Should Patients with Waldenström Macroglobulinemia Receive a BTK Inhibitor as Frontline Therapy?
Abstract
1. Introduction
2. First Line Therapies
2.1. Cytotoxic Agents and AntiCD20 Monoclonal Antibodies
2.2. Proteasome Inhibitor-Based Therapy
2.3. Rituximab Maintenance
2.4. BTK Inhibitors
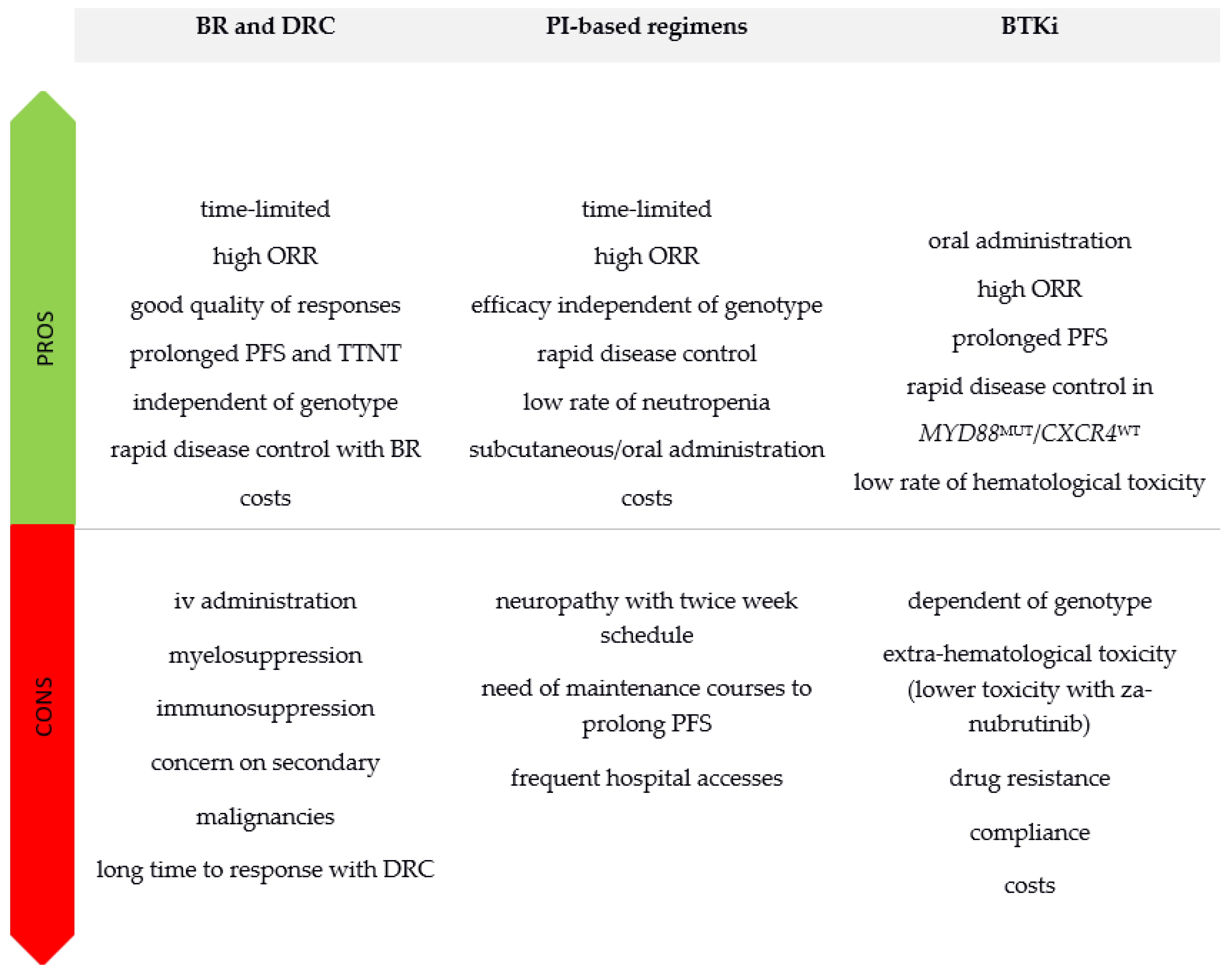
3. Making a Choice in First Line
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Panayiotidis, P.; Moulopoulos, L.A.; Sfikakis, P.; Dalakas, M. Waldenström’s macroglobulinemia: Clinical features, complications, and management. J. Clin. Oncol. 2000, 18, 214–226. [Google Scholar] [CrossRef]
- Kyle, R.A.; Treon, S.P.; Alexanian, R.; Barlogie, B.; Björkholm, M.; Dhodapkar, M.; Lister, T.A.; Merlini, G.; Morel, P.; Stone, M.; et al. Prognostic markers and criteria to initiate therapy in Waldenstrom’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003, 30, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.J.; Bogen, S.A. Role of plasmapheresis in Waldenström’s macroglobulinemia. Clin. Lymphoma Myeloma Leuk 2013, 13, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Pal Singh, S.; Dammeijer, F.; Hendriks, R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Leblond, V.; Kastritis, E.; Advani, R.; Ansell, S.M.; Buske, C.; Castillo, J.J.; García-Sanz, R.; Gertz, M.; Kimby, E.; Kyriakou, C.; et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s Macroglobulinemia. Blood 2016, 128, 1321–1328. [Google Scholar] [CrossRef]
- Leblond, V.; Johnson, S.; Chevret, S.; Copplestone, A.; Rule, S.; Tournilhac, O.; Seymour, J.F.; Patmore, R.D.; Wright, D.; Morel, P.; et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J. Clin. Oncol. 2013, 31, 301–307. [Google Scholar] [CrossRef]
- Rummel, M.J.; Niederle, N.; Maschmeyer, G.; Banat, G.A.; von Grünhagen, U.; Losem, C.; Kofahl-Krause, D.; Heil, G.; Welslau, M.; Balser, C.; et al. Study group indolent Lymphomas (StiL) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013, 381, 1203–1210. [Google Scholar] [CrossRef]
- Buske, C.; Dimopoulos, M.A.; Grunenberg, A.; Kastritis, E.; Tomowiak, C.; Mahé, B.; Troussard, X.; Hajek, R.; Viardot, A.; Tournilhac, O.; et al. Bortezomib in Combination with Dexamethasone, Rituximab and Cyclophosphamide (B-DRC) As First—Line Treatment of Waldenstrom’s Macroglobulinemia: Results of a Prospectively Randomized Multicenter European Phase II Trial. Blood 2020, 136, 26. [Google Scholar] [CrossRef]
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Sanz, R.G.; et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef]
