Recombinant Clostridium acetobutylicum Endoxylanase for Xylooligosaccharide Production from Pretreated Lignocellulosic Biomass
Abstract
1. Introduction
2. Materials and Methods
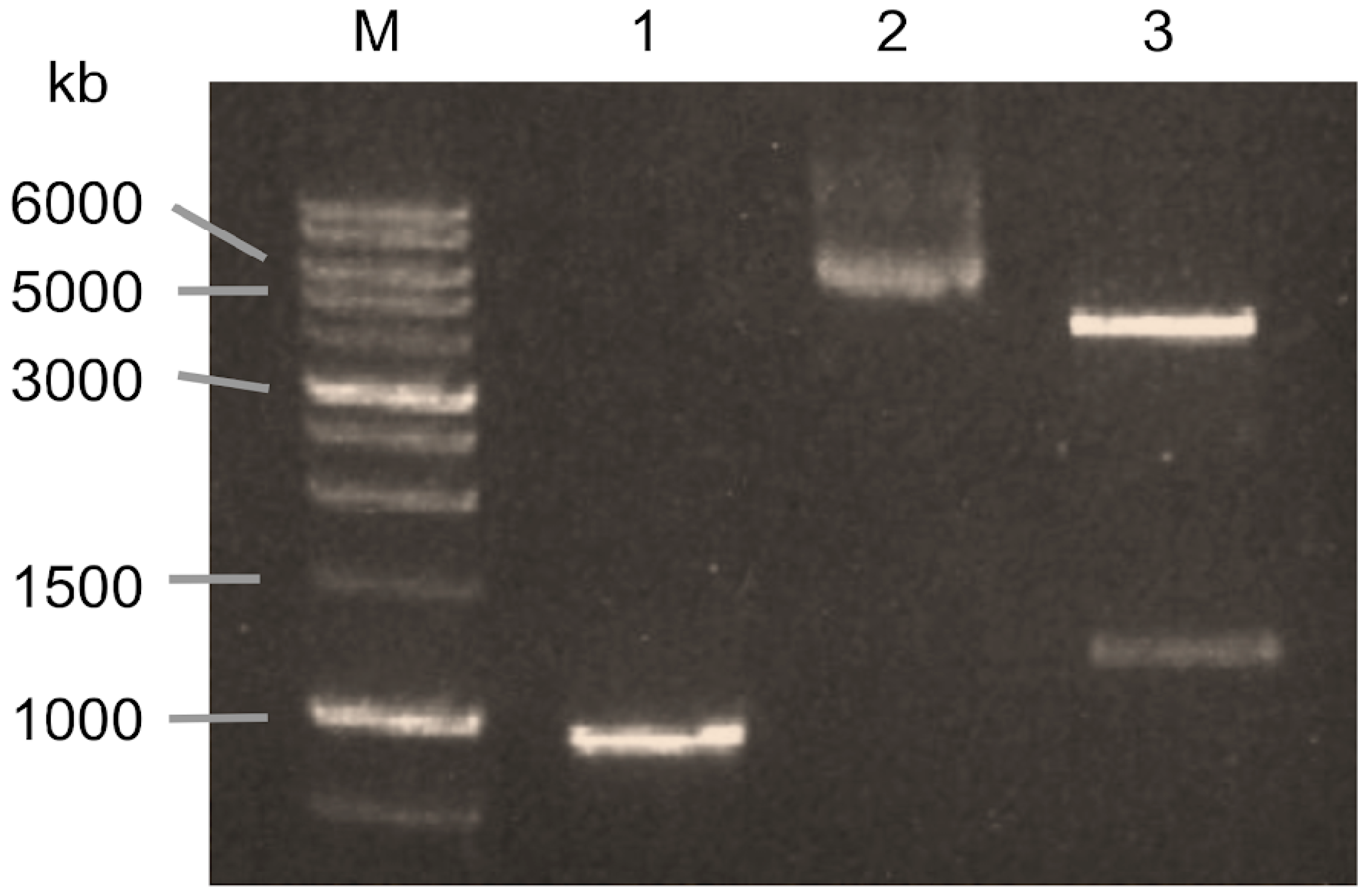
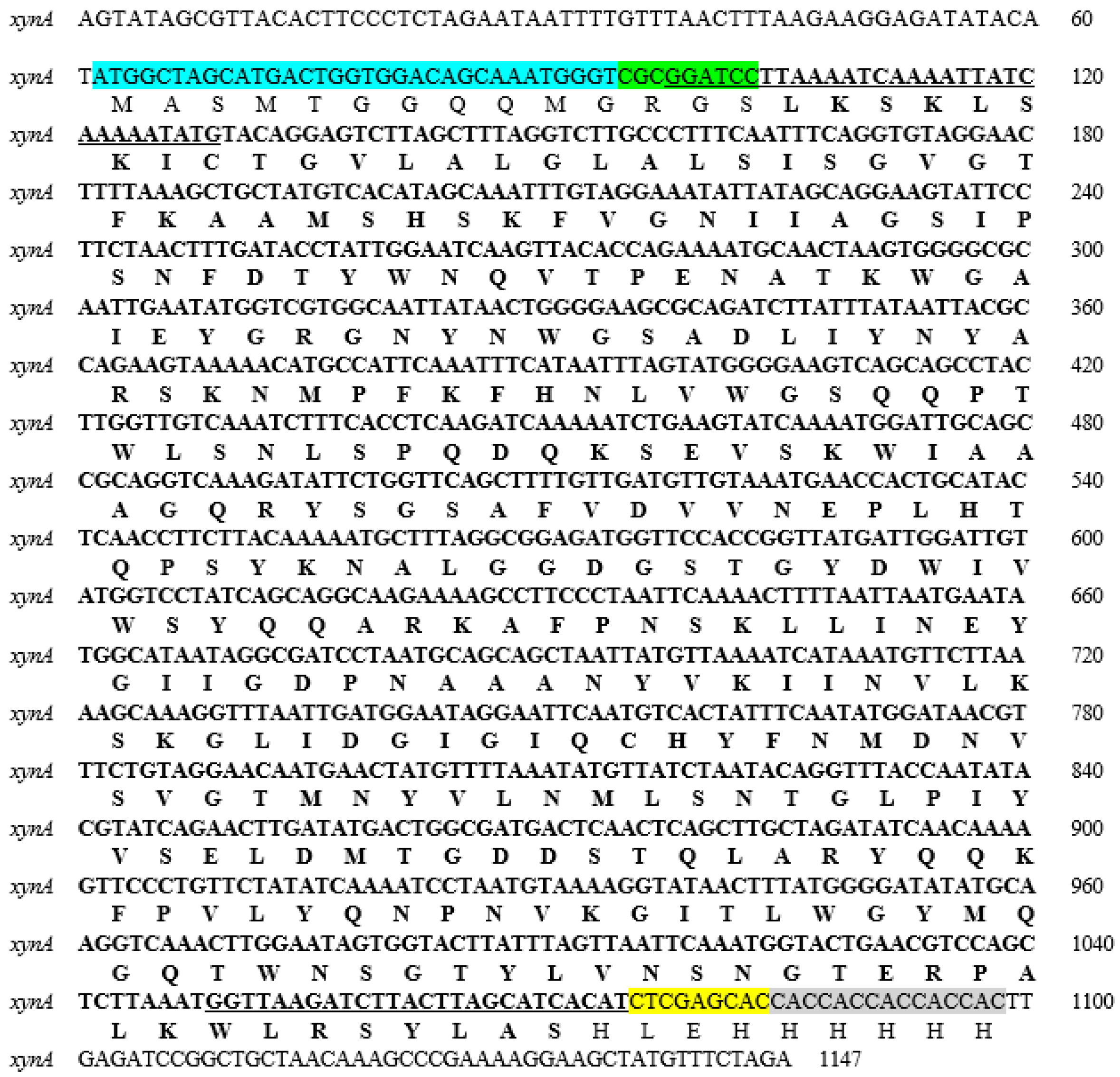
2.1. Cloning and Expression of Endo-Xylanase from C. acetobutylicum
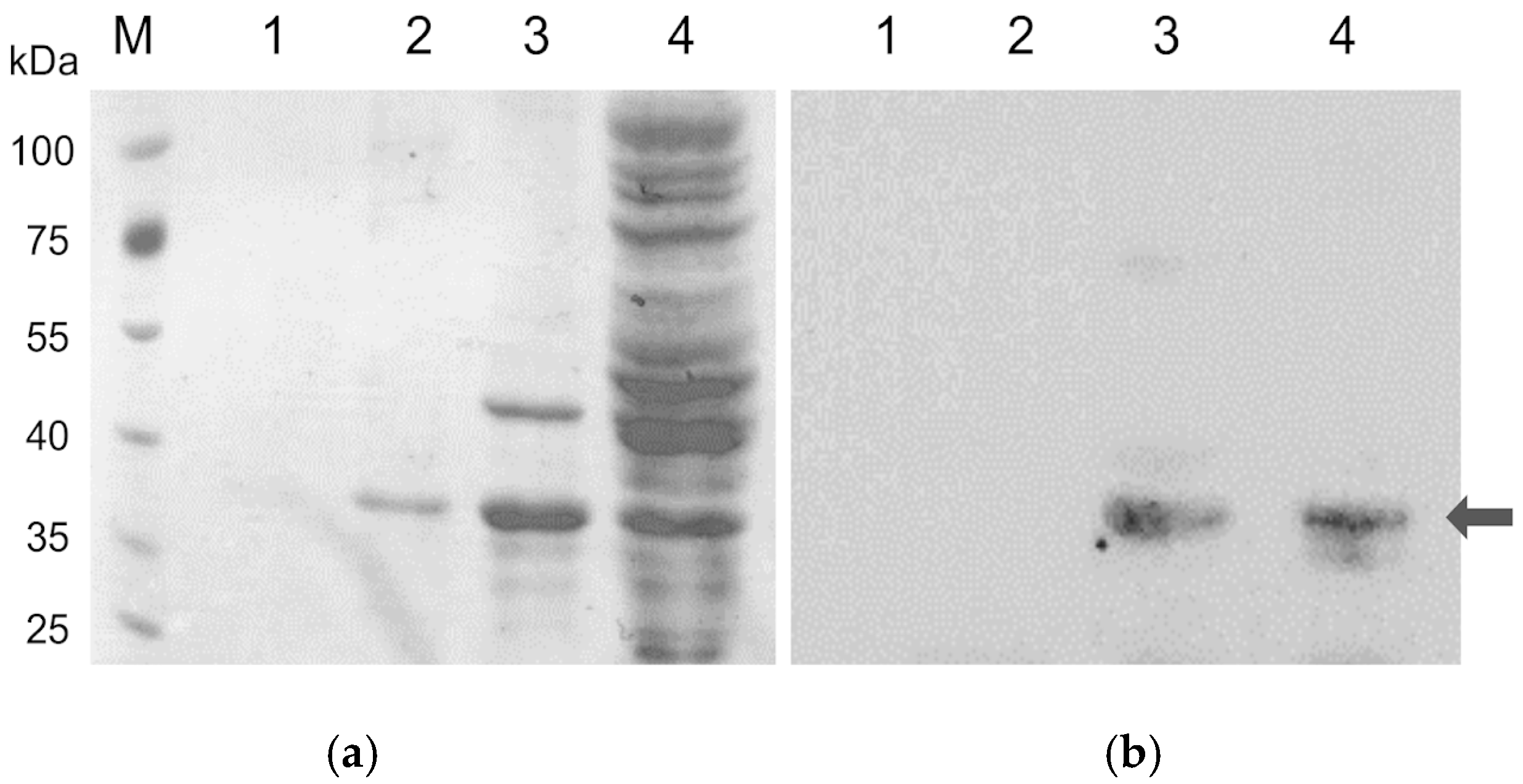
2.2. SDS-PAGE and Western Blot Analysis
2.3. Enzyme Activity and Substrate Specificity Assay
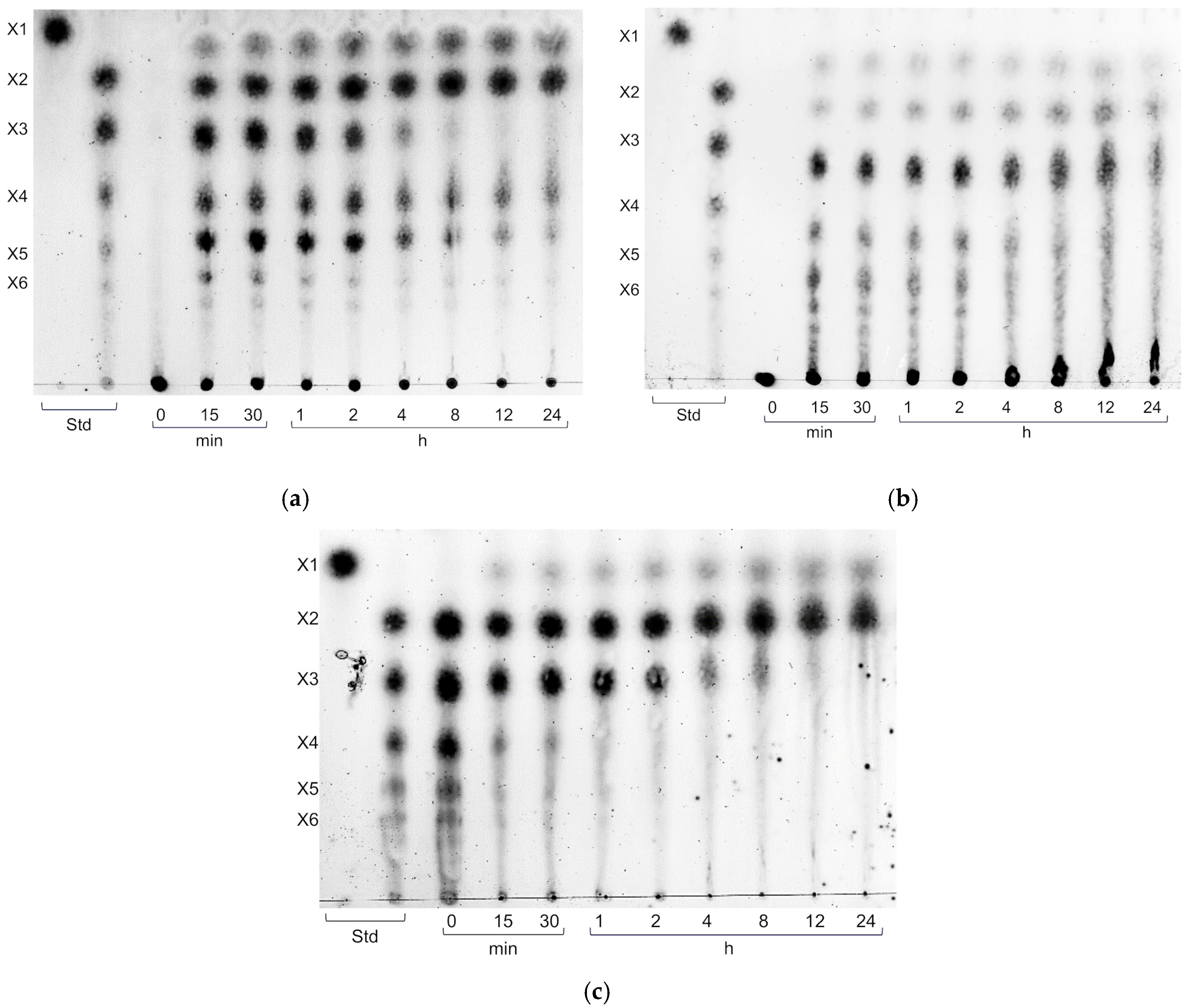
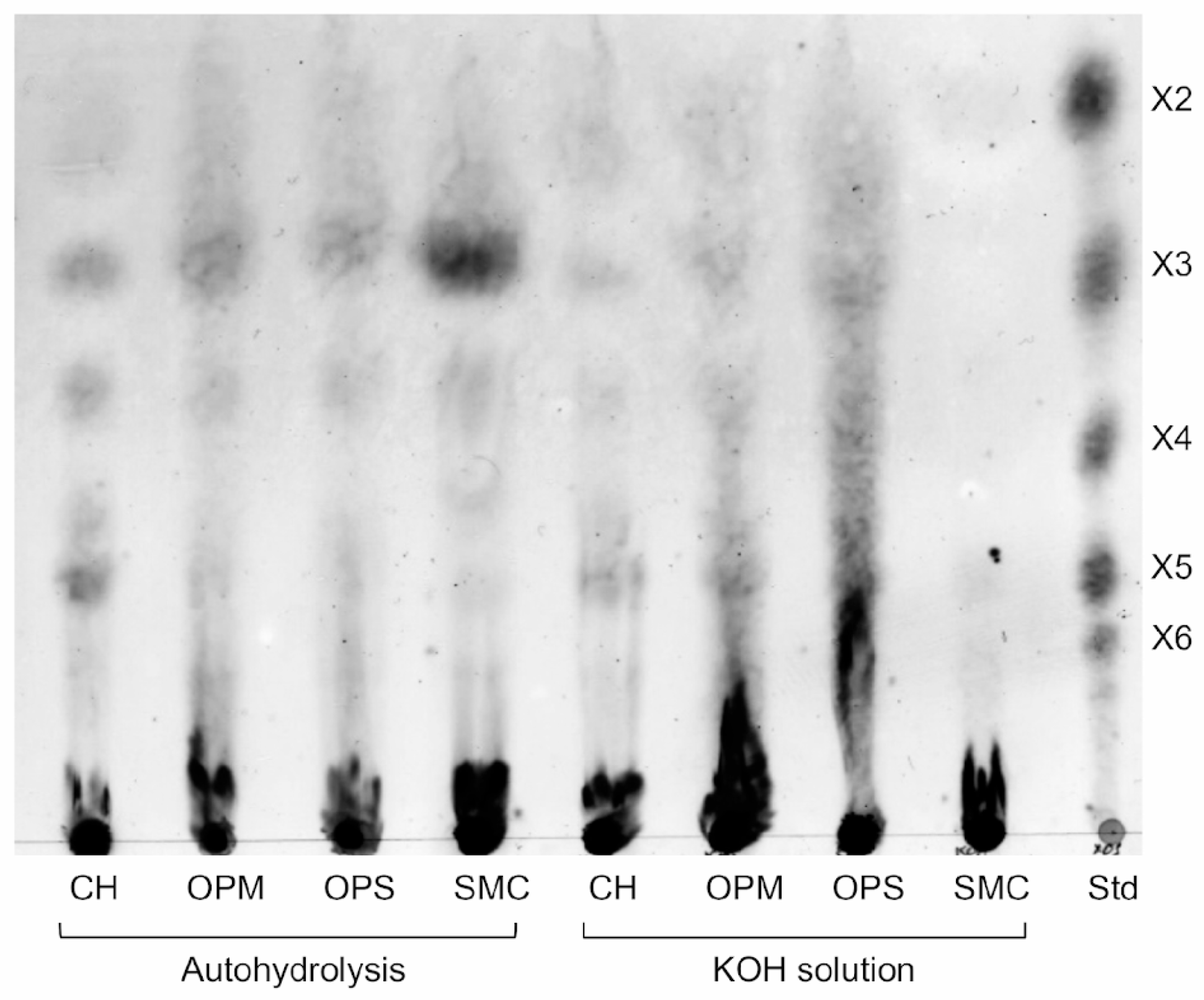
2.4. Hydrolysis Product Analysis
2.5. Biomass Selection and Pretreatment
3. Results
3.1. Cloning and Expression of XynA from C. acetobutylicum
3.2. Endo-Xylanase Activity of XynA
3.3. Pretreatment of Agricultural Biomass and Enzymatic Hydrolysis by XynA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| XOS | Xylooligosaccharides |
| TLC | Thin-Layer Chromatography |
| SDS-PAGE | Sodium Dodecyl Sulphate-Poly Acrylamide Gel Electrophoresis |
| IPTG | isopropyl β-D-1-thiogalactopyranoside |
| OPS | Oil Palm Shell |
| OPM | Oil Palm Mesocarp |
| CH | Coconut Husk |
| SMC | Spent Mushroom Compost |
| DP | Degree of Polymerization |
| CMC | Carboxyl Methyl Cellulose |
