Mediastinal Gray-Zone Lymphoma: Still an Open Issue
Abstract
1. Introduction
2. Evolution of the Concept of Gray-Zone Lymphoma
3. Clinical Characteristics
4. Biopsy Issues
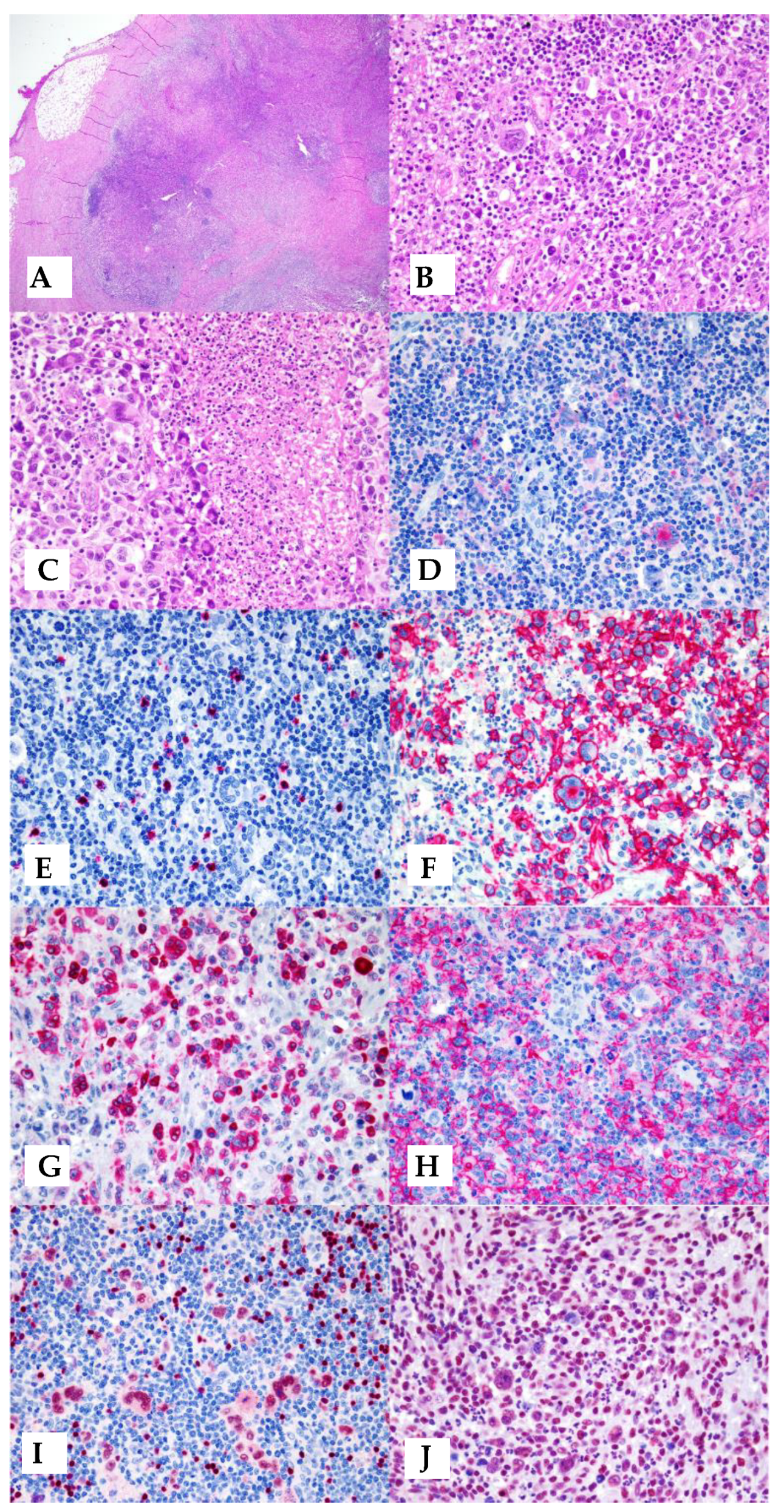
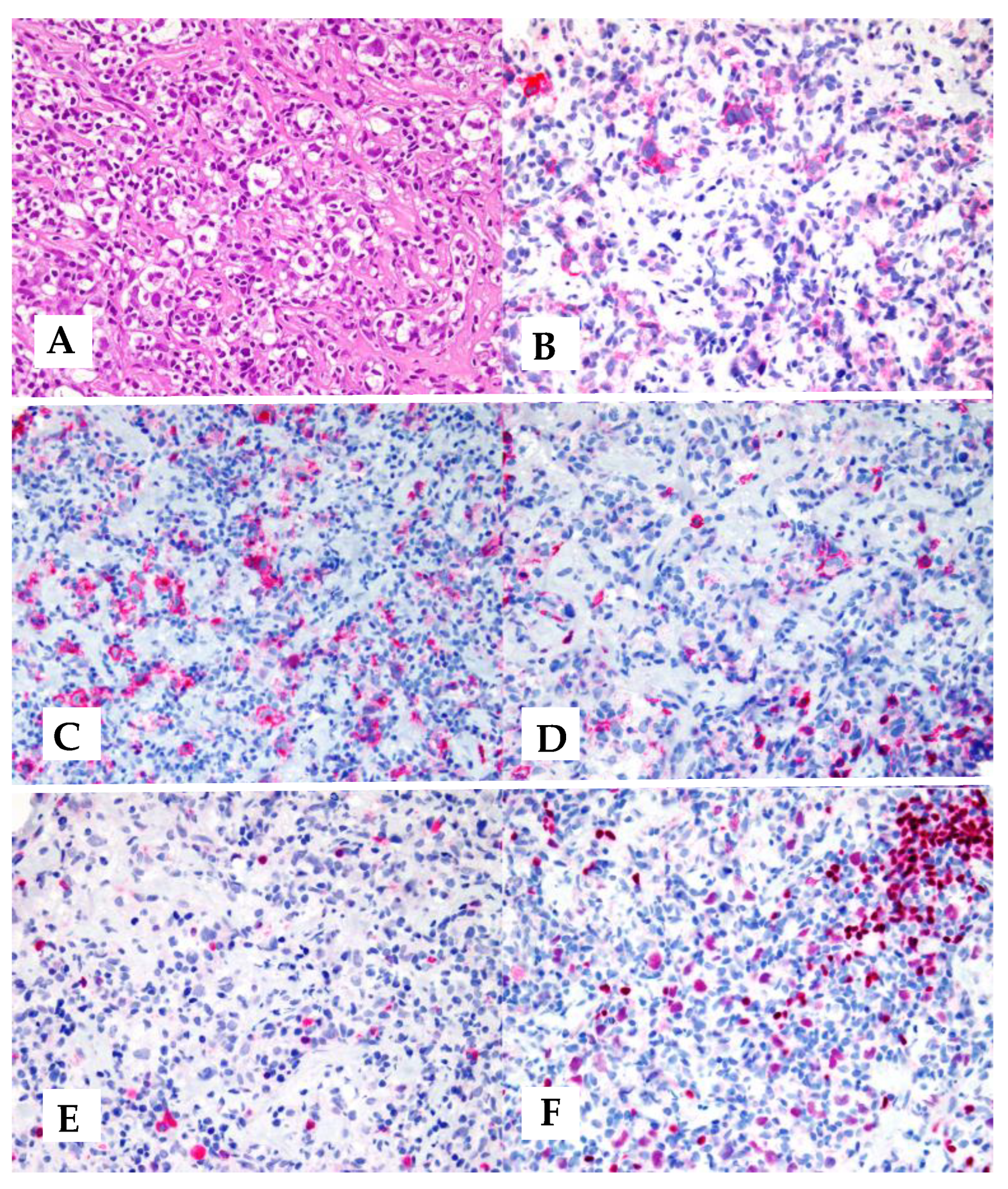
5. Morphology and Phenotype
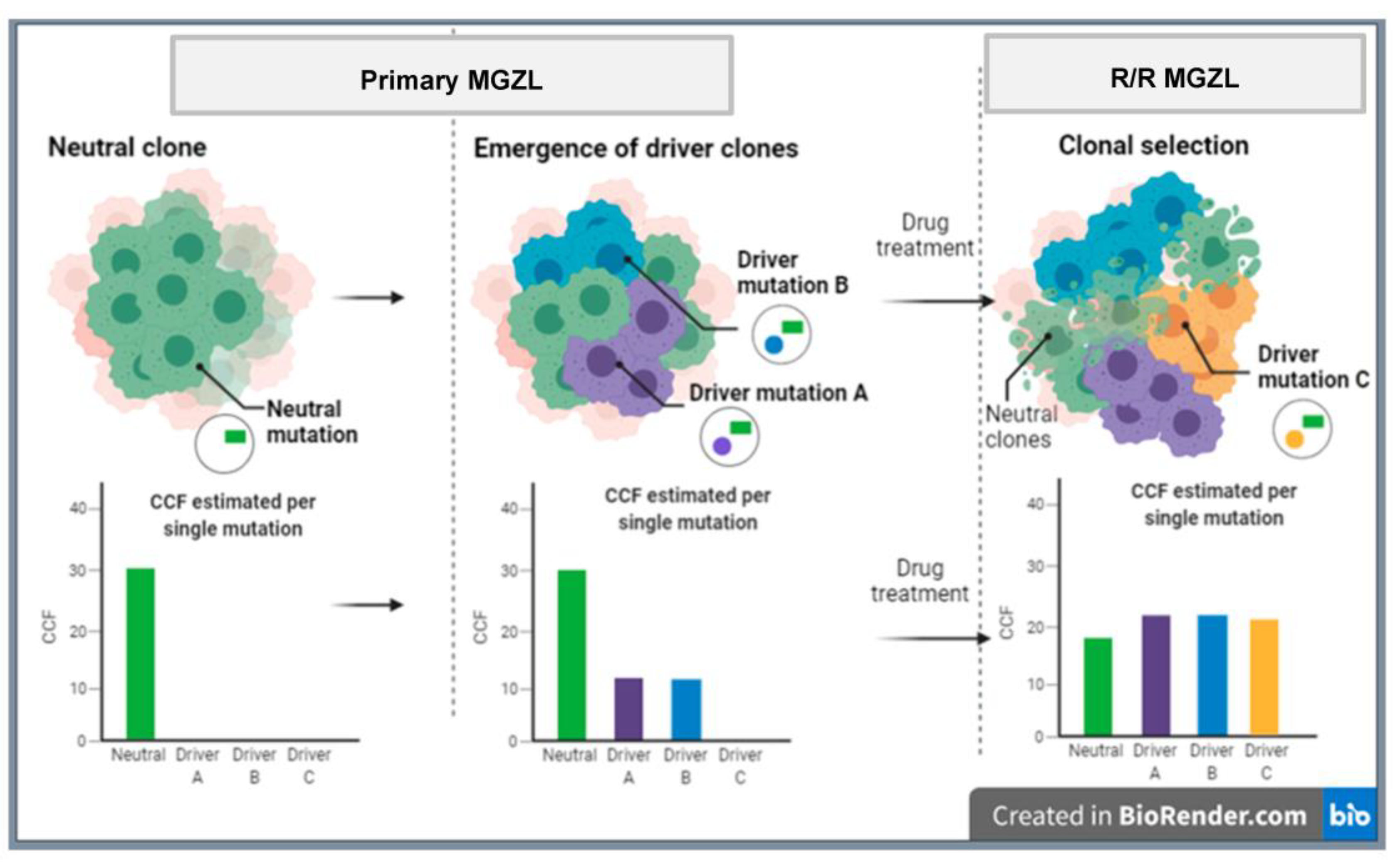
6. Molecular Features
7. Therapeutic Strategies
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rüdiger, T.; Jaffe, E.S.; Delsol, G.; deWolf-Peeters, C.; Gascoyne, R.D.; Georgii, A.; Harris, N.L.; Kadin, M.E.; MacLennan, K.A.; Poppema, S.; et al. Workshop report on Hodgkin’s disease and related diseases (‘gray zone’ lymphoma). Ann. Oncol. 1998, 9 (Suppl. 5), s31–s38. [Google Scholar] [CrossRef] [PubMed]
- Poppema, S.; Kluiver, J.L.; Atayar, C.; van den Berg, A.; Rosenwald, A.; Hummel, M.; Lenze, D.; Lammert, H.; Stein, H.; Joos, S.; et al. Report: Workshop on mediastinal gray zone lymphoma. Eur. J. Haematol. 2005, 75 (Suppl. 66), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Leroy, K.; Yu, X.; Gaulard, P.; Gascoyne, R.D.; Chan, W.C.; Zhao, T.; Haioun, C.; Greiner, T.C.; et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J. Exp. Med. 2003, 198, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.L. Shades of gray between large B-cell lymphomas and Hodgkin lymphomas: Differential diagnosis and biological implications. Mod. Pathol. 2013, 26, s57–s70. [Google Scholar] [CrossRef]
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Campo, E.; Stefano Pileri, S.A.; Swerdlow, S.H. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classic Hodgkin lymphoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Vardiman, J.W., Eds.; IARC press: Lyon, France, 2008; pp. 267–268. [Google Scholar]