- Treon, S.P.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Sheehy, P.; Manning, R.J.; Patterson, C.J.; Tripsas, C.; et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N. Engl. J. Med. 2012, 367, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Venet-Caillault, A.; Figeac, M.; Herbaux, C.; Marot, G.; Doye, E.; Bertrand, E.; Geffroy, S.; Lepretre, F.; et al. Genomic Landscape of CXCR4 Mutations in Waldenström Macroglobulinemia. Clin. Cancer Res. 2016, 22, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Hunter, Z.R.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Manning, R.J.; Tripsas, C.; Patterson, C.J.; Sheehy, P.; et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014, 123, 1637–1646. [Google Scholar] [CrossRef]
- Treon, S.P.; Cao, Y.; Xu, L.; Yang, G.; Liu, X.; Hunter, Z.R. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood 2014, 123, 2791–2796. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Zervas, C.; Zomas, A.; Kiamouris, C.; Viniou, N.A.; Grigoraki, V.; Karkantaris, C.; Mitsouli, C.; Gika, D.; Christakis, J.; et al. Treatment of Waldenstrom’s macroglobulinemia with rituximab. J. Clin. Oncol. 2002, 20, 2327–2333. [Google Scholar] [CrossRef]
- Gertz, M.A.; Rue, M.; Blood, E.; Kaminer, L.S.; Vesole, D.H.; Greipp, P.R. Multicenter phase 2 trial of rituximab for Waldenstrom macroglobulinemia (WM): An Eastern Cooperative Oncology Group Study (E3A98). Leuk Lymphoma 2004, 45, 2047–2055. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Zervas, C.; Zomas, A.; Hamilos, G.; Gika, D.; Efstathiou, E.; Panayiotidis, P.; Vervessou, E.; Anagnostopoulos, N.; Christakis, J. Extended rituximab therapy for previously untreated patients with Waldenstrom’s macroglobulinemia. Clin. Lymphoma 2002, 3, 163–166. [Google Scholar] [CrossRef]
- Treon, S.P.; Emmanouilides, C.; Kimby, E.; Kelliher, A.; Prefer, F.; Branagan, A.R.; Anderson, K.C.; Frankel, S.R. Waldenström’s Macroglobulinemia Clinical Trials Group. Extended rituximab therapy in Waldenström’s macroglobulinemia. Ann. Oncol. 2005, 16, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Branagan, A.R.; Ioakimidis, L.; Soumerai, J.D.; Patterson, C.J.; Turnbull, B.; Wasi, P.; Emmanouilides, C.; Frankel, S.R.; Lister, A.; et al. Long-term outcomes to fludarabine and rituximab in Waldenström macroglobulinemia. Blood 2009, 113, 3673–3678. [Google Scholar] [CrossRef][Green Version]
- Tedeschi, A.; Benevolo, G.; Varettoni, M.; Battista, M.L.; Zinzani, P.L.; Visco, C.; Meneghini, V.; Pioltelli, P.; Sacchi, S.; Ricci, F.; et al. Fludarabine plus cyclophosphamide and rituximab in Waldenstrom macroglobulinemia: An effective but myelosuppressive regimen to be offered to patients with advanced disease. Cancer 2012, 118, 434–443. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E. How I treat Waldenström macroglobulinemia. Blood 2019, 134, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Anagnostopoulos, A.; Kyrtsonis, M.C.; Zervas, K.; Tsatalas, C.; Kokkinis, G.; Repoussis, P.; Symeonidis, A.; Delimpasi, S.; Katodritou, E.; et al. Primary treatment of Waldenstrom’s macroglobulinemia with dexamethasone, rituximab and cyclophosphamide. J. Clin. Oncol. 2007, 25, 3344–3349. [Google Scholar] [CrossRef]
- Kastritis, E.; Gavriatopoulou, M.; Kyrtsonis, M.C.; Roussou, M.; Hadjiharissi, E.; Symeonidis, A.; Repoussis, P.; Michalis, E.; Delimpasi, S.; Tsatalas, K.; et al. Dexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenström macroglobulinemia: Final analysis of a phase 2 study. Blood 2015, 126, 1392–1394. [Google Scholar] [CrossRef] [PubMed]
- Laribi, K.; Poulain, S.; Willems, L.; Merabet, F.; Herbaux, C.; Roos-Weil, D.; Baugier de Materre, A.; Roussel, X.; Tricot, S.; Dupuis, J.; et al. The Bendamustine Plus Rituximab Regimen Is Active and Safe in Previously Untreated Patients with Waldenström Macroglobulinemia, a Study on Behalf of the French Innovative Leukemia Organization (FILO). Blood 2017, 130, 4046. [Google Scholar]
- Zanwar, S.; Abeykoon, J.; Castillo, J.; Durot, E.; Kastritis, E.; Uppal, E.; Morel, P.; Tawfiq, R.; Montes, L.; Paludo, J.; et al. A Multicenter, International Collaborative Study Evaluating Frontline Therapy with Bendamustine Rituximab for Waldenström Macroglobulinemia. HemaSphere 2022, 6, 1046–1047. [Google Scholar] [CrossRef]
- Paludo, J.; Abeykoon, J.P.; Shreders, A.; Ansell, S.M.; Kumar, S.; Ailawadhi, S.; King, R.L.; Koehler, A.B.; Reeder, C.B.; Buadi, F.K.; et al. Bendamustine and rituximab (BR) versus dexamethasone, rituximab, and cyclophosphamide (DRC) in patients with Waldenström macroglobulinemia. Ann. Hematol. 2018, 97, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Caravita, T.; Siniscalchi, A.; Tendas, A.; Cupelli, L.; Dentamaro, T.; Natale, G.; Spagnoli, A.; de Fabritiis, P. High-dose therapy with autologous PBSC transplantation in the front-line treatment of Waldenstrom’s macroglobulinemia. Bone Marrow Transplant. 2009, 43, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Dhodapkar, M.; Siegel, D.; Fassas, A.; Singh, J.; Singhal, S.; Mehta, J.; Vesole, D.; Tricot, G.; Jagannath, S.; et al. High-dose therapy with autologous haemopoietic stem cell support for Waldenström’s macroglobulinaemia. Br. J. Haematol. 1999, 105, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Barlogie, B. Role for high-dose therapy with autologous hematopoietic stem cell support in Waldenstrom’s macroglobulinemia. Semin Oncol. 2003, 30, 282–285. [Google Scholar] [CrossRef]
- Dreger, P.; Schmitz, N. Autologous stem cell transplantation as part of first-line treatment of Waldenström’s macroglobulinemia. Biol. Blood Marrow Transplant. 2007, 13, 623–624. [Google Scholar] [CrossRef]
- Pratt, G.; El-Sharkawi, D.; Kothari, J.; D’Sa, S.; Auer, R.; McCarthy, H.; Krishna, R.; Miles, O.; Kyriakou, C.; Owen, R. Diagnosis and management of Waldenström macroglobulinaemia-A British Society for Haematology guideline. Br. J. Haematol. 2022, 197, 171–187. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines. Available online: https://www.nccn.org/guidelines (accessed on 17 September 2022).
