Alterations of Peripheral Blood T Cell Subsets following Donor Lymphocyte Infusion in Patients after Allogeneic Stem Cell Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Donor Lymphocyte Infusions
2.3. Analysis of Lymphocytes and Subsets by Immunophenotyping
2.4. Definitions
2.5. Statistics
3. Results
3.1. Patient Characteristics and Clinical Outcomes
3.2. Analysis of Lymphocytes and Subsets
3.2.1. Baseline levels
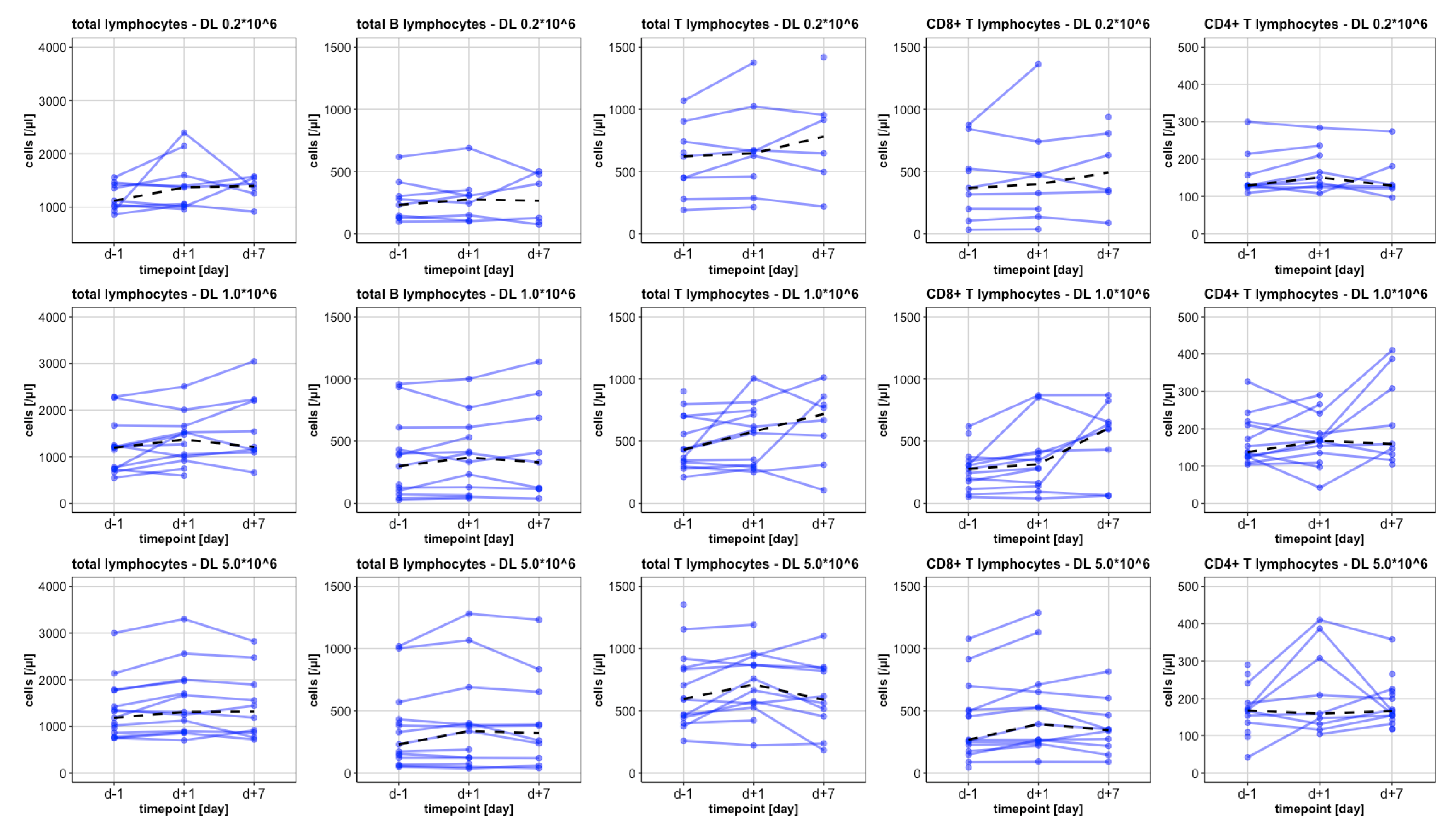
3.2.2. The Total Lymphocytes and CD3+ Lymphocytes
3.2.3. CD4+ Lymphocytes
3.2.4. CD8+ Lymphocytes
3.2.5. NK Cells
3.2.6. B Lymphocytes
3.2.7. Subgroup Analysis on Recipients of Preemptive DLI
3.2.8. Subgroup Analysis on Patients Developing GvHD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kolb, H.J.; Mittermuller, J.; Clemm, C.; Holler, E.; Ledderose, G.; Brehm, G.; Heim, M.; Wilmanns, W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990, 76, 2462–2465. [Google Scholar] [CrossRef] [Green Version]
- Deol, A.; Lum, L.G. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat. Rev. 2010, 36, 528–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, C.; Labopin, M.; Nagler, A.; Bornhäuser, M.; Finke, J.; Fassas, A.; Volin, L.; Gürman, G.; Maertens, J.; Bordigoni, P.; et al. Donor Lymphocyte Infusion in the Treatment of First Hematological Relapse After Allogeneic Stem-Cell Transplantation in Adults with Acute Myeloid Leukemia: A Retrospective Risk Factors Analysis and Comparison with Other Strategies by the EBMT Acute Leukemia Working Party. J. Clin. Oncol. 2007, 25, 4938–4945. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Huang, X.-J. Donor lymphocyte infusions for relapse after allogeneic transplantation. When, if and for whom? Blood Rev. 2013, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Schleuning, M.; Stadler, M.; Finke, J.; Hurst, E.; Baron, F.; Ringden, O.; et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—A matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2019, 184, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Wang, Y.; Liu, D.-H.; Xu, L.-P.; Chen, H.; Chen, Y.-H.; Han, W.; Shi, H.-X.; Liu, K.-Y. Modified Donor Lymphocyte Infusion (DLI) for the Prophylaxis of Leukemia Relapse after Hematopoietic Stem Cell Transplantation in Patients with Advanced Leukemia—Feasibility and Safety Study. J. Clin. Immunol. 2008, 28, 390–397. [Google Scholar] [CrossRef]
- Dominietto, A.; Pozzi, S.; Miglino, M.; Albarracin, F.; Piaggio, G.; Bertolotti, F.; Grasso, R.; Zupo, S.; Raiola, A.M.; Gobbi, M.; et al. Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood 2007, 109, 5063–5064. [Google Scholar] [CrossRef] [Green Version]