- Jaffe, E.S.; Stein, H.; Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classic Hodgkin lymphoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Revised fourth, edition; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., Le Beau, M.M., et al., Eds.; IARC press: Lyon, France, 2017; pp. 342–344. [Google Scholar]
- Sarkozy, C.; Copie-Bergman, C.; Damotte, D.; Ben-Neriah, S.; Burroni, B.; Cornillon, J.; Lemal, R.; Golfier, C.; Fabiani, B.; Chassagne-Clément, C.; et al. Gray-zone Lymphoma Between cHL and Large B-Cell Lymphoma: A Histopathologic Series From the LYSA. Am. J. Surg. Pathol. 2019, 43, 341–351. [Google Scholar] [CrossRef]
- Sarkozy, C.; Chong, L.; Takata, K.; Chavez, E.A.; Miyata-Takata, T.; Duns, G.; Telenius, A.; Boyle, M.; Slack, G.W.; Laurent, C.; et al. Gene expression profiling of gray zone lymphoma. Blood Adv. 2020, 4, 2523–2535. [Google Scholar] [CrossRef]
- Sarkozy, C.; Hung, S.S.; Chavez, E.A.; Duns, G.; Takata, K.; Chong, L.C.; Aoki, T.; Jiang, A.; Miyata-Takata, T.; Telenius, A.; et al. Mutational landscape of gray zone lymphoma. Blood 2021, 137, 1765–1776. [Google Scholar] [CrossRef]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Campo, E.; Jaffe, E.S. Taking gray zone lymphomas out of the shadows. Blood 2021, 137, 1703–1704. [Google Scholar] [CrossRef]
- Aussedat, G.; Traverse-Glehen, A.; Stamatoullas, A.; Molina, T.; Safar, V.; Laurent, C.; Michot, J.M.; Hirsch, P.; Nicolas-Virelizier, E.; Lamure, S.; et al. Composite and sequential lymphoma between classical Hodgkin lymphoma and primary mediastinal lymphoma/diffuse large B-cell lymphoma, a clinico-pathological series of 25 cases. Br. J. Haematol. 2020, 189, 244–256. [Google Scholar] [CrossRef]