- Treon, S.P.; Ioakimidis, L.; Soumerai, J.D.; Patterson, C.J.; Sheehy, P.; Nelson, M.; Willen, M.; Matous, J.; Mattern, J.; Diener, J.G.; et al. Primary therapy of Waldenstrom’s macroglobulinemia with Bortezomib, Dexamethasone and Rituximab: Results of WMCTG clinical trial 05–180. J. Clin. Oncol. 2009, 27, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Meid, K.; Gustine, J.; Patterson, C.J.; Matous, J.F.; Ghobrial, I.M.; Castillo, J.J. Long-Term Outcome of a Prospective Study of Bortezomib, Dexamethasone and Rituximab (BDR) in Previously Untreated, Symptomatic Patients with Waldenstrom’s Macroglobulinemia. Blood 2015, 126, 1833. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Xie, W.; Padmanabhan, S.; Badros, A.; Rourke, M.; Leduc, R.; Chuma, S.; Kunsman, J.; Warren, D.; Poon, T.; et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenstrom macroglobulinemia. Am. J. Hematol. 2010, 85, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; García-Sanz, R.; Gavriatopoulou, M.; Morel, P.; Kyrtsonis, M.C.; Michalis, E.; Kartasis, Z.; Leleu, X.; Palladini, G.; Tedeschi, A.; et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): Long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood 2013, 122, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; García-Sanz, R.; Kastritis, E.; Morel, P.; Kyrtsonis, M.C.; Michalis, E.; Kartasis, Z.; Leleu, X.; Palladini, G.; Tedeschi, A.; et al. BDR in newly diagnosed patients with WM: Final analysis of a phase 2 study after a minimum follow-up of 6 years. Blood 2017, 129, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Abeykoon, J.P.; Zanwar, S.; Ansell, S.M.; Muchtar, E.; He, R.; Greipp, P.T.; King, R.L.; Ailawadhi, S.; Paludo, J.; Larsen, J.T.; et al. Assessment of fixed-duration therapies for treatment-naïve Waldenström macroglobulinemia. Am. J. Hematol. 2021, 96, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Gustine, J.N.; Meid, K.; Dubeau, T.E.; Severns, P.; Xu, L.; Yang, G.; Hunter, Z.R.; Treon, S.P. Response and survival for primary therapy combination regimens and maintenance rituximab in Waldenström macroglobulinaemia. Br. J. Haematol. 2018, 181, 77–85. [Google Scholar] [CrossRef]
- Meid, K.; Dubeau, T.; Severns, P.; Gustine, J.; Ghobrial, I.M.; Castillo, J.J.; Treon, S.P. Long-Term Follow-up of a Prospective Clinical Trial of Carfilzomib, Rituximab and Dexamethasone (CaRD) in Waldenstrom’s Macroglobulinemia. Blood 2017, 130, 2772. [Google Scholar]
- Castillo, J.J.; Meid, K.; Gustine, J.N.; Dubeau, T.; Severns, P.; Hunter, Z.R.; Yang, G.; Xu, L.; Treon, S.P. Prospective Clinical Trial of Ixazomib, Dexamethasone, and Rituximab as Primary Therapy in Waldenström Macroglobulinemia. Clin. Cancer Res. 2018, 24, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Rummel, M.J.; Lerchenmüller, C.; Hensel, M.; Goerner, M.; Buske, C.; Schulz, H.; Schmidt, B.; Kojouharoff, G.; Lange, E.; Willenbacher, W.; et al. Two Years Rituximab Maintenance Vs. Observation after First Line Treatment with Bendamustine Plus Rituximab (B-R) in Patients with Waldenström’s Macroglobulinemia (MW): Results of a Prospective, Randomized, Multicenter Phase 3 Study (the StiL NHL7-2008 MAINTAIN trial). Blood 2019, 134, 343. [Google Scholar]
- IMBRUVICA™ (ibrutinib) US Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205552Orig2lbl.pdf (accessed on 21 October 2022).
- IMBRUVICA™ (ibrutinib) European Medical Agency. Available online: https://www.ema.europa.eu/en/documents/overview/imbruvica-epar-medicine-overview_en.pdf (accessed on 21 October 2022).