References
- Kumar, S.; Lohan, S.K.; Parihar, D.S. Biomass energy from agriculture: Conversion techniques and use. In Handbook of Energy Management in Agriculture; Rakshit, A., Biswas, A., Sarkar, D., Meena, V.S., Datta, R., Eds.; Springer Nature: Singapore, 2023; pp. 3–38. [Google Scholar] [CrossRef]
- Kumari, K.; Singh, A.; Marathe, D.; Pariyar, P. Agricultural biomass as value chain developers in different sectors. In Advanced Technology for the Conversion of Waste into Fuels and Chemicals; Khan, A., Jawaid, M., Pizzi, A., Azum, N., Asiri, A., Isa, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 467–509. [Google Scholar]
- Kumari, K.; Nagar, S.; Goyal, S.; Maan, S.; Kumar, V.; Kharor, N.; Sindhu, M.; Kumar, V. A fast, reliable, low-cost, and efficient xylan extraction for xylooligosaccharides production. Biofuels Bioprod. Bioref. 2024, 18, 1355–1368. [Google Scholar] [CrossRef]
- Peralta, A.G.; Venkatachalam, S.; Stone, S.C.; Pattathil, S. Xylan epitope profiling: An enhanced approach to study organ development-dependent changes in xylan structure, biosynthesis, and deposition in plant cell walls. Biotechnol. Biofuels 2017, 10, 245. [Google Scholar] [CrossRef]
