1. Introduction
In recent years, there has been an increase in cases of death from multidrug-resistant bacteria [
1]. This bacterial resistance can be caused by the indiscriminate use of drugs and by genetic mutations [
2]. This resistance makes conventional medicines ineffective, thus posing a threat to human health [
3].
In 2024, The World Health Organization (WHO) expanded the list of microorganisms that present a major threat to society.
Pseudomonas aeruginosa,
Acinetobacter baumannii, and
Klebisiella pneumoniae are still considered priorities on the updated list [
4]. These microorganisms are associated with severe hospital admissions and high mortality rates, underscoring the critical need for effective control methods against these pathogens, which are implicated in the development of pneumonia, bacteremia, meningitis, and urinary tract infections [
5].
These bacteria have a high capacity to spread in hospital environments and develop resistance to a wide variety of antibiotics due to several mechanisms, including the reduced permeability of the outer membrane of cells, enzyme inactivation, efflux pump systems, and the ability to form biofilms [
6,
7]. Bacterial biofilms are a survival strategy that bacteria use to resist the effects of antibiotics. Reports in the literature indicate that the types of species present in the biofilm and the specific antibiotics used can significantly influence the effectiveness of the treatment in disrupting the biofilm [
8].
When discussing biofilms associated with nosocomial infections, the fungus
Candida albicans is directly related, as it is commonly found in large quantities on urinary catheters and endotracheal tubes used in hospitalized patients [
9,
10]. Effective antifungal therapy is crucial for successful treatment. However, cases of antifungal resistance are becoming increasingly frequent, particularly with
Candida albicans [
11,
12]. This fungus species is also found in the oral cavity and is the primary cause of oral candidiasis. Its ability to adhere to epithelial cells and its potential to transform into hyphal forms make it prevalent in polymicrobial biofilms [
13,
14].
Polymicrobial infections resulting from the interaction of bacteria, fungi, