Enhanced Succinate Production in Actinobacillus succinogenes via Neutral Red Bypass Reduction in a Novel Bioelectrochemical System
Abstract
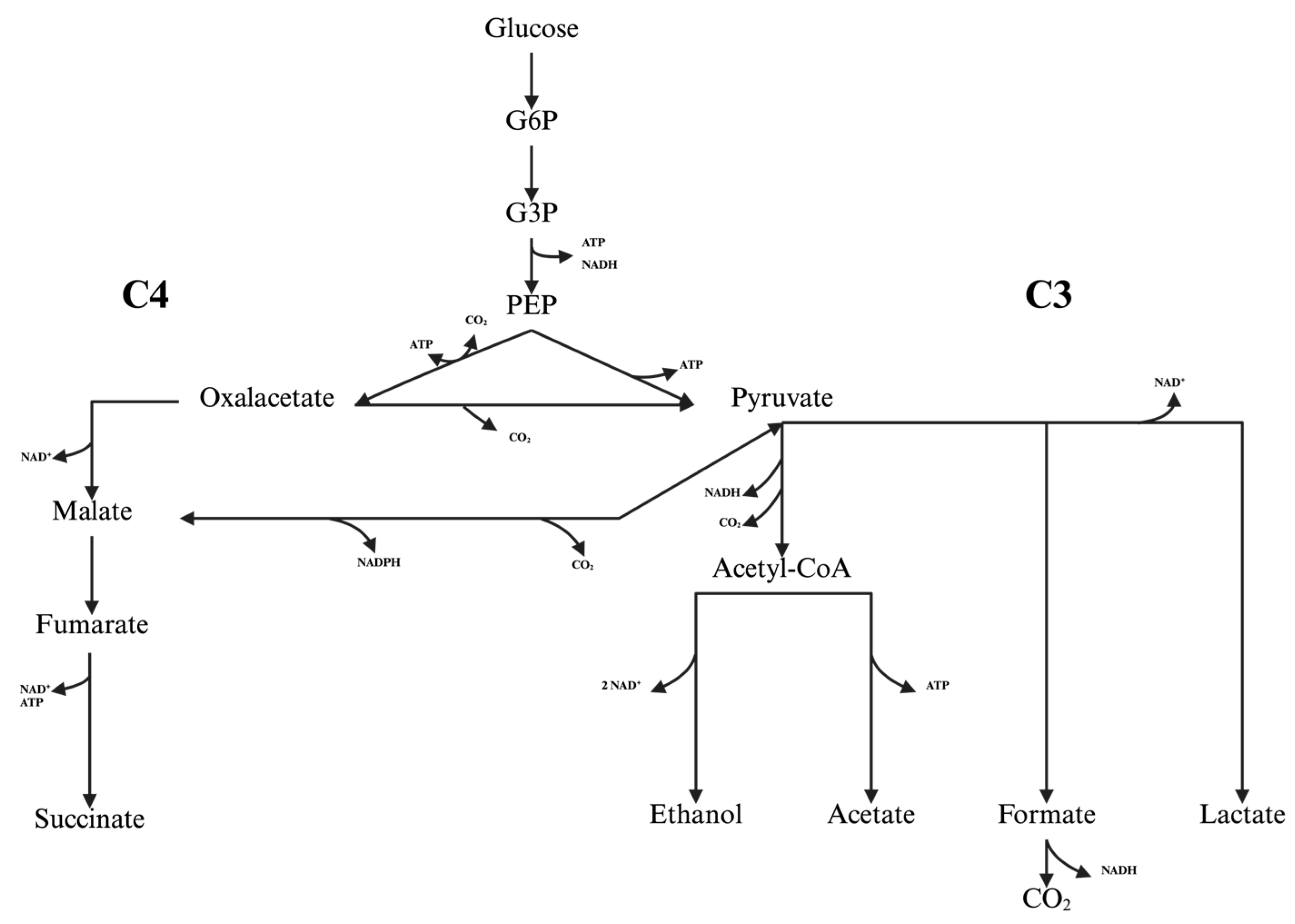
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Bioreactor Operation and Experimental Setup
2.3. Analytical Procedures
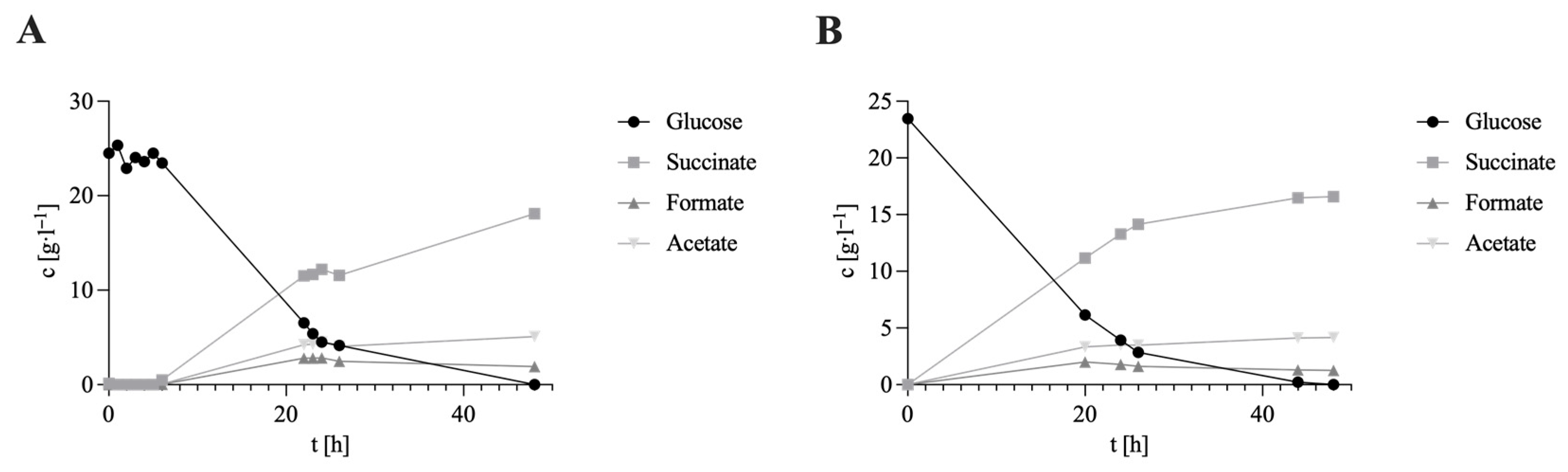
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hengsbach, J.; Cwienczek, M.; Laudensack, W.; Stiefelmaier, J.; Tippkötter, N.; Ulber, R. Succinic Acid Production With Actinobacillus succinogenes–Influence of an Electric Potential on the Intercellular NADH/NAD+ Balance. Eng. Life Sci. 2024, 25, e202400053. [Google Scholar] [CrossRef] [PubMed]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an Intermediate in Metabolism, Signal Transduction, ROS, Hypoxia, and Tumorigenesis. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-W.; Tang, Y.-J. Current Advances of Succinate Biosynthesis in Metabolically Engineered Escherichia coli. Biotechnol. Adv. 2017, 35, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of Succinic Acid by Metabolically Engineered Microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Succinic Acid: New Bio-Based Building Block with a Huge Market and Environmental Potential? Available online: https://renewable-carbon.eu/news/succinic-acid-new-bio-based-building-block-with-a-huge-market-and-environmental-potential/ (accessed on 2 September 2025).
- Global Bio-Based Succinic Acid Market: Snapshot. Available online: https://www.transparencymarketresearch.com/bio-succinic-acid.html (accessed on 11 July 2024).