- Kaprelyants, L.; Zhurlova, O.; Shpyrko, T.; Pozhitkova, L. Xylooligosaccharides from agricultural by-products: Characterisation, production and physiological effects. Harčova Nauka Tehnol. 2017, 11, 25–34. [Google Scholar] [CrossRef]
- Praveen, K.G.; Pushpa, A.; Prabha, H. Value addition of orange fruit wastes in the enzymatic production of xylooligosaccharides. Afr. J. Biotechnol. 2017, 16, 1324–1330. [Google Scholar] [CrossRef][Green Version]
- Chaiyates, R.; Mahakhan, P.; Sawaengkaew, J. Production of xylo-oligosaccharides from corncob using high efficiency xylanase from Trichoderma harzianum 4FR8. Biomass Conv. Bioref. 2024, 15, 19175–19187. [Google Scholar] [CrossRef]
- Li, T.; Lei, X.; Wang, L.; Liu, C.; Qiu, Q.; Li, Y.; Song, X.; Xiong, X.; Zang, Y.; Qu, M.; et al. Production of xylo-oligosaccharides with degree of polymerization 3–5 from wheat straw xylan by a xylanase derived from rumen metagenome and utilization by probiotics. Food Biosci. 2023, 56, 103360. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.; Moniz, P. Hydrothermal/Liquid Hot Water Pretreatment (Autohydrolysis). In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 315–347. [Google Scholar] [CrossRef]