- Rettinger, E.; Willasch, A.M.; Kreyenberg, H.; Borkhardt, A.; Holter, W.; Kremens, B.; Strahm, B.; Woessmann, W.; Mauz-Koerholz, C.; Gruhn, B.; et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood 2011, 118, 5681–5688. [Google Scholar] [CrossRef] [Green Version]
- Tsirigotis, P.; Byrne, M.; Schmid, C.; Baron, F.; Ciceri, F.; Esteve, J.; Gorin, N.C.; Giebel, S.; Mohty, M.; Savani, B.N.; et al. Relapse of AML after hematopoietic stem cell transplantation: Methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016, 51, 1431–1438. [Google Scholar] [CrossRef]
- Zeiser, R.; Beelen, D.W.; Bethge, W.; Bornhäuser, M.; Bug, G.; Burchert, A.; Christopeit, M.; Duyster, J.; Finke, J.; Gerbitz, A.; et al. Biology-Driven Approaches to Prevent and Treat Relapse of Myeloid Neoplasia after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, e128–e140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, A.M.; Norden, J.; Li, S.; Hromadnikova, I.; Schmid, C.; Schmetzer, H.; Jochem-Kolb, H. Graft-versus-Leukemia Effect Following Hematopoietic Stem Cell Transplantation for Leukemia. Front. Immunol. 2017, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Matte-Martone, C.; Jain, D.; McNiff, J.; Shlomchik, W.D. Central Memory CD8+T Cells Induce Graft-versus-Host Disease and Mediate Graft-versus-Leukemia. J. Immunol. 2009, 182, 5938–5948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulmy, E. Human minor histocompatibility antigens: New concepts for marrow transplantation and adoptive immunotherapy. Immunol. Rev. 1997, 157, 125–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkenburg, J.H.F.; van de Corput, L.; Marijt, E.W.A.; Willemze, R. Minor histocompatibility antigens in human stem cell transplantation. Exp. Hematol. 2003, 31, 743–751. [Google Scholar] [CrossRef]
- Miller, J.S.; Warren, E.H.; van den Brink, M.R.M.; Ritz, J.; Shlomchik, W.D.; Murphy, W.J.; Barrett, A.J.; Kolb, H.J.; Giralt, S.; Bishop, M.R.; et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol. Blood Marrow Transplant. 2010, 16, 565–586. [Google Scholar] [CrossRef] [Green Version]
- Schmid, C.; Schleuning, M.; Ledderose, G.; Tischer, J.; Kolb, H.-J. Sequential Regimen of Chemotherapy, Reduced-Intensity Conditioning for Allogeneic Stem-Cell Transplantation, and Prophylactic Donor Lymphocyte Transfusion in High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. J. Clin. Oncol. 2005, 23, 5675–5687. [Google Scholar] [CrossRef]
- Jedlickova, Z.; Schmid, C.; Koenecke, C.; Hertenstein, B.; Baurmann, H.; Schwerdtfeger, R.; Tischer, J.; Kolb, H.-J.; Schleuning, M. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 663–667. [Google Scholar] [CrossRef]