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Warren, D.; Varma, G.; Green, R.; Argyropoulos, K.V.; Yang, G.; Cao, Y.; Xu, L.; et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N. Engl. J. Med. 2015, 372, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Gustine, J.; Meid, K.; Yang, G.; Xu, L.; Liu, X.; Demos, M.; Kofides, A.; Tsakmaklis, N.; Chen, J.G.; et al. Ibrutinib Monotherapy in Symptomatic, Treatment-Naïve Patients With Waldenström Macroglobulinemia. J. Clin. Oncol. 2018, 36, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Meid, K.; Gustine, J.N.; Leventoff, C.; White, T.; Flynn, C.A.; Sarosiek, S.; Demos, M.G.; Guerrera, M.L.; Kofides, A.; et al. Long-term follow-up of ibrutinib monotherapy in treatment-naive patients with Waldenstrom macroglobulinemia. Leukemia 2022, 36, 532–539. [Google Scholar] [CrossRef]
- Buske, C.; Tedeschi, A.; Trotman, J.; García-Sanz, R.; MacDonald, D.; Leblond, V.; Mahe, B.; Herbaux, C.; Matous, J.V.; Tam, C.S.; et al. Ibrutinib Plus Rituximab Versus Placebo Plus Rituximab for Waldenström’s Macroglobulinemia: Final Analysis From the Randomized Phase III iNNOVATE Study. J. Clin. Oncol. 2022, 40, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.; McCarthy, H.; Rule, S.; D’Sa, S.; Thomas, S.; Tournilhac, O.; Forconi, F.; Kersten, M.; Zinzani, P.; Iyengar, S.; et al. Acalabrutinib In Treatment-Naive Or Relapsed/Refractory Waldenström Macroglobulinemia: 5-Year Follow-Up Of A Phase 2, Single-Arm Study. HemaSphere 2022, 6, 1020–1021. [Google Scholar] [CrossRef]
- Trotman, J.; Opat, S.; Gottlieb, D.; Simpson, D.; Marlton, P.; Cull, G.; Munoz, J.; Tedeschi, A.; Roberts, A.W.; Seymour, J.F.; et al. Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow-up. Blood 2020, 136, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Garcia-Sanz, R.; et al. ASPEN: Long-Term Follow-Up Results Of A Phase 3 Randomized Trial Of Zanubrutinib (Zanu) Vs Ibrutinib (Ibr) In Patients (Pts) With Waldenström Macroglobulinemia (Wm). HemaSphere 2022, 6, 1048–1049. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Sanz, R.G.; Lee, H.P.; Trneny, M.; Varettoni, M.; Opat, S.; D’Sa, S.; Owen, R.G.; Cull, G.; Mulligan, S.; et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenström macroglobulinemia: A substudy of the phase 3 ASPEN trial. Blood Adv. 2020, 4, 6009–6018. [Google Scholar] [CrossRef]
- Abeykoon, P.J.; Kumar, S.; Castillo, J.J.; D’Sa, S.; Kastritis, E.; Durot, E.; Uppal, E.; Morel, P.; Paludo, J.; Tawfiq Sarosiek, S.; et al. Bendamustine rituximab (BR) versus ibrutinib (Ibr) as primary therapy for Waldenström macroglobulinemia (WM): An international collaborative study. J. Clin. Oncol. 2022, 40, 7566. [Google Scholar] [CrossRef]
- ESMO Guidelines. Available online: https://www.esmo.org/guidelines (accessed on 17 September 2022).
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Novak, J.; Strugov, V.; Gill, D.; et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: Final analysis of the randomized, phase III iLLUMINATE trial. Haematologica 2022, 107, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.S.; Shati, M.; Keshtkar, A.; Malakouti, S.K.; Bazargan, M.; Assari, S. Defining polypharmacy in the elderly: A systematic review protocol. BMJ Open 2016, 6, e010989. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Sadullah, S.; Kastritis, E.; Tedeschi, A.; García-Sanz, R.; Bolkun, L.; Leleu, X.; Willenbacher, W.; Hájek, R.; Minnema, M.C.; et al. Treatment and outcome patterns in European patients with Waldenström’s macroglobulinaemia: A large, observational, retrospective chart review. Lancet Haematol. 2018, 5, e299–e309. [Google Scholar] [CrossRef]
- Varettoni, M.; Tedeschi, A.; Arcaini, L.; Pascutto, C.; Vismara, E.; Orlandi, E.; Ricci, F.; Corso, A.; Greco, A.; Mangiacavalli, S.; et al. Risk of second cancers in Waldenström macroglobulinemia. Ann. Oncol. 2012, 23, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Imbruvica [Package Insert]. Available online: https://www.imbruvica.com/files/prescribing-information.pdf (accessed on 21 October 2022).
