Biomaterials and Tissue Engineering in Neurosurgery: Current Innovations and Future Directions
Abstract
1. Introduction
2. Materials and Methods
3. Results
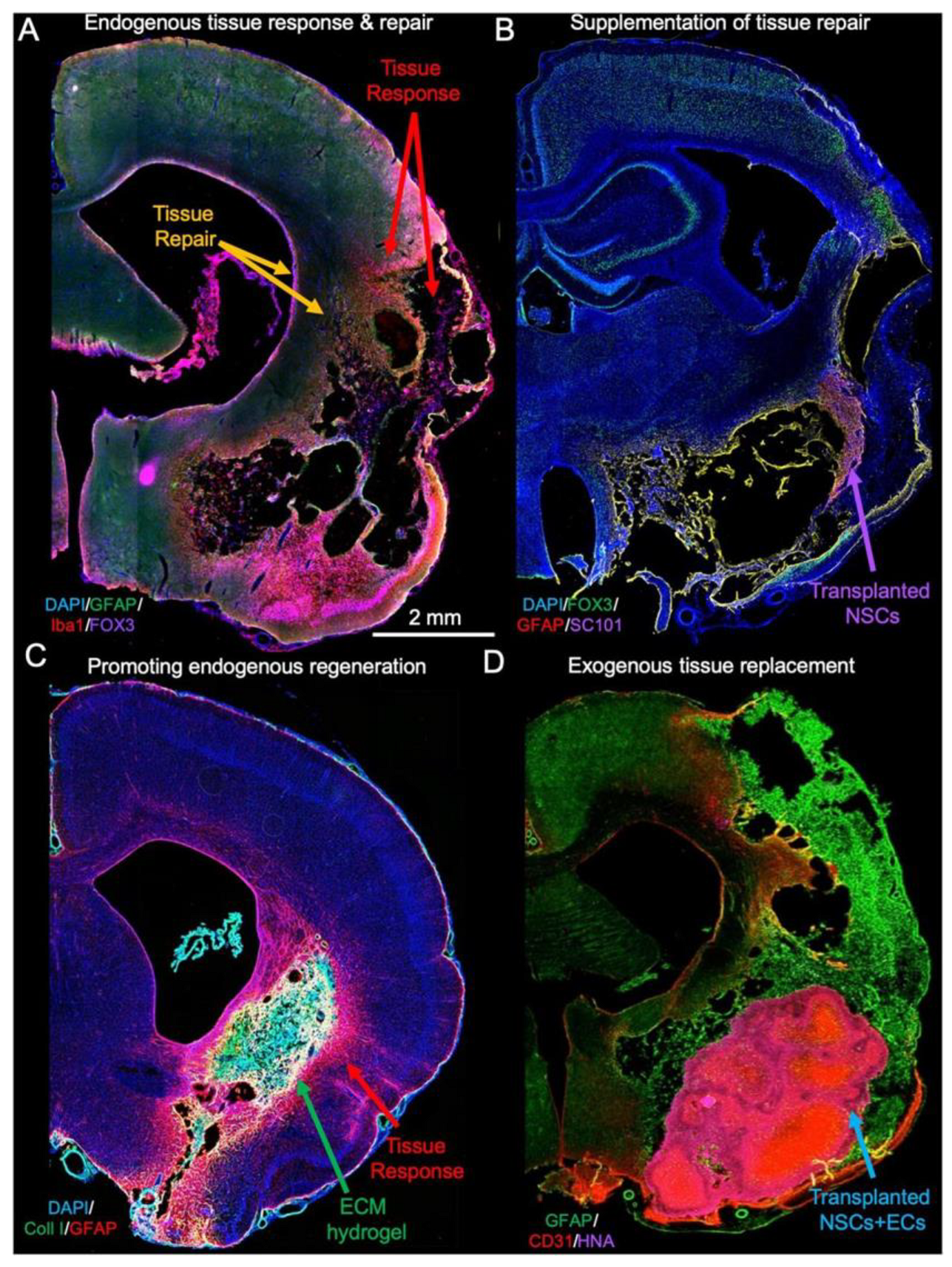
3.1. Brain Tissue Repair
3.1.1. Hydrogels
3.1.2. Nanomaterials and Fiber Scaffolds
3.1.3. Biocompatibility and Immune Modulation
3.2. Spinal Cord Injury and Spinal Implants
3.2.1. Tissue Engineering for Spinal Cord Repair
3.2.2. Bioactive Molecules and Controlled Delivery in SCI
3.3. Peripheral Nerve Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBI | Traumatic Brain Injury |
| SCI | Spinal Cord Injury |
| CNS | Central Nervous System |
| ECM | Extracellular Matrix |
| PLA | Poly(lactic acid) |
| PGA | Poly(glycolic acid) |
| PCL | Polycaprolactone |
| PEG | Polyethylene Glycol |
| HA | Hyaluronic Acid |
| BDNF | Brain-Derived Neurotrophic Factor |
| NGF | Nerve Growth Factor |
| CNTs | Carbon Nanotubes |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| dECM | Decellularized Extracellular Matrix |
| IL-10 | Interleukin-10 |
| PEEK | Polyether Ether Ketone |
| NSCs | Neural Stem Cells |
| MSCs | Mesenchymal Stem Cells |
| iPSC | Induced Pluripotent Stem Cell |
| NT-3 | Neurotrophin-3 |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| CSPGs | Chondroitin Sulfate Proteoglycans |
| NGCs | Nerve Guidance Conduits |
| PLGA | Poly(lactic-co-glycolic acid) |
| FDA | Food and Drug Administration |
References
- Liao, W.; Shi, Y.; Li, Z.; Yin, X. Advances in 3D printing combined with tissue engineering for nerve regeneration and repair. J. Nanobiotechnol. 2025, 23, 5. [Google Scholar] [CrossRef]
- Hammam, I.A.; Winters, R.; Hong, Z. Advancements in the Application of Biomaterials in Neural Tissue Engineering: A Review. Biomed. Eng. Adv. 2024, 8, 100132. [Google Scholar] [CrossRef]
- Zeng, C.-W. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Li, F.; Ducker, M.; Sun, B.; Szele, F.G.; Czernuszka, J.T. Interpenetrating polymer networks of collagen, hyaluronic acid, and chondroitin sulfate as scaffolds for brain tissue engineering. Acta Biomater. 2020, 112, 122–135. [Google Scholar] [CrossRef]
- Mishchenko, T.A.; Klimenko, M.O.; Kuznetsova, A.I.; Yarkov, R.S.; Savelyev, A.G.; Sochilina, A.V.; Mariyanats, A.O.; Popov, V.K.; Khaydukov, E.V.; Zvyagin, A.V.; et al. 3D-printed hyaluronic acid hydrogel scaffolds impregnated with neurotrophic factors (BDNF, GDNF) for post-traumatic brain tissue reconstruction. Front. Bioeng. Biotechnol. 2022, 10, 895406. [Google Scholar] [CrossRef]