- Hao, N.; Bezerra, T.L.; Wu, Q.; Ben, H.; Sun, Q.; Adhikari, S.; Ragauskas, A.J. Effect of autohydrolysis pretreatment on biomass structure and the resulting bio-oil from a pyrolysis process. Fuel 2017, 206, 494–503. [Google Scholar] [CrossRef]
- Zhang, W.; You, Y.; Lei, F.; Li, P.; Jiang, J. Acetyl-assisted autohydrolysis of sugarcane bagasse for the production of xylo-oligosaccharides without additional chemicals. Bioresour. Technol. 2018, 265, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Rigual, V.; Santos, T.M.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Evaluation of hardwood and softwood fractionation using autohydrolysis and ionic liquid microwave pretreatment. Biomass Bioenerg. 2018, 117, 190–197. [Google Scholar] [CrossRef]
- Ali, M.K.; Rudolph, F.B.; Bennett, G.N. Thermostable xylanase10B from Clostridium acetobutylicum ATCC824. J. Ind. Microbiol. Biotechnol. 2004, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Rudolph, F.B.; Bennett, G.N. Characterization of thermostable Xyn10A enzyme from mesophilic Clostridium acetobutylicum ATCC 824. J. Ind. Microbiol. Biotechnol. 2005, 32, 12–18. [Google Scholar] [CrossRef]