- Gustine, J.N.; Sarosiek, S.; Flynn, C.A.; Meid, K.; Leventoff, C.; White, T.; Guerrera, M.L.; Xu, L.; Kofides, A.; Tsakmaklis, N.; et al. Natural history of Waldenström macroglobulinemia following acquired resistance to ibrutinib monotherapy. Haematologica 2022, 107, 1163–1171. [Google Scholar] [CrossRef]
| Author | Regimen | N. pts | Responses (%) | Survival Rates | Genotype Impact | F/U (m) |
|---|---|---|---|---|---|---|
| Leblond 2013 [7] | Chlorambucil 8 mg/m2 daily for 10 d every 28 d vs. Fludarabine 40 mg/m2 daily for 5 d every 28 d | 170 169 | ORR 36% ORR 46% | mPFS 27 m mPFS 38 m | NA | 36 m |
| Treon 2009 [19] | R + Fludarabine: R 375 mg/m2/w at w 1 to 4, 17, 18, 30, 31 + 6 cycles of F 25 mg/m2 daily for 5 d at w 5, 9, 13, 19, 23, and 27 | 43 (27 TN) | ORR 95% MRR 86% CR + VGPR% 37% | mPFS 77.6 m | NA | 40.3 m |
| Tedeschi 2012 [20] | FCR: R 375 mg/m2 on d 1 + F 25 mg/m2 and C 250 mg/m2 on d 2–4, every 28 d for 6 cycles | 43 (28 TN) | ORR 79% MRR 74% CR + VGPR% 32% | mPFS NR 2 y OS 88.4% 4 y OS 69.1% | NA | 37.2 m |
| Kastritis 2015 [23] | DRC: D 20 mg + R 375 mg/m2 on d 1 + C 100 mg/m2 bid on d 1–5, every 21 d for 6 cycles | 72 | ORR 83% MRR 74% CR 7% | mPFS 35 m mOS 95 m | NA | 7 y |
| Rummel 2013 [8] | BR: Bendamustine 90 mg/m2 on d 1, 2 + R 375 mg/m2 on d 1, every 4 w for 6 cycles | 22 | ORR 93% CR 40% | mPFS 69.5 m | NA | 45 m |
| Laribi 2018 [24] | BR: Bendamustine 90 mg/m2 on d 1, 2 + R 375 mg/m2 on d 1, every 4 w for 6 cycles | 69 | ORR 97% MRR 96% CR + VGPR 56% | 2 y PFS 87% 2 y OS 97.1% | MYD88L265P vs. MYD88WT: ORR, PFS: NS CXCR4MUT vs. CXCR4WT: ORR; PFS: NS | 23 m |
| Zanwar 2022 [25] | BR: Bendamustine 90 mg/m2 on d 1, 2 + R 375 mg/m2 on d 1, every 4 w for 6 cycles | 208 | ORR 95% MRR 93% VGPR 31% | Est mPFS 5.9 y Est 5 y OS 90% | MYD88L265P vs. MYD88WT: ORR, PFS: NS CXCR4MUT vs. CXCR4WT: ORR; PFS: NS | 4 y |
| Paludo 2018 [26] | BR: Bendamustine 90 mg/m2 on d 1, 2 + R 375 mg/m2 on d 1, every 4 w for 6 cycles vs. DRC: D 20 mg + R 375 mg/m2 on d 1 + C 100 mg/m2 bid on d 1–5, every 21 d for 6 cycles | 60 (17 TN) 100 (50 TN) | ORR 93% MRR 86% VGPR 29% ORR 96% MRR 87% VGPR 17% | 2 y PFS 88% 2 y PFS 61% | MYD88L265P vs. MYD88WT: ORR, PFS: NS | 30 m |
| Treon 2015 [34] | BDR: Bortezomib 1.3 mg/m2 IV + D 40 mg on d 1, 4, 8, 11 + R 375 mg/m2 on d 11. A total of 4 cycles of induction + 4 cycles, each 3 months apart, for maintenance | 23 | ORR 96% MRR 91% CR + VGPR 52% | 5 y PFS 57% 5 y OS 95% | NA | 8.5 y |
| Ghobrial 2010 [35] | Bortezomib + R: Bortezomib 1.6 mg/m2 IV on d 1, 8, 15, every 28 d for 6 cycles + R 375 mg/m2/w on cycles 1 and 4 | 26 | ORR 89% MRR 66% CR + VGPR 8% | 1 y EFS 79% | NA | 14 m |