- Hasanzadeh, E.; Seifalian, A.; Mellati, A.; Saremi, J.; Asadpour, S.; Enderami, S.E.; Nekounam, H.; Mahmoodi, N. Injectable hydrogels in central nervous system: Unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater. Today Bio 2023, 20, 100614. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Atapour-Mashhad, H.; Shahvali, S.; Salehi, B.; Shaban, M.; Shirzad, M.; Salahvarzi, A.; Mohammadi, M. Hydrogels as advanced drug delivery platforms for cancer immunotherapy: Promising innovations and future outlook. J. Nanobiotechnol. 2025, 23, 545. [Google Scholar] [CrossRef]
- Modo, M. Bioscaffold-Induced Brain Tissue Regeneration. Front. Neurosci. 2019, 13, 1156. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Luo, Z.; Yan, S.; You, R. Biofunctionalized silk fibroin nanofibers for directional and long neurite outgrowth. Biointerphases 2019, 14, 061001. [Google Scholar] [CrossRef]
- Xu, X.-M.; Deng, L.-X.; Liu, N.-K.; Wen, R.N.; Yang, S.-N.; Wen, X. Laminin-coated multifilament entubulation, combined with Schwann cells and glial cell line-derived neurotrophic factor, promotes unidirectional axonal regeneration in a rat model of thoracic spinal cord hemisection. Neural Regen. Res. 2020, 16, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Stadler, F.J.; Fu, M. Biomimetic electrospun tubular PLLA/gelatin nanofiber scaffold promoting regeneration of sciatic nerve transection in SD rat. Mater. Sci. Eng. C 2021, 121, 111858. [Google Scholar] [CrossRef] [PubMed]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Mungenast, L.; Nieminen, R.; Gaiser, C.; Faia-Torres, A.B.; Rühe, J.; Suter-Dick, L. Electrospun decellularized extracellular matrix scaffolds promote the regeneration of injured neurons. Biomater. Biosyst. 2023, 11, 100081. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Guan, X.; Ma, Z.; Shi, Q.; Panteleev, M.; Ataullakhanov, F.I. Bioactive fibrous scaffolds with programmable release of polypeptides regulate inflammation and extracellular matrix remodeling. Regen. Biomater. 2023, 10, rbad010. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, Y.; Yuan, T.; Zhang, Z.; Ge, J.; Meng, Q.; Li, Z.; Sun, S. Macrophages in aseptic loosening: Characteristics, functions, and mechanisms. Front. Immunol. 2023, 14, 1122057. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; He, M.; Mao, L.; Li, T.; Zhang, L.; Liu, L.; Feng, G.; Song, Y. Titanium-interlayer mediated hydroxyapatite coating on polyetheretherketone: A prospective study in patients with single-level cervical degenerative disc disease. J. Transl. Med. 2021, 19, 14. [Google Scholar] [CrossRef]