- Traverse-Glehen, A.; Pittaluga, S.; Gaulard, P.; Sorbara, L.; Alonso, M.A.; Raffeld, M.; Jaffe, E.S. Mediastinal gray zone lymphoma: The missing link between classic Hodgkin’s lymphoma and mediastinal large B-cell lymphoma. Am. J. Surg. Pathol. 2005, 29, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Kritharis, A.; Pilichowska, M.; Evens, A.M. How I manage patients with gray zone lymphoma. Br. J. Haematol. 2016, 174, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Pilichowska, M.; Pittaluga, S.; Ferry, J.A.; Hemminger, J.; Chang, H.; Kanakry, J.A.; Sehn, L.H.; Feldman, T.; Abramson, J.S.; Kritharis, A.; et al. Clinicopathologic consensus study of grey zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv. 2017, 1, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Westin, J.R.; Miranda, R.N.; Cheah, C.Y.; Oki, Y.; Turturro, F.; Romaguera, J.E.; Neelapu, S.S.; Nastoupil, L.J.; Fayad, L.E.; et al. Dose adjusted-EPOCH-R and mediastinal disease may improve outcomes for patients with gray-zone lymphoma. Br. J. Haematol. 2017, 179, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, G.; Cretella, P.; Zeppa, P.; Caputo, A. Large-cell lymphoma with features intermediate between Hodgkin’s, primary mediastinal B-cell and gray-zone lymphoma: A conundrum on fine needle aspiration cytology. Cytopathology 2020, 31, 325–328. [Google Scholar] [CrossRef]
- Egan, C.; Pittaluga, S. Into the gray-zone: Update on the diagnosis and classification of a rare lymphoma. Expert Rev. Hematol. 2020, 13, 1–3. [Google Scholar] [CrossRef]
- Tabanelli, V.; Melle, F.; Motta, G.; Mazzara, S.; Fabbri, M.; Agostinelli, C.; Calleri, A.; Del Corvo, M.; Fiori, S.; Lorenzini, D.; et al. The identification of TCF1+ progenitor exhausted T cells in THRLBCL may predict a better response to PD-1/PD-L1 blockade. Blood Adv. 2022, 6, 4634–4644. [Google Scholar] [CrossRef]