| Gavriatopoulou 2017 [37] | BDR: Bortezomib 1.3 mg/m2 IV on d 1, 4, 8, 11 (cycle 1), B 1.6 mg/m2 IV on d 1, 8, 15, 22 (cycles 2–5) + D 40 mg and R 375 mg/m2/w in cycle 2 and 5. Every 35 d for 5 cycles | 59 | ORR 85% MRR 68% CR + VGPR 10% | mPFS 43 m 7 y OS 66% | NA | 86 m |
| Abeykoon 2021 [38] | BR: Bendamustine 90 mg/m2 on d 1, 2 + R 375 mg/m2 on d 1, every 4 w for 6 cycles vs. BDR: Bortezomib 1.3 mg/m2 IV on d 1, 4, 8, 11 (cycle 1), B 1.6 mg/m2 IV on d 1, 8, 15, 22 (cycles 2–5) + D 40 mg and R 375 mg/m2/w in cycle 2 vs. DRC: D 20 mg + R 375 mg/m2 on d 1 + C 100 mg/m2 bid on d 1–5, every 21 d for 6 cycles | 83 45 92 | ORR 98% MRR 96% ORR 84% MRR 68% ORR 78% MRR 53% | mPFS 5.2 y 4 y OS 90% mPFS 1.8 y 4 y OS 87% mPFS 4.3 y 4 y OS 87% | MYD88L265P vs. MYD88WT: ORR, PFS: NS | 2.3 y 5.2 y 6.3 y |
| Castillo 2018 [39] | BR: Bendamustine 90 mg/m2 on d 1, 2 + R 375 mg/m2 on d 1, every 4 w for 6 cycles vs. BDR: bortezomib 1.6 mg/m2 + D 20 mg on d 1, 4, 8 and 11 or 1, 8, 15 and 22, every 3 and 4 w, respectively, + rituximab 375 mg/m2 on d 11 or 22, respectively, for 4 cycles vs. DRC: C 1000 mg/m2 + plus D 20 mg + R 375 mg/m2 on d 1, for 6 cycles | 57 87 38 | ORR 98% MRR 94% CR + VGPR 45% ORR 90% MRR 83% CR + VGPR 35% ORR 89% MRR 84% CR + VGPR 42% | mPFS 5.5 m 10 y OS 95% mPFS 5.8 m 10 y OS 96% mPFS 4.8 m 10 y OS 81% | NA | 3 y 4 y 5 y |
| Buske 2020 [9] | B-DRC: DRC + Bortezomib sc 1,6 mg/m2 on d 1, 8, 15, every 28 d for 6 cycles vs. DRC: D 20 mg + R 375 mg/m2 on d 1 + C 100 mg/m2 bid on d 1–5, every 21 d for 6 cycles | 100 100 | ORR 91% MRR 79% CR + VGPR 19% ORR 87% MRR 69% CR + VGPR 11% | mPFS NR mPFS 50.1 m | MYD88L265P vs. MYD88WT: ORR, PFS: NS CXCR4MUT vs. CXCR4WT: ORR; PFS: NS | 27.5 m |
| Meid 2017 [40] | CaRD: carfilzomib 20 mg/m2 (cycle 1) and 36 mg/m2 (cycles 2 and beyond), D 20 mg on d 1, 2, 8, 9; Rituximab 375 mg/m2 on d 2, 9 of each 21-d cycle. Six induction cycles +8 cycles, each 8 w apart for maintenance | 31 | ORR 81% MRR 71% CR + VGPR 39% | mPFS 46 m | MYD88L265P vs. MYD88WT: ORR: NS CXCR4MUT vs. CXCR4WT: ORR: NS | NA |
| Castillo 2018 [41] | IDR: ixazomib 4 mg and D 20 mg on d 1, 8, and 15 every 4 w for cycles 1 and 2, + R 375 mg/m2 on d 1, e 4 weeks for cycles 3 to 6. Then 6 cycles, each 8 w apart, for maintenance | 26 | ORR 96% MRR 77% | mPFS NR | CXCR4MUT vs. CXCR4WT: ORR: NS | 22 m |
| Author | BTKi | N. pts | Responses | Survival Rates | Genotype Impact | F/U |
|---|---|---|---|---|---|---|
| Castillo 2022 [47] | Ibrutinib 420 mg daily | 30 | ORR 100% MRR 87% VGPR 30% | 4 y PFS 76% | longer TTMR in CXCR4MUT vs. CXCR4WT | 50.1 m |