- Streitz, M.; Miloud, T.; Kapinsky, M.; Reed, M.R.; Magari, R.; Geissler, E.K.; Hutchinson, J.A.; Vogt, K.; Schlickeiser, S.; Kverneland, A.H.; et al. Standardization of whole blood immune phenotype monitoring for clinical trials: Panels and methods from the ONE study. Transplant. Res. 2013, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Waidhauser, J.; Schuh, A.; Trepel, M.; Schmälter, A.-K.; Rank, A. Chemotherapy markedly reduces B cells but not T cells and NK cells in patients with cancer. Cancer Immunol. Immunother. 2020, 69, 147–157. [Google Scholar] [CrossRef]
- Rank, A.; Löhr, P.; Hoffmann, R.; Ebigbo, A.; Grützner, S.; Schmid, C.; Claus, R. Sustained cellular immunity in adults recovered from mild COVID-19. Cytom. Part A 2021, 99, 429–434. [Google Scholar] [CrossRef]
- Schmaelter, A.-K.; Labopin, M.; Socié, G.; Itälä-Remes, M.; Blaise, D.; Yakoub-Agha, I.; Forcade, E.; Cornelissen, J.; Ganser, A.; Beelen, D.; et al. Inferior outcome of allogeneic stem cell transplantation for secondary acute myeloid leukemia in first complete remission as compared to de novo acute myeloid leukemia. Blood Cancer J. 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Brink, M.R.M.; Velardi, E.; Perales, M.-A. Immune reconstitution following stem cell transplantation. Hematology 2015, 2015, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, S.; Vasileiou, S.; Aguayo-Hiraldo, P.; Mukhi, S.; Sasa, G.; Martinez, C.; Krance, R.A.; Gottschalk, S.; Leen, A. Toward Functional Immune Monitoring in Allogeneic Stem Cell Transplant Recipients. Biol. Blood Marrow Transplant. 2020, 26, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Ogonek, J.; Juric, M.K.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, S.; Schmitt, M.; Götz, M.; Döhner, H.; Wiesneth, M.; Bunjes, D.; Greiner, J. Donor lymphocyte infusion leads to diversity of specific T cell responses and reduces regulatory T cell frequency in clinical responders. Int. J. Cancer 2019, 144, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Van Balen, P.; Van Bergen, C.A.M.; Van Luxemburg-Heijs, S.A.P.; De Klerk, W.; Van Egmond, E.H.M.; Veld, S.A.J.; Halkes, C.J.M.; Zwaginga, J.-J.; Griffioen, M.; Jedema, I.; et al. CD4 Donor Lymphocyte Infusion Can Cause Conversion of Chimerism Without GVHD by Inducing Immune Responses Targeting Minor Histocompatibility Antigens in HLA Class II. Front. Immunol. 2018, 9, 3016. [Google Scholar] [CrossRef] [Green Version]