- Lee, S.F.; Forsberg, C.W.; Gibbins, L.N. Xylanolytic activity of Clostridium acetobutylicum. Appl. Environ. Microbiol. 1985, 50, 1068–1076. [Google Scholar] [CrossRef]
- Kramer, M.F.; Coen, D.M. Enzymatic amplification of DNA by PCR: Standard procedures and optimization. Curr. Protoc. Cell Biol. 2001, 10, A.3F.1–A.3F.14. [Google Scholar] [CrossRef]
- Růčková, E.; Müller, P.; Vojtěšek, B. Protein expression and purification. Klin. Onkol. 2014, 27 (Suppl. S1), S92–S98. [Google Scholar] [CrossRef][Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Cotta, M.A. Utilization of xylooligosaccharides by selected ruminal bacteria. Appl. Environ. Microbiol. 1993, 59, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Effect of autohydrolysis and enzymatic treatment on oil palm (Elaeis guineensis Jacq.) frond fibres for xylose and xylooligosaccharides production. Bioresour. Technol. 2011, 102, 1234–1239. [Google Scholar] [CrossRef]
- Nasir, M.A.M.; Saleh, S.H. Characterization of hemicelluloses from oil palm empty fruit bunches obtained by alkaline extraction and ethanol precipitation. Malays. J. Anal. Sci. 2016, 20, 849–855. [Google Scholar] [CrossRef]