| Buske 2022 [48] | ibrutinib 420 mg daily + R 375 mg/m2 on d1 of w 1–4, 17–20 vs. placebo + R 375 mg/m2 on d1 of w 1–4, 17–20 | 75 (45 TN) 75 (45 TN) | ORR 91% MRR 76% CR + VGPR: 27% ORR 53% MRR 41% CR + VGPR 9% | 48 m PFS 70% 48 m PFS 32% | MYD88L265P vs. MYD88WT: ORR, PFS: NS CXCR4MUT vs. CXCR4WT: ORR; PFS: NS | 50 m |
| Owen 2022 [49] | Acalabrutinib 100 mg bid | 106 (14 TN) | ORR 93% MRR 79% | 66 m PFS 84% 66 m OS 91% | NA | 63.7 m |
| Trotman 2020 [50] | zanubrutinib 160 mg bid | 77 (24 TN) | ORR 100% MRR 87.5% CR + VGPR 33% | Est 2 y EFS 92% | MYD88L265P vs. MYD88WT: ORR, PFS: NS CXCR4MUT vs. CXCR4WT: ORR; PFS: NS | 23.5 m |
| Tam 2020 [10] | ibrutinib 420 mg daily vs. zanubrutinib 160 mg bid | 99 (18 TN) 102 (19 TN) | ORR 89% MRR 67% CR + VGPR 14% ORR 95% MRR 74% CR + VGPR 26% | mPFS NR Est 18 m OS 97% mPFS NR Est 18 m OS 93% | CXCR4MUT vs. CXCR4WT: ORR: NS | 18.5 m |
| Dimopoulos 2022 [51] | ibrutinib 420 mg daily vs. zanubrutinib 160 mg bid | 99 (18 TN) 102 (19 TN) | All pts CR + VGPR 22% All pts CR + VGPR 36% | All pts mPFS NR mOS NR All pts mPFS NR mOS NR | CXCR4WT Ibr CR + VGPR 28% Zanu CR + VGPR 45% (p = 0.04) CXCR4MUT ibr CR + VGPR 5% Zanu CR + VGPR 21% (p = 0.15) * | 43 m |
| Dimopoulos 2020, 2022 [51,52] | zanubrutinib 160 mg bid | 28 (5 TN) | ORR 80% MRR 40% VGPR 20% | All pts 43 m PFS 53.8% 43 m OS 83.9% | only MYD88WT pts | 43 m |
| Abeykoon 2022 [53] | BR: Bendamustine 90 mg/m2 on d 1, 2 + R 375 mg/m2 on d 1, every 4 w for 6 cycles Vs. ibrutinib 420 mg daily | 208 139 | ORR 94% MRR 92% CR + VGPR 50% ORR 94% MRR 83% CR + VGPR 33% | 4 y PFS 73% 4 y OS 94% 4 y PFS 73% 4 y OS 82% | NA | 4.2 y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deodato, M.; Frustaci, A.M.; Zamprogna, G.; Cotilli, G.; Cairoli, R.; Tedeschi, A. Should Patients with Waldenström Macroglobulinemia Receive a BTK Inhibitor as Frontline Therapy? Hemato 2022, 3, 689-703. https://doi.org/10.3390/hemato3040046
Deodato M, Frustaci AM, Zamprogna G, Cotilli G, Cairoli R, Tedeschi A. Should Patients with Waldenström Macroglobulinemia Receive a BTK Inhibitor as Frontline Therapy? Hemato. 2022; 3(4):689-703. https://doi.org/10.3390/hemato3040046
Chicago/Turabian StyleDeodato, Marina, Anna Maria Frustaci, Giulia Zamprogna, Giulia Cotilli, Roberto Cairoli, and Alessandra Tedeschi. 2022. "Should Patients with Waldenström Macroglobulinemia Receive a BTK Inhibitor as Frontline Therapy?" Hemato 3, no. 4: 689-703. https://doi.org/10.3390/hemato3040046
APA StyleDeodato, M., Frustaci, A. M., Zamprogna, G., Cotilli, G., Cairoli, R., & Tedeschi, A. (2022). Should Patients with Waldenström Macroglobulinemia Receive a BTK Inhibitor as Frontline Therapy? Hemato, 3(4), 689-703. https://doi.org/10.3390/hemato3040046