- Van Bergen, C.A.M.; Van Luxemburg-Heijs, S.A.P.; De Wreede, L.C.; Eefting, M.; Von Dem Borne, P.A.; Van Balen, P.; Heemskerk, M.H.M.; Mulder, A.; Claas, F.H.J.; Navarrete, M.A.; et al. Selective graft-versus-leukemia depends on magnitude and diversity of the alloreactive T cell response. J. Clin. Investig. 2017, 127, 517–529. [Google Scholar] [CrossRef]
- Bleakley, M.; Riddell, S.R. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat. Rev. Cancer 2004, 4, 371–380. [Google Scholar] [CrossRef]
- Bouvier, A.; Riou, J.; Thepot, S.; Del Galy, A.S.; François, S.; Schmidt, A.; Orvain, C.; Estienne, M.-H.; Villate, A.; Paz, D.L.; et al. Quantitative chimerism in CD3-negative mononuclear cells predicts prognosis in acute myeloid leukemia patients after hematopoietic stem cell transplantation. Leukemia 2020, 34, 1342–1353. [Google Scholar] [CrossRef]
- Zorn, E.; Wang, K.S.; Hochberg, E.P.; Canning, C.; Alyea, E.P.; Soiffer, R.J.; Ritz, J. Infusion of CD4+ donor lymphocytes induces the expansion of CD8+ donor T cells with cytolytic activity directed against recipient hematopoietic cells. Clin. Cancer Res. 2002, 8, 2052–2060. [Google Scholar] [PubMed]
- Greiner, J.; Ono, Y.; Hofmann, S.; Schmitt, A.; Mehring, E.; Götz, M.; Guillaume, P.; Döhner, K.; Mytilineos, J.; Döhner, H.; et al. Mutated regions of nucleophosmin 1 elicit both CD4(+) and CD8(+) T-cell responses in patients with acute myeloid leukemia. Blood 2012, 120, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Soiffer, R.J.; Alyea, E.P.; Hochberg, E.; Wu, C.; Canning, C.; Parikh, B.; Zahrieh, D.; Webb, I.; Antin, J.; Ritz, J. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol. Blood Marrow Transplant. 2002, 8, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Orr, M.T.; Lanier, L.L. Natural Killer Cell Education and Tolerance. Cell 2010, 142, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, L.; Mancusi, A.; Burchielli, E.; Capanni, M.; Carotti, A.; Aloisi, T.; Aversa, F.; Martelli, M.F.; Velardi, A. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol. Dis. 2008, 40, 84–90. [Google Scholar] [CrossRef]
- Uzhachenko, R.V.; Shanker, A. CD8+ T Lymphocyte and NK Cell Network: Circuitry in the Cytotoxic Domain of Immunity. Front. Immunol. 2019, 10, 1906. [Google Scholar] [CrossRef] [Green Version]
- Paz Morante, M.; Briones, J.; Canto, E.; Sabzevari, H.; Martino, R.; Sierra, J.; Rodriguez-Sanchez, J.L.; Vidal, S. Activation-associated phenotype of CD3+ T cells in acute graft-versus-host disease. Clin. Exp. Immunol. 2006, 145, 36–43. [Google Scholar] [CrossRef]