- Chakdar, H.; Kumar, M.; Pandiyan, K.; Singh, A.; Nanjappan, K.; Kashyap, P.L.; Srivastava, A.K. Bacterial xylanases: Biology to biotechnology. 3 Biotech 2016, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Hoffmam, Z.B.; de Matos Martins, V.P.; Zanphorlin, L.M.; De Paula Assis, L.H.; Honorato, R.V. Molecular mechanisms associated with xylan degradation by Xanthomonas plant pathogens. J. Biol. Chem. 2014, 289, 32186–32200. [Google Scholar] [CrossRef] [PubMed]
- Jurak, E.; Patyshakuliyeva, A.; Kapsokalyvas, D.; Xing, L.; Van Zandvoort, M.A.M.J.; De Vries, R.P.; Gruppen, H.; Kabel, M.A. Accumulation of recalcitrant xylan in mushroom-compost is due to a lack of xylan substituent removing enzyme activities of Agaricus bisporus. Carbohydr. Polym. 2015, 132, 359–368. [Google Scholar] [CrossRef]
- Jurak, E.; Patyshakuliyeva, A.; De Vries, R.P.; Gruppen, H.; Kabel, M.A. Compost grown Agaricus bisporus lacks the ability to degrade and consume highly substituted xylan fragments. PLoS ONE 2015, 10, e0134169. [Google Scholar] [CrossRef] [PubMed]
- Kapu, N.U.S.; Manning, M.; Hurley, T.B.; Voigt, J.; Cosgrove, D.J.; Romaine, C.P. Surfactant-assisted pretreatment and enzymatic hydrolysis of spent mushroom compost for the production of sugars. Bioresour. Technol. 2012, 114, 399–405. [Google Scholar] [CrossRef]