- McKinlay, J.B.; Zeikus, J.G.; Vieille, C. Insights into Actinobacil- Lus Succinogenes Fermentative Metabolism in a Chemically Defined Growth Medium. Appl. Environ. Microbiol. 2005, 71, 6651–6656. [Google Scholar] [CrossRef]
- Guettler, M.V.; Rumler, D.; Jain, M.K. Actinobacillus succinogenes Sp. Nov., a Novel Succinic-Acid-Producing Strain from the Bovine Rumen. Int. J. Syst. Evol. Microbiol. 1999, 49, 207–216. [Google Scholar] [CrossRef]
- Lee, P.; Lee, S.; Hong, S.; Chang, H. Isolation and Characterization of a New Succinic Acid-Producing Bacterium, Mannheimia succiniciproducens MBEL55E, from Bovine Rumen. Appl. Microbiol. Biotechnol. 2002, 58, 663–668. [Google Scholar] [CrossRef]
- Thakker, C.; Martínez, I.; San, K.; Bennett, G.N. Succinate Production in Escherichia coli. Biotechnol. J. 2012, 7, 213–224. [Google Scholar] [CrossRef]
- Chung, S.-C.; Park, J.-S.; Yun, J.; Park, J.H. Improvement of Succinate Production by Release of End-Product Inhibition in Corynebacterium Glutamicum. Metab. Eng. 2017, 40, 157–164. [Google Scholar] [CrossRef]
- Otero, J.M.; Cimini, D.; Patil, K.R.; Poulsen, S.G.; Olsson, L.; Nielsen, J. Industrial Systems Biology of Saccharomyces Cerevisiae Enables Novel Succinic Acid Cell Factory. PLoS ONE 2013, 8, e54144. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, J.B.; Laivenieks, M.; Schindler, B.D.; McKinlay, A.A.; Siddaramappa, S.; Challacombe, J.F.; Lowry, S.R.; Clum, A.; Lapidus, A.L.; Burkhart, K.B.; et al. A Genomic Perspective on the Potential of Actinobacillus succinogenes for Industrial Succinate Production. BMC Genom. 2010, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Miguel, J.; Vilaça, P.; Soares, S.; Rocha, I.; Carneiro, S. Reconstruction of a Genome-Scale Metabolic Model for Actinobacillus succinogenes 130Z. BMC Syst. Biol. 2018, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J.; Stryer, L. Stryer Biochemie; SpringerLink Bücher; 8. Auflage; Springer Spektrum: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-54619-2. [Google Scholar]
- Almqvist, H.; Pateraki, C.; Alexandri, M.; Koutinas, A.; Lidén, G. Succinic Acid Production by Actinobacillus succinogenes from Batch Fermentation of Mixed Sugars. J. Ind. Microbiol. Biotechnol. 2016, 43, 1117–1130. [Google Scholar] [CrossRef]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How To Drive Fermentation Using Electrochemical Systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbes Electricity To Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. mBio 2010, 1, e00103-10. [Google Scholar] [CrossRef]
- Dutta, P.K.; Keller, J.; Yuan, Z.; Rozendal, R.A.; Rabaey, K. Role of Sulfur during Acetate Oxidation in Biological Anodes. Environ. Sci. Technol. 2009, 43, 3839–3845. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella Secretes Flavins That Mediate Extracellular Elec- Tron Transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Meng, J.; Sun, K.; Yan, H. A Neutral Red Mediated Electro-Fermentation System of Clostridium Beijerinckii for Effective Co-Production of Butanol and Hydrogen. Bioresour. Technol. 2021, 332, 125097. [Google Scholar] [CrossRef]
- Park, D.H.; Zeikus, J.G. Utilization of Electrically Reduced Neutral Red by Actinobacillus succinogenes: Physiological Function of Neutral Red in Membrane-Driven Fumarate Reduction and Energy Conservation. J. Bacteriol. 1999, 181, 2403–2410. [Google Scholar] [CrossRef]