- Ortí, G.; Palacio-Garcia, C.; García-Cadenas, I.; Sánchez-Ortega, I.; Jimenez, M.J.; Azqueta, C.; Villacampa, G.; Ferrà, C.; Parody, R.; Martino, R.; et al. Analysis of Cell Subsets in Donor Lymphocyte Infusions from HLA Identical Sibling Donors after Allogeneic Hematopoietic Cell Transplant. Transplant. Cell. Ther. 2020, 27, 53.e1–53.e8. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, C.; Arcese, W.; Dazzi, F.; Brand, R.; Bunjes, D.; Verdonck, L.F.; Schattenberg, A.; Kolb, H.-J.; Ljungman, P.; Devergie, A.; et al. Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: Prognostic relevance of the initial cell dose. Blood 2002, 100, 397–405. [Google Scholar] [CrossRef] [PubMed]
| Variables | Results | ||
|---|---|---|---|
| Age; median/average (range) | 55.5/56.9 (41–70) | ||
| Gender | |||
| male; n (%) | 11 (68.75) | ||
| female; n (%) | 5 (31.25) | ||
| Donor | |||
| URD; n (%) | 10 (62.5) | ||
| MSD; n (%) | 3 (18.8) | ||
| Haplo; n (%) | 3 (18.8) | ||
| Indication for alloSCT | |||
| AML; n (%) | 10 (62.5) | ||
| de novo AML; n (% of AML) | 3 (30) | ||
| sAML; n (% of AML) | 6 (60) | ||
| tAML; n (% of AML) | 1 (10) | ||
| CML; n (%) | 2 (12.5) | ||
| Multiple Myeloma; n (%) | 3 (18.75) | ||
| Pro-B-ALL; n (%) | 1 (6.25) | ||
| Indication for DLI | |||
| Prophylactic *; n (%) | 5 (31.3) | ||
| Therapeutic **; n (%) | 3 (18.8) | ||
| Preemptive *; n (%) | 8 (50) | ||
| Mixed chimerism; n | 6 | ||
| Molecular relapse; n | 1 | ||
| Mixed chimerism and molecular relapse; n | 1 | ||
| Initial DLI dose | |||
| 2 × 105 CD3+ lymphocytes/kg | 10 (62.5) | ||
| 1 × 106 CD3+ lymphocytes/kg | 5 (31.3) | ||
| 5 × 106 CD3+ lymphocytes/kg | 1 * (6) | ||
| Time alloSCT to 1. DLI; median (range in months) | 8 (5–44) | ||
| Time 1. DLI to 2. DLI; median (range in days) | 28 (21–215) | ||
| Time 2. DLI to 3. DLI; median (range in days) | 28 (27–177) | ||
| Dose | 1 × 106 | 5 × 106 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d − 1 | d + 1 | p (d − 1 vs. d + 1) | d + 7 | p (d − 1 vs. d + 7) | d − 1 | d + 1 | p (d − 1 vs. d + 1) | d + 7 | p (d − 1 vs. d + 7) | |
| CD4+ Lymphocytes | 137 [104;326] | 166 [42;290] | 0.929 | 159 [104;410] | 0.11 | 166 [117;358] | 219 [119;400] | 0.033 | 165 [50;383] | 0.721 |
| memory | 125 [101;286] | 146 [39;254] | 0.929 | 156 [85;365] | 0.066 | 155 [107;318] | 197 [155;359] | 0.028 | 158 [38;340] | 0.859 |
| central memory | 62 [21;618] | 69 [18;141] | 0.424 | 65 [33;225] | 0.093 | 69 [30;191] | 95 [32;232] | 0.019 | 83 [19;210] | 0.953 |
| naive | 8 [1;45] | 8 [1;55] | 0.859 | 9 [5;47] | 0.011 | 10 [1;57] | 11 [1;46] | 0.182 | 9 [2;52] | 0.441 |
| effector memory | 64 [37;151] | 77 [21;164] | 1 | 84 [45;249] | 0.214 | 80 [44;140] | 86 [37;138] | 0.286 | 71 [22;139] | 0.953 |
| EMRA | 24 [1;90] | 22 [2;70] | 0.213 | 37 [2;108] | 0.441 | 31 [3;77] | 31 [3;69] | 0.093 | 26 [1;75] | 0.26 |