- Frankenberger, T.; Graw, C.L.; Engel, N.; Gerber, T.; Frerich, B.; Dau, M. Sustainable Surface Modification of Polyetheretherketone (PEEK) Implants by Hydroxyapatite/Silica Coating—An In Vivo Animal Study. Materials 2021, 14, 4589. [Google Scholar] [CrossRef]
- Chang, S.Y.; Kang, D.-H.; Cho, S.K. Innovative Developments in Lumbar Interbody Cage Materials and Design: A Comprehensive Narrative Review. Asian Spine J. 2024, 18, 444–457. [Google Scholar] [CrossRef]
- Sanchez, C.V.; Krag, A.E.; Barnett, S.; Welch, B.G.; Rozen, S.M. Polyetheretherketone Implant Cranioplasty for Large Cranial Defects: A Seven-Year Follow-Up. J. Craniofacial Surg. 2024, 35, 903–907. [Google Scholar] [CrossRef]
- Laperle, A.H.; Moser, V.A.; Avalos, P.; Lu, B.; Wu, A.; Fulton, A.; Ramirez, S.; Garcia, V.J.; Bell, S.; Ho, R.; et al. Human iPSC-derived neural progenitor cells secreting GDNF provide protection in rodent models of ALS and retinal degeneration. Stem Cell Rep. 2023, 18, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.-C.; Liu, Y.-Y.; Zhu, F.-Q.; Xiong, M.-J.; Hu, D.-X.; Zhang, W.-J. Therapeutic role of neural stem cells in neurological diseases. Front. Bioeng. Biotechnol. 2024, 12, 1329712. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Revuelta, J.; Bastida, A.; Gómez-Casado, V.; Civera, C.; Garrido, L.; García-Junceda, E.; Heras, Á.; Alcántara, A.R.; et al. Fast and Sustained Axonal Growth by BDNF Released from Chitosan Microspheres. Mar. Drugs 2023, 21, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Z.; Qiu, G.; Song, Y. Combination of Chondroitinase ABC, Glial Cell Line–Derived Neurotrophic Factor and Nogo A Antibody Delayed-Release Microspheres Promotes the Functional Recovery of Spinal Cord Injury. J. Craniofacial Surg. 2013, 24, 2153–2157. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, C.; Xu, J.; Zhai, H.; Liu, S.; Xu, Y.; Hu, Y.; Long, H.; Bai, Y.; Quan, D. Neurotrophin-3-Loaded Multichannel Nanofibrous Scaffolds Promoted Anti-Inflammation, Neuronal Differentiation, and Functional Recovery after Spinal Cord Injury. ACS Biomater. Sci. Eng. 2020, 6, 1228–1238. [Google Scholar] [CrossRef]