- Guerrero, K.; Gallardo, R.; González, E.; Veliz, F.; Conejeros, R.; Gentina, J.C.; Aroca, G. Butanol Production by Clostridium Aceto- Butylicum ATCC 824 Using Electro-Fermentation in Culture Medium Supplemented with Butyrate and Neutral Red. J. Chem. Tech. Biotech. 2022, 97, 1526–1535. [Google Scholar] [CrossRef]
- Krieg, T.; Madjarov, J.; Rosa, L.F.M.; Enzmann, F.; Harnisch, F.; Holtmann, D.; Rabaey, K. Reactors for Microbial Electrobiotechnology. In Bioelectrosynthesis; Harnisch, F., Holtmann, D., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2018; Volume 167, pp. 231–271. ISBN 978-3-030-03298-2. [Google Scholar]
- Huang, J.; Zeng, C.; Luo, H.; Bai, J.; Liu, G.; Zhang, R. Enhanced Sulfur Recovery and Sulfate Reduction Using Single-Chamber Bioelectrochemical System. Sci. Total Environ. 2022, 823, 153789. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morita, M.; Sasaki, D.; Matsumoto, N.; Ohmura, N.; Igarashi, Y. Single-Chamber Bioelectrochemical Hydrogen Fermentation from Garbage Slurry. Biochem. Eng. J. 2012, 68, 104–108. [Google Scholar] [CrossRef]
- Logroño, W.; Pérez, M.; Urquizo, G.; Kadier, A.; Echeverría, M.; Recalde, C.; Rákhely, G. Single Chamber Microbial Fuel Cell (SCMFC) with a Cathodic Microalgal Biofilm: A Preliminary Assessment of the Generation of Bioelectricity and Biodegradation of Real Dye Textile Wastewater. Chemosphere 2017, 176, 378–388. [Google Scholar] [CrossRef]
- Anoy, M.M.I.; Hill, E.A.; Garcia, M.R.; Kim, W.-J.; Beliaev, A.S.; Beyenal, H. A Directional Electrode Separator Improves Anodic Biofilm Current Density in a Well-Mixed Single-Chamber Bioelectrochemical System. Enzym. Microb. Technol. 2024, 180, 110502. [Google Scholar] [CrossRef]
- Rader, G.K.; Logan, B.E. Multi-Electrode Continuous Flow Microbial Electrolysis Cell for Biogas Production from Acetate. Int. J. Hydrogen Energy 2010, 35, 8848–8854. [Google Scholar] [CrossRef]
- Yan, H.; Saito, T.; Regan, J.M. Nitrogen Removal in a Single-Chamber Microbial Fuel Cell with Nitrifying Biofilm Enriched at the Air Cathode. Water Res. 2012, 46, 2215–2224. [Google Scholar] [CrossRef]
- Raghavulu, S.V.; Mohan, S.V.; Goud, R.K.; Sarma, P.N. Effect of Anodic pH Microenvironment on Microbial Fuel Cell (MFC) Performance in Concurrence with Aerated and Ferricyanide Catholytes. Electrochem. Commun. 2009, 11, 371–375. [Google Scholar] [CrossRef]
- Liu, S.Y.; Charles, W.; Ho, G.; Cord-Ruwisch, R.; Cheng, K.Y. Bioelectrochemical Enhancement of Anaerobic Digestion: Comparing Single- and Two-Chamber Reactor Configurations at Thermophilic Conditions. Bioresour. Technol. 2017, 245, 1168–1175. [Google Scholar] [CrossRef]
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef]
- Tix, J.; Gotthardt, L.; Bode, J.; Karabacak, B.; Nordmann, J.; Hengsbach, J.-N.; Ulber, R.; Tippkötter, N. Enhancement of Succinic Acid Production by Actinobacillus succinogenes in an Electro-Bioreactor. Fermentation 2024, 10, 504. [Google Scholar] [CrossRef]
- Pateraki, C.; Magdalinou, E.; Skliros, D.; Flemetakis, E.; Rabaey, K.; Koutinas, A. Transcriptional Regulation in Key Metabolic Pathways of Actinobacillus succinogenes in the Presence of Electricity. Bioelectrochemistry 2023, 151, 108376. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, S.; Li, Y.; Wang, Z.; Chen, L. Enhanced Succinic Acid Production and Electronic Utilization Efficiency by Actinobacillus succinogenes 130Z in an ORP-Controlled Microbial Electrolysis Cell System. Fermentation 2024, 10, 109. [Google Scholar] [CrossRef]
- 545: TRYPTONE SOYA BROTH (TSB). Available online: https://www.dsmz.de/microorganisms/medium/pdf/DSMZ_Medium545.pdf (accessed on 3 July 2024).