- Steidl, C.; Gascoyne, R.D. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood 2011, 118, 2659–2669. [Google Scholar] [CrossRef]
- Eberle, F.C.; Rodriguez-Canales, J.; Wei, L.; Hanson, J.C.; Killian, J.K.; Sun, H.W.; Adams, L.G.; Hewitt, S.M.; Wilson, W.H.; Pittaluga, S.; et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. Haematologica 2011, 96, 558–566. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K. Primary mediastinal B-cell lymphoma: Biology and evolving therapeutic strategies. Hematol. Am. Soc Hematol. Educ. Program 2017, 1, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Kanakry, J.A.; Sehn, L.H.; Kritharis, A.; Feldman, T.; Kroll, A.; Gascoyne, R.D.; Abramson, J.S.; Petrich, A.M.; Hernandez-Ilizaliturri, F.J.; et al. Gray zone lymphoma with features intermediate between classical Hodgkin lymphoma and diffuse large B-cell lymphoma: Characteristics, outcomes, and prognostication among a large multicenter cohort. Am. J. Hematol. 2015, 90, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Pittaluga, S.; Nicolae, A.; Camphausen, K.; Shovlin, M.; Steinberg, S.M.; Roschewski, M.; Staudt, L.M.; Jaffe, E.S.; Dunleavy, K. A prospective study of mediastinal gray-zone lymphoma. Blood 2014, 124, 1563–1569. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Santoro, A.; Gritti, G.; Brice, P.; Barr, P.M.; Kuruvilla, J.; Cunningham, D.; Kline, J.; Johnson, N.A.; Mehta-Shah, N.; et al. Nivolumab Combined With Brentuximab Vedotin for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Efficacy and Safety From the Phase II CheckMate 436 Study. J. Clin. Oncol. 