- Hey, G.; Willman, M.; Patel, A.; Goutnik, M.; Willman, J.; Lucke-Wold, B. Stem Cell Scaffolds for the Treatment of Spinal Cord Injury—A Review. Biomechanics 2023, 3, 322–342. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Wu, Y.; Liu, S. Biomaterials targeting the microenvironment for spinal cord injury repair: Progression and perspectives. Front. Cell. Neurosci. 2024, 18, 1362494. [Google Scholar] [CrossRef]
- Li, G.; Han, Q.; Lu, P.; Zhang, L.; Zhang, Y.; Chen, S.; Zhang, P.; Zhang, L.; Cui, W.; Wang, H.; et al. Construction of Dual-Biofunctionalized Chitosan/Collagen Scaffolds for Simultaneous Neovascularization and Nerve Regeneration. Research 2020, 2020, 2603048. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef]
- Lazarevic, M.; Petrovic, S.; Pierfelice, T.V.; Ignjatovic, N.; Piattelli, A.; Tovilovic, T.V.; Radunovic, M. Antimicrobial and Osteogenic Effects of Collagen Membrane Decorated with Chitosan–Nano-Hydroxyapatite. Biomolecules 2023, 13, 579. [Google Scholar] [CrossRef]
- Meder, T.; Prest, T.; Skillen, C.; Marchal, L.; Yupanqui, V.T.; Soletti, L.; Gardner, P.; Cheetham, J.; Brown, B.N. Nerve-specific extracellular matrix hydrogel promotes functional regeneration following nerve gap injury. NPJ Regen. Med. 2021, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, H.; Wan, T.; Jiang, H.-R.; Yang, M.; Wen, Y.-Q.; Zhang, P.-X. Micron track chitosan conduit fabricated by 3D-printed model topography provides bionic microenvironment for peripheral nerve regeneration. Int. J. Bioprinting 2023, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, R.J.; Chen, M.; Liu, X.Y.; Ma, K.; Xu, H.Y.; Deng, W.S.; Ye, Y.C.; Li, W.X.; Chen, X.Y.; et al. Collagen/heparan sulfate porous scaffolds loaded with neural stem cells improve neurological function in a rat model of traumatic brain injury. Neural Regen. Res. 2021, 16, 1068–1077. [Google Scholar] [CrossRef]
- Xu, J.; Hsu, S.-h. Self-healing hydrogel as an injectable implant: Translation in brain diseases. J. Biomed. Sci. 2023, 30, 43. [Google Scholar] [CrossRef]
- Licciardello, M.; Traldi, C.; Bortolameazzi, M.; Testore, D.; Ciardelli, G.; Tonda-Turo, C. Aligned polycaprolactone/polyaniline electrospun nanofibers for directing neural stem cell differentiation and neuron arrangement. Front. Biomater. Sci. 2024, 3, 1362599. [Google Scholar] [CrossRef]