- Wang, Z.; Li, H.; Feng, J.; Zhang, A.; Ying, H.; He, X.; Jiang, M.; Chen, K.; Ouyang, P. Enhanced Succinic Acid Production from Polyacrylamide-pretreated Cane Molasses in Microbial Electrolysis Cells. J. Chem. Tech. Biotech. 2018, 93, 855–860. [Google Scholar] [CrossRef]
- Tix, J.; Moll, F.; Krafft, S.; Betsch, M.; Tippkötter, N. Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga Neapolitana. Energies 2024, 17, 2938. [Google Scholar] [CrossRef]
- Fathey, R.; Gomaa, O.M.; Ali, A.E.-H.; El Kareem, H.A.; Zaid, M.A. Neutral Red as a Mediator for the Enhancement of Electricity Production Using a Domestic Wastewater Double Chamber Microbial Fuel Cell. Ann. Microbiol. 2016, 66, 695–702. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Chou, Y.-C.; Salvachúa, D.; Mohagheghi, A.; St. John, P.C.; Peterson, D.J.; Bomble, Y.J.; Beckham, G.T. Metabolic Engineering of Actinobacillus succinogenes Provides Insights into Succinic Acid Biosynthesis. Appl. Environ. Microbiol. 2017, 83, e00996-17. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, M.; Li, J.; Zheng, X.; Yang, Z.; Fang, X.; Ye, G. Effect of adding intermediate metabolites on succinate production by Actinobacillus succinogenes. Sheng Wu Gong Cheng Xue Bao 2010, 26, 1249–1256. [Google Scholar]
- McKinlay, J.B.; Vieille, C. 13C-Metabolic Flux Analysis of Actinobacillus succinogenes Fermentative Metabolism at Different NaHCO3 and H2 Concentrations. Metab. Eng. 2008, 10, 55–68. [Google Scholar] [CrossRef]
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus succinogenes: Advances on Succinic Acid Production and Prospects for Development of Integrated Biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Park, D.H.; Zeikus, J.G. Electricity Generation in Microbial Fuel Cells Using Neutral Red as an Electronophore. Appl. Environ. Microbiol. 2000, 66, 1292–1297. [Google Scholar] [CrossRef]
- Harrington, T.D.; Tran, V.N.; Mohamed, A.; Renslow, R.; Biria, S.; Orfe, L.; Call, D.R.; Beyenal, H. The Mechanism of Neutral Red-Mediated Microbial Electrosynthesis in Escherichia coli: Menaquinone Reduction. Bioresour. Technol. 2015, 192, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Tix, J.; Hengsbach, J.-N.; Bode, J.; Pedraza, F.; Willer, J.; Park, S.J.; Reardon, K.F.; Ulber, R.; Tippkötter, N. Actinobacillus succinogenes in Bioelectrochemical Systems: Influence of Electric Potentials and Carbon Fabric Electrodes on Fermentation Performance. Microorganisms 2025, 13, 1720. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, J.; Arkatkar, A. Anodic Catalyst and Mediators for Tailoring the Biochemical Behaviour of Microbial Fuel Cell System. Discov. Electrochem. 2025, 2, 41. [Google Scholar] [CrossRef]
- Gemünde, A.; Lai, B.; Pause, L.; Krömer, J.; Holtmann, D. Redox Mediators in Microbial Electrochemical Systems. ChemElectroChem 2022, 9, e202200216. [Google Scholar] [CrossRef]