2019, 37, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Moskowitz, A.J.; Ferrari, S.; Carlo-Stella, C.; Lisano, J.M.; Francis, S.; Wen, R.; Akyol, A.; Savage, K.J. Nivolumab combined with brentuximab vedotin for relapsed/refractory mediastinal gray zone lymphoma. Blood 2023. online ahead of print. [Google Scholar] [CrossRef]
- Terao, T.; Yuda, J.; Yamauchi, N.; Guo, Y.-M.; Shimada, K.; Sugano, M.; Ishii, G.; Minami, Y. Brentuximal vedotin after autologous stem cell transplantation for refractory gray zone lymphoma with long term remission. Mol. Clin. Oncol. 2021, 14, 125–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pileri, S.; Tabanelli, V.; Chiarle, R.; Calleri, A.; Melle, F.; Motta, G.; Sapienza, M.R.; Sabattini, E.; Zinzani, P.L.; Derenzini, E. Mediastinal Gray-Zone Lymphoma: Still an Open Issue. Hemato 2023, 4, 196-206. https://doi.org/10.3390/hemato4030016
Pileri S, Tabanelli V, Chiarle R, Calleri A, Melle F, Motta G, Sapienza MR, Sabattini E, Zinzani PL, Derenzini E. Mediastinal Gray-Zone Lymphoma: Still an Open Issue. Hemato. 2023; 4(3):196-206. https://doi.org/10.3390/hemato4030016
Chicago/Turabian StylePileri, Stefano, Valentina Tabanelli, Roberto Chiarle, Angelica Calleri, Federica Melle, Giovanna Motta, Maria Rosaria Sapienza, Elena Sabattini, Pier Luigi Zinzani, and Enrico Derenzini. 2023. "Mediastinal Gray-Zone Lymphoma: Still an Open Issue" Hemato 4, no. 3: 196-206. https://doi.org/10.3390/hemato4030016
APA StylePileri, S., Tabanelli, V., Chiarle, R., Calleri, A., Melle, F., Motta, G., Sapienza, M. R., Sabattini, E., Zinzani, P. L., & Derenzini, E. (2023). Mediastinal Gray-Zone Lymphoma: Still an Open Issue. Hemato, 4(3), 196-206. https://doi.org/10.3390/hemato4030016