| HLA-DR+ | 62 [12;90] | 56 [9;103] | 0.722 | 74 [10;149] | 0.327 | 68 [12;145] | 83 [11;150] | 0.117 | 66 [2;143] | 0.333 |
| CD69+ | 11 [3;22] | 8 [2;20] | 0.424 | 9 [4;166] | 0.26 | 6 [3;20] | 9 [3;28] | 0.272 | 6 [4;19] | 0.959 |
| Th1 | 14 [1;62] | 17 [3;39] | 0.286 | 19 [6;87] | 0.26 | 21 [1;55] | 30 [2;72] | 0.209 | 21 [1;54] | 0.594 |
| Th2 | 13 [2;56] | 9 [6;42] | 0.213 | 21 [2;131] | 0.401 | 15 [1;53] | 15 [1;53] | 0.099 | 16 [2;42] | 0.646 |
| Th17 | 12 [0;64] | 7 [0;57] | 0.333 | 7 [2;76] | 0.889 | 11 [2;74] | 13 [2;70] | 0.155 | 11 [0;78] | 0.878 |
| regulatory | 2 [1;26] | 2 [1;22] | 1 | 2 [1;27] | 0.678 | 2 [1;19] | 3 [1;20] | 0.003 | 2 [1;5] | 0.721 |
| CD25+ | 1 [0;13] | 1 [0;13] | 0.374 | 1 [0;10] | 0.594 | 1 [0;7] | 2 [0;8] | 0.019 | 1 [1;2] | 0.959 |
| Dose | 1 × 106 | 5 × 106 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d − 1 | d + 1 | p (d − 1 vs. d + 1) | d + 7 | p (d − 1 vs. d + 7) | d − 1 | d + 1 | p (d − 1 vs. d + 1) | d + 7 | p (d − 1 vs. d + 7) | |
| CD8+ Lymphocytes | 276 [51;617] | 345 [39;868] | 0.023 | 604 [62;869] | 0.011 | 267 [44;1078] | 394 [91;1289] | 0.007 | 344 [90;816] | 0.575 |
| CD4+CD8+ | 1 [0;0] | 2 [0;11] | 0.285 | 3 [0;15] | 0.327 | 2 [0;12] | 3 [0;9] | 0.959 | 3 [0;11] | 0.674 |
| memory | 136 [10;439] | 204 [21;625] | 0.11 | 216 [31;620] | 0.038 | 155 [20;670] | 168 [68;803] | 0.019 | 176 [58;523] | 0.374 |
| central memory | 38 [0;143] | 49 [0;130] | 0.075 | 56.5 [0;114] | 0.314 | 57 [2;193] | 62 [2;164] | 0.173 | 65 [1;126] | 0.26 |
| naive | 17 [1;67] | 18 [1;90] | 0.041 | 32 [3;119] | 0.028 | 20 [7;225] | 31 [10;218] | 0.099 | 26 [8;124] | 0.515 |
| effector memory | 147 [29;396] | 158 [23;581] | 0.05 | 264 [29;615] | 0.028 | 120 [22;524] | 130 [44;704] | 0.075 | 127 [42;590] | 0.515 |
| EMRA | 43 [6;281] | 46 [9;369] | 0.041 | 117 [4;376] | 0.011 | 87 [13;602] | 89 [8;757] | 0.015 | 49 [6;131] | 0.953 |
| early | 97 [20;273] | 100 [15;236] | 0.123 | 82 [22;668] | 0.038 | 111 [25;390] | 150 [54;398] | 0.05 | 113 [52;255] | 0.878 |
| intermediate | 40 [16;79] | 52 [11;118] | 0.015 | 46 [11;130] | 0.015 | 34 [12;147] | 51 [19;205] | 0.039 | 49 [17;95] | 0.386 |
| late | 53 [10;441] | 43 [12;633] | 0.05 | 317 [8;644] | 0.021 | 60 [6;703] | 93 [13;910] | 0.039 | 71 [14;640] | 0.285 |
| exhausted | 134 [32;459] | 210 [26;675] | 0.034 | 266 [41;558] | 0.021 | 170 [26;465] | 232 [63;437] | 0.013 | 240 [65;383] | 0.878 |
| terminal effector | 31 [1;141] | 29 [2;341] | 0.075 | 182 [1;229] | 0.011 | 30 [1;549] | 38 [1;678] | 0.131 | 34 [1;389] | 0.139 |
| HLA-DR+ | 168 [11;549] | 165 [8;740] | 0.041 | 371 [12;766] | 0.009 | 205 [9;701] | 225 [16;813] | 0.007 | 220 [7;602] | 0.799 |