- Kastury, F.; Juhasz, A.; Beckmann, S.; Manefield, M. Ecotoxicity of Neutral Red (Dye) and Its Environmental Applications. Ecotoxicol. Environ. Saf. 2015, 122, 186–192. [Google Scholar] [CrossRef]
- Van der Werf, M.J.V.; Guettler, M.V.; Jain, M.K.; Zeikus, J.G. Environmental and Physiological Factors Affecting the Succinate Product Ratio during Carbohydrate Fermentation by Actinobacillus Sp. 130Z. Arch. Microbiol. 1997, 167, 332–342. [Google Scholar] [CrossRef]
- Sund, C.J.; McMasters, S.; Crittenden, S.R.; Harrell, L.E.; Sumner, J.J. Effect of Electron Mediators on Current Generation and Fermentation in a Microbial Fuel Cell. Appl. Microbiol. Biotechnol. 2007, 76, 561–568. [Google Scholar] [CrossRef]


| Study (Year) | Reactor/Chamber Design | Electric Operation (Potential/Voltage) | Mediator | Notes from Methods |
|---|---|---|---|---|
| Park & Zeikus (1999) [23] | Two-chamber H-cell (membrane-separated) | Cathodic supply; ~−1.5 V | NR | First demonstration of electrically reduced NR serving as an external electron donor for A. succinogenes metabolism. |
| Pateraki et al. (2023) [36] | BES (no bypass) | Applied potential (exact value n.d.) | n.d. | Investigated transcriptional regulation in A. succinogenes under electricity; confirms redox-linked gene expression shifts. |
| Peng et al. (2024) [37] | Two-chamber MEC | ORP-controlled (−400 mV) and pulsed −1 V (0.5 s on/off) | NR (0.1 mM) | Cathodic enhancement of succinate formation; carbon-felt electrodes, 280 mL per chamber. |
| Tix et al. (2024) [35] | Single-chamber electro-bioreactor (STR-based) | −600 mV vs. Ag/AgCl | NR (0.1 µM) and more tested | 2 L STR; 0.25 vvm (80% N2/20% CO2); 37 °C; pH 6.8; controlled via BioFlo 120; carbon-fiber electrodes. |
| Hengsbach et al. (2024) [1] | BES (no bypass) | Controlled potential (~−600 mV range) | NR (0.1 mM) | Investigated potential effects on NAD+/NADH ratios; carbon fabric electrodes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tix, J.; Pedraza, F.; Ulber, R.; Tippkötter, N. Enhanced Succinate Production in Actinobacillus succinogenes via Neutral Red Bypass Reduction in a Novel Bioelectrochemical System. BioTech 2025, 14, 84. https://doi.org/10.3390/biotech14040084
Tix J, Pedraza F, Ulber R, Tippkötter N. Enhanced Succinate Production in Actinobacillus succinogenes via Neutral Red Bypass Reduction in a Novel Bioelectrochemical System. BioTech. 2025; 14(4):84. https://doi.org/10.3390/biotech14040084
Chicago/Turabian StyleTix, Julian, Fernando Pedraza, Roland Ulber, and Nils Tippkötter. 2025. "Enhanced Succinate Production in Actinobacillus succinogenes via Neutral Red Bypass Reduction in a Novel Bioelectrochemical System" BioTech 14, no. 4: 84. https://doi.org/10.3390/biotech14040084
APA StyleTix, J., Pedraza, F., Ulber, R., & Tippkötter, N. (2025). Enhanced Succinate Production in Actinobacillus succinogenes via Neutral Red Bypass Reduction in a Novel Bioelectrochemical System. BioTech, 14(4), 84. https://doi.org/10.3390/biotech14040084