- Zhao, S.; Lau, R.; Chen, M.H. Influence of chain length on the colonic fermentation of xylooligosaccharides. Carbohydr. Polym. 2024, 331, 121869. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, M.J.; Seo, S.; Yoo, S.; Hong, J.H. Relative sweetness and sweetness quality of xylobiose. Food Sci. Biotechnol. 2017, 26, 689–696. [Google Scholar] [CrossRef]
| Substrate | Reducing Sugar Produced (mg/mL) |
|---|---|
| oat spelt xylan | 1489.78 ± 3.33 |
| beechwood xylan | 1560.61 ± 3.21 |
| rye arabinoxylan | 1119.78 ± 26.13 |
| wheat arabinoxylan | 345.89 ± 12.88 |
| XOS | 291.72 ± 3.85 |
| β-glucan | ND 1 |
| CMC | ND |
| lichenan | ND |
| avicel | ND |
| cellulose | ND |
| p-Nitrophenyl Xylopiranoside | ND |
| Pretreatment Method | Biomass | Hemicellulose Yield (%) | Reducing Sugar Produced (mg/mL) |
|---|---|---|---|
| Autohydrolysis | OPM 2 | 4.37 ± 0.20 | 155.33 ± 10.09 |
| OPS 3 | 1.19 ± 0.18 | 93.67 ± 10.00 | |
| CH 4 | 3.32 ± 0.25 | 179.78 ± 26.69 | |
| SMC 5 | 8.90 ± 0.14 | 1 ND | |
| KOH soaking | OPM | 16.10 ± 0.58 | 77.00 ± 2.22 |
| OPS | 3.32 ± 0.74 | 59.59 ± 4.49 | |
| CH | 5.30 ± 0.59 | 185.15 ± 9.45 | |
| SMC | 74.83 ± 0.71 | ND | |
| KOH soaking with ethanol precipitation | OPM | 10.73 ± 0.28 | 299.22 ± 8.68 |
| OPS | 1.84 ± 0.79 | 299.59 ± 10.26 | |
| CH | 16.10 ± 1.15 | ND | |
| SMC | 17.50 ± 0.51 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husna, A.; Wardani, A.K.; Hu, C.-Y.; Chen, Y.-C. Recombinant Clostridium acetobutylicum Endoxylanase for Xylooligosaccharide Production from Pretreated Lignocellulosic Biomass. BioTech 2025, 14, 85. https://doi.org/10.3390/biotech14040085
Husna A, Wardani AK, Hu C-Y, Chen Y-C. Recombinant Clostridium acetobutylicum Endoxylanase for Xylooligosaccharide Production from Pretreated Lignocellulosic Biomass. BioTech. 2025; 14(4):85. https://doi.org/10.3390/biotech14040085
Chicago/Turabian StyleHusna, Afifa, Agustin Krisna Wardani, Chun-Yi Hu, and Yo-Chia Chen. 2025. "Recombinant Clostridium acetobutylicum Endoxylanase for Xylooligosaccharide Production from Pretreated Lignocellulosic Biomass" BioTech 14, no. 4: 85. https://doi.org/10.3390/biotech14040085
APA StyleHusna, A., Wardani, A. K., Hu, C.-Y., & Chen, Y.-C. (2025). Recombinant Clostridium acetobutylicum Endoxylanase for Xylooligosaccharide Production from Pretreated Lignocellulosic Biomass. BioTech, 14(4), 85. https://doi.org/10.3390/biotech14040085








