higher transfusion rates in patients from wealthy communities, lower odds of transfusion in teaching hospitals, and lower 30-day readmission rates among transfused patients. The observed variations in transfusion practices underscore the ongoing imperative of adhering to current treatment guidelines, particularly in standardizing transfusion practices for SCD management across diverse healthcare settings. This emphasizes the call for the broader implementation of quality improvement initiatives, with a specific focus on transfusional thresholds. Furthermore, future research is warranted to investigate the impact of emerging therapeutic options such as voxelotor and pyruvate kinase activators on the observed RBC transfusion patterns in SCD admissions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/hemato5010004/s1, Table S1: Diagnoses and procedure codes used in the study.
Author Contributions
Conceptualization, V.S. and A.S. (Aditi Sharma); methodology, V.S. and A.S. (Aditi Sharma); software, resources and formal analysis, A.S. (Aditi Sharma); validation, A.S. (Aditi Sharma), A.A. and V.S.; data curation, A.D., A.S. (Aditya Sharma), J.K. and A.S. (Aditi Sharma); writing—original draft preparation, A.D., V.S., J.K., A.S. (Aditi Sharma) and A.S. (Aditya Sharma); writing—review and editing, A.S. (Aditi Sharma), A.D., V.S., A.A., A.S. (Aditya Sharma), J.K. and I.W.; supervision, V.S., I.W. and A.A.; project administration, A.S. (Aditi Sharma) and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
As the database contains publicly available, depersonalized information, the study was exempt from review by Institutional Review Board.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this study are available upon request from the authors.
Acknowledgments
This article is a revised and expanded version of a paper entitled “Epidemiology and Predictors of Blood Transfusion in Hospitalizations for Sickle Cell Disease”, which was presented at the 63rd ASH Annual Meeting and Exposition in Atlanta, GA, USA from 11–14 December 2021, and published in Blood on 24 December 2021, Volume 138, Supplement 1, 23 November 2021, Page 3103.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Quirolo, K.C. Current issues in blood transfusion for sickle cell disease. Curr. Opin. Pediatr. 2009, 21, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Josephson, C.D.; Su, L.L.; Hillyer, K.L.; Hillyer, C.D. Transfusion in the patient with sickle cell disease: A critical review of the literature and transfusion guidelines. Transfus. Med. Rev. 2007, 21, 118–133. [Google Scholar] [CrossRef]
- Smith-Whitley, K.; Thompson, A.A. Indications and complications of transfusions in sickle cell disease. Pediatr. Blood Cancer 2012, 59, 358–364. [Google Scholar] [CrossRef]
- Vichinsky, E.P.; Haberkern, C.M.; Neumayr, L.; Earles, A.N.; Black, D.; Koshy, M.; Pegelow, C.; Abboud, M.; Ohene-Frempong, K.; Iyer, R.V. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N. Engl. J. Med. 1995, 333, 206–213. [Google Scholar] [CrossRef]
- Adams, R.J.; Brambilla, D. Optimizing Primary Stroke Prevention in Sickle Cell Anemia Trial I. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N. Engl. J. Med. 2005, 353, 2769–2778. [Google Scholar]
- Adams, R.J.; McKie, V.C.; Hsu, L.; Files, B.; Vichinsky, E.; Pegelow, C.; Abboud, M.; Gallagher, D.; Kutlar, A.; Nichols, F.T.; et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N. Engl. J. Med. 1998, 339, 5–11. [Google Scholar] [CrossRef]
- Miller, S.T.; Wright, E.; Abboud, M.; Berman, B.; Files, B.; Scher, C.D.; Styles, L.; Adams, R.J. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J. Pediatr. 2001, 139, 785–789. [Google Scholar] [CrossRef]
- Howard, J. Sickle cell disease: When and how to transfuse. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 625–631. [Google Scholar] [CrossRef]
- Fasano, R.M.; Leong, T.; Kaushal, M.; Sagiv, E.; Luban, N.L.; Meier, E.R. Effectiveness of red blood cell exchange, partial manual exchange, and simple transfusion concurrently with iron chelation therapy in reducing iron overload in chronically transfused sickle cell anemia patients. Transfusion 2016, 56, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Drasar, E.; Igbineweka, N.; Vasavda, N.; Free, M.; Awogbade, M.; Allman, M.; Mijovic, A.; Thein, S.L. Blood transfusion usage among adults with sickle cell disease-a single institution experience over ten years. Br. J. Haematol. 2011, 152, 766–770. [Google Scholar] [CrossRef]
- Raphael, J.L.; Oyeku, S.O.; Kowalkowski, M.A.; Mueller, B.U.; Ellison, A.M. Trends in blood transfusion among hospitalized children with sickle cell disease. Pediatr. Blood Cancer 2013, 60, 1753–1758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, A.; Singh, V.; Alavi, A. Epidemiology and Predictors of Blood Transfusion in Hospitalizations for Sickle Cell Disease. Blood. 2021, 138 (Suppl. 1), 3103. [Google Scholar] [CrossRef]
- NRD Overview. Healthcare Cost and Utilization Project (HCUP); Agency for Healthcare Research and Quality: Rockville, MD, USA. Available online: www.hcup-us.ahrq.gov/nrdoverview.jsp (accessed on 1 December 2022).
- Cunningham, F.G.; Pritchard, J.A.; Mason, R.; Chase, G. Prophylactic transfusions of normal red blood cells during pregnancies complicated by sickle cell hemoglobinopathies. Am. J. Obstet. Gynecol. 1979, 135, 994–1003. [Google Scholar] [CrossRef]
- McKerrell, T.D.; Cohen, H.W.; Billett, H.H. The older sickle cell patient. Am. J. Hematol. 2004, 76, 101–106. [Google Scholar] [CrossRef]
- Sherwood, J.B.; Goldwasser, E.; Chilcote, R.; Carmichael, L.D.; Nagel, R.L. Sickle cell anemia patients have low erythropoietin levels for their degree of anemia. Blood 1986, 67, 46–49. [Google Scholar] [CrossRef]
- Rao, K.R.; Patel, A.R.; McGinnis, P.; Patel, M.K. Iron stores in adults with sickle cell anemia. J. Lab. Clin. Med. 1984, 103, 792–797. [Google Scholar]
- Giordano, P.; Urbano, F.; Lassandro, G.; Faienza, M.F. Mechanisms of Bone Impairment in Sickle Bone Disease. Int. J. Environ. Res. Public Health 2021, 18, 1832. [Google Scholar] [CrossRef]
- Choosing Wisely. Available online: https://www.hematology.org/education/clinicians/guidelines-and-quality-care/choosing-wisely (accessed on 12 January 2023).
- Vichinsky, E.; Hoppe, C.C.; Ataga, K.I.; Ware, R.E.; Nduba, V.; El-Beshlawy, A.; Hassab, H.; Achebe, M.M.; Alkindi, S.; Brown, R.C.; et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N. Engl. J. Med. 2019, 381, 509–519. [Google Scholar] [CrossRef]
- Naik, R.P.; Streiff, M.B.; Haywood, C., Jr.; Nelson, J.A.; Lanzkron, S. Venous thromboembolism in adults with sickle cell disease: A serious and under-recognized complication. Am. J. Med. 2013, 126, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Manci, E.A.; Culberson, D.E.; Yang, Y.M.; Gardner, T.M.; Powell, R.; Haynes, J., Jr.; Shah, A.K.; Mankad, V.N.; Investigators of the Cooperative Study of Sickle Cell Disease. Causes of death in sickle cell disease: An autopsy study. Br. J. Haematol. 2003, 123, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Ballas, S.K.; Lewis, C.N.; Noone, A.M.; Krasnow, S.H.; Kamarulzaman, E.; Burka, E.R. Clinical, hematological, and biochemical features of Hb SC disease. Am. J. Hematol. 1982, 13, 37–51. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).