| CD69+ | 15 [3;66] | 12 [6;69] | 0.534 | 25 [4;192] | 0.26 | 16 [4;208] | 19 [9;203] | 0.182 | 15 [4;73] | 0.721 |
| regulatory | 0 [0;7] | 0 [0;7] | 0.678 | 0 [0;10] | 0.463 | 0 [0;1] | 0 [0;4] | 0.075 | 0 [0;4] | 0.5 |
| CD25+ | 0 [0;1] | 0 [0;2] | 0.878 | 0 [0;1] | 0.249 | 0 [0;1] | 0 [0;1] | 0.721 | 0 [0;0] | 0.499 |
| CD56+ T cells | 7 [1;59] | 9 [0;41] | 0.041 | 7 [2;60] | 0.021 | 6 [1;52] | 9 [2;48] | 0.017 | 7 [1;35] | 0.767 |
| Dose | 1 × 106 | 5 × 106 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d − 1 | d + 1 | p (d − 1 vs. d + 1) | d + 7 | p (d − 1 vs. d + 7) | d − 1 | d + 1 | p (d − 1 vs. d + 1) | d + 7 | p (d − 1 vs. d + 7) | |
| NK cells | 158 [88;441] | 196 [125;369] | 1.0 | 231 [66;322] | 0.374 | 162 [93;401] | 208 [93;387] | 0.917 | 189 [127;438] | 0.037 |
| CD56+CD16+ | 97 [44;289] | 106 [62;252] | 0.695 | 112 [43;251] | 0.26 | 104 [49;289] | 104 [66;280] | 0.463 | 130 [64;305] | 0.017 |
| CD56bright CD16dim | 36 [18;229] | 42 [17;203] | 0.328 | 36 [13;223] | 0.859 | 36 [13;211] | 37 [16;76] | 0.552 | 29 [15;101] | 0.285 |
| CD56dim CD16bright | 23 [10;158] | 31 [8;86] | 0.859 | 37 [6;78] | 0.374 | 27 [7;120] | 20 [4;101] | 0.917 | 23 [12;164] | 0.445 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmaelter, A.-K.; Waidhauser, J.; Kaiser, D.; Lenskaja, T.; Gruetzner, S.; Claus, R.; Trepel, M.; Schmid, C.; Rank, A. Alterations of Peripheral Blood T Cell Subsets following Donor Lymphocyte Infusion in Patients after Allogeneic Stem Cell Transplantation. Hemato 2021, 2, 692-702. https://doi.org/10.3390/hemato2040046
Schmaelter A-K, Waidhauser J, Kaiser D, Lenskaja T, Gruetzner S, Claus R, Trepel M, Schmid C, Rank A. Alterations of Peripheral Blood T Cell Subsets following Donor Lymphocyte Infusion in Patients after Allogeneic Stem Cell Transplantation. Hemato. 2021; 2(4):692-702. https://doi.org/10.3390/hemato2040046
Chicago/Turabian StyleSchmaelter, Ann-Kristin, Johanna Waidhauser, Dina Kaiser, Tatjana Lenskaja, Stefanie Gruetzner, Rainer Claus, Martin Trepel, Christoph Schmid, and Andreas Rank. 2021. "Alterations of Peripheral Blood T Cell Subsets following Donor Lymphocyte Infusion in Patients after Allogeneic Stem Cell Transplantation" Hemato 2, no. 4: 692-702. https://doi.org/10.3390/hemato2040046
APA StyleSchmaelter, A.-K., Waidhauser, J., Kaiser, D., Lenskaja, T., Gruetzner, S., Claus, R., Trepel, M., Schmid, C., & Rank, A. (2021). Alterations of Peripheral Blood T Cell Subsets following Donor Lymphocyte Infusion in Patients after Allogeneic Stem Cell Transplantation. Hemato, 2(4), 692-702. https://doi.org/10.3390/hemato2040046