- Kim, D.; Park, D.; Kim, T.H.; Chung, J.J.; Jung, Y.; Kim, S.H. Substance P/Heparin-Conjugated PLCL Mitigate Acute Gliosis on Neural Implants and Improve Neuronal Regeneration via Recruitment of Neural Stem Cells. Adv. Healthc. Mater. 2021, 10, 2100107. [Google Scholar] [CrossRef]
- Cheers, G.M.; Weimer, L.P.; Neuerburg, C.; Arnholdt, J.; Gilbert, F.; Thorwächter, C.; Holzapfel, B.M.; Mayer-Wagner, S.; Laubach, M. Advances in implants and bone graft types for lumbar spinal fusion surgery. Biomater. Sci. 2024, 12, 4875–4902. [Google Scholar] [CrossRef]
- da Silva, V.A.; Bobotis, B.C.; Correia, F.F.; Lima-Vasconcellos, T.H.; Chiarantin, G.M.D.; De La Vega, L.; Lombello, C.B.; Willerth, S.M.; Malmonge, S.M.; Paschon, V.; et al. The Impact of Biomaterial Surface Properties on Engineering Neural Tissue for Spinal Cord Regeneration. Int. J. Mol. Sci. 2023, 24, 13642. [Google Scholar] [CrossRef]
- Yu, L.; Bennett, C.J.; Lin, C.-H.; Yan, S.; Yang, J. Scaffold design considerations for peripheral nerve regeneration. J. Neural Eng. 2024, 21, 041001. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Yushan, M.; Yusufu, A. Enhanced Nerve Regeneration by Bionic Conductive Nerve Scaffold Under Electrical Stimulation. Front. Neurosci. 2022, 16, 810676. [Google Scholar] [CrossRef]
- Toader, C.; Eva, L.; Tataru, C.-I.; Covache-Busuioc, R.-A.; Bratu, B.-G.; Dumitrascu, D.-I.; Costin, H.P.; Glavan, L.-A.; Ciurea, A.V. Frontiers of Cranial Base Surgery: Integrating Technique, Technology, and Teamwork for the Future of Neurosurgery. Brain Sci. 2023, 13, 1495. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Garcia-Atutxa, I.; Santos, A.; Armendariz-Borunda, J. Toward a New Generation of Bio-Scaffolds for Neural Tissue Engineering: Challenges and Perspectives. Pharmaceutics 2023, 15, 1750. [Google Scholar] [CrossRef]
- Yael, D. Addressing the High Costs of Stem Cell Therapies. Stem. Cell. Res. Reg. Med. 2024, 7, 255–256. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.J.; Zhang, X.-a.; Hu, M. Advances in hyaluronic acid hydrogel for meniscus repair. Front. Bioeng. Biotechnol. 2025, 13, 1639034. [Google Scholar] [CrossRef]
- Patel, G.; Bouchard, L.-S. Applications of aligned nanofiber for tissue engineering. arXiv 2024, arXiv:2408.07909. [Google Scholar] [CrossRef]
- Shash, Y.H. Assessment of cranial reconstruction utilizing various implant materials: Finite element study. J. Mater. Sci. Mater. Med. 2024, 35, 50. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Deng, L.; Yong, Y.-Y.; Wu, J.-M.; Qin, D.-L.; Yu, L.; Zhou, X.-G.; Wu, A.-G. The Application of Biomaterials in Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhu, S.; Sun, B.; Nan, C.; Cong, L.; Zhao, Z.; Liu, L. 3D cell-laden scaffold printed with brain acellular matrix bioink. J. Nanobiotechnol. 2025, 23, 564. [Google Scholar] [CrossRef]
- Rajendran, A.; Rajan, R.A.; Balasubramaniyam, S.; Elumalai, K. Nano Delivery Systems in Stem Cell Therapy: Transforming Regenerative Medicine and Overcoming Clinical Challenges. Nano TransMed. 2025, 4, 100069. [Google Scholar] [CrossRef]
- Zeng, C.-W. Multipotent Mesenchymal Stem Cell-Based Therapies for Spinal Cord Injury: Current Progress and Future Prospects. Biology 2023, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Liu, J.; Wang, B.; Chen, S.; Liu, C.; Chen, X. The Effect of Aligned and Random Electrospun Fibers Derived from Porcine Decellularized ECM on Mesenchymal Stem Cell-Based Treatments for Spinal Cord Injury. Bioengineering 2024, 11, 772. [Google Scholar] [CrossRef] [PubMed]
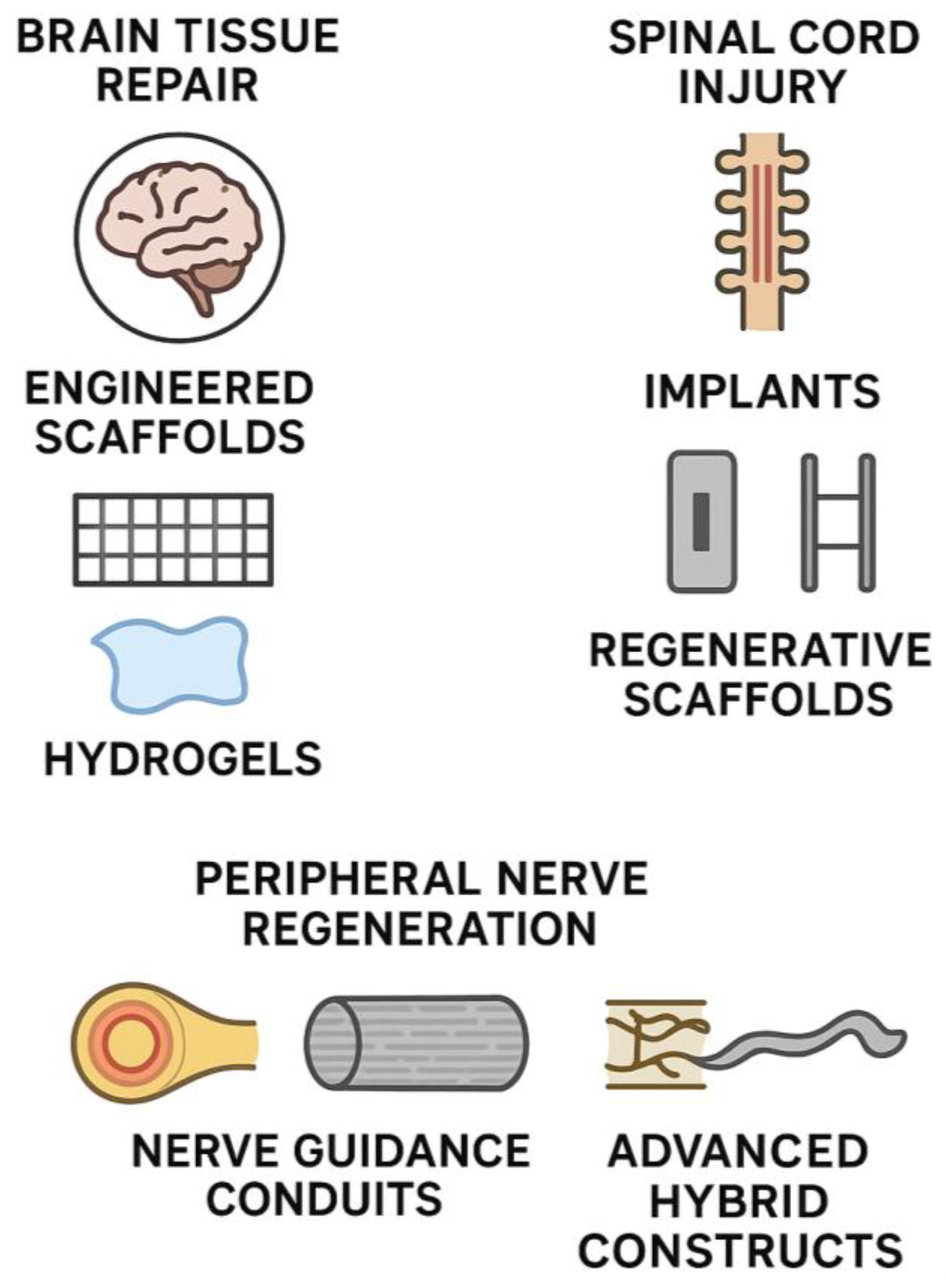
| Category | Approach/Subtype | Key Materials/Strategies | Clinical/Experimental Notes | Reference |
|---|---|---|---|---|
| Brain Tissue Repair | Engineered scaffolds | Natural biomaterials (collagen, fibrin, chitosan); synthetic polymers (PLA, PGA, PCL); composites (e.g., collagen–PGA) | Provide mechanical support + bioactive environment; used to fill resection cavities; mimic ECM to promote growth. | Smith et al., 2025. [33] |
| Hydrogels | Agarose, alginate, PEG, hyaluronic acid hydrogels; in situ crosslinking; drug/antibody-loaded (BDNF, NGF, anti-Nogo-A) | Injectable; conform to cavity shape; depot for controlled release; shown effective in rodent stroke models. | Xu et al., 2023. [34] | |
| Nanomaterials and fiber scaffolds | Conductive scaffolds (graphene, PEDOT, CNTs); electrospun gelatin/laminin fibers | Conductivity enhances neurite outgrowth; aligned fibers guide axons; limited infiltration unless modified. | Licciardello et al., 2024. [35] | |
| Biocompatibility/immune modulation | Decellularized neural ECM; PEGylated surfaces; scaffolds with IL-10 or M2-polarizing peptides; slow-release dexamethasone | Reduces glial scarring and chronic inflammation; improves cell adhesion and integration in CNS tissue. | Kim et al., 2021. [36] | |
| Spinal Cord Injury and Implants | Spinal stabilization implants | Titanium and PEEK cages/rods; hydroxyapatite coatings; 3D-printed patient-specific spinal implants | Mechanical stability in trauma/degeneration; PEEK reduces stress shielding; imaging compatibility. | Cheers et al., 2024. [37] |
| Tissue-engineered cord repair | Multi-channel collagen/polymer scaffolds seeded with NSCs, MSCs, Schwann cells | Guides axon regrowth in transected cord models; cells differentiate and integrate; partial motor recovery achieved. | Da Silva et al., 2023. [38] | |
| Bioactive molecule delivery | Scaffolds/microspheres releasing BDNF, NT-3, GDNF, chondroitinase ABC | Sustains local factor delivery; degrades scar CSPGs; promotes remyelination and axon extension. | Mungenast et al., 2023. [14] | |
| Peripheral Nerve Regeneration | Nerve guidance conduits (NGCs) | Biodegradable tubes (collagen, gelatin, chitosan, PCL, PLGA); FDA-approved collagen conduits; microchannel or fiber-lined conduits | Bridge short gaps (<20 mm); support axon guidance; alternatives to autografts; some FDA-approved devices. | Yu et al., 2023. [39] |
| Advanced conduits | The 3D-printed customized conduits; nanofiber-coated or electroconductive hybrids; Schwann cell- or NGF/GDNF-loaded conduits | Improve regeneration across longer gaps; mimic ECM cues; show muscle reinnervation in animal models. | Liu et al., 2024. [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubović, J.; Vučurović, D. Biomaterials and Tissue Engineering in Neurosurgery: Current Innovations and Future Directions. BioTech 2025, 14, 65. https://doi.org/10.3390/biotech14030065
Golubović J, Vučurović D. Biomaterials and Tissue Engineering in Neurosurgery: Current Innovations and Future Directions. BioTech. 2025; 14(3):65. https://doi.org/10.3390/biotech14030065
Chicago/Turabian StyleGolubović, Jagoš, and Damjan Vučurović. 2025. "Biomaterials and Tissue Engineering in Neurosurgery: Current Innovations and Future Directions" BioTech 14, no. 3: 65. https://doi.org/10.3390/biotech14030065
APA StyleGolubović, J., & Vučurović, D. (2025). Biomaterials and Tissue Engineering in Neurosurgery: Current Innovations and Future Directions. BioTech, 14(3), 65. https://doi.org/10.3390/biotech14030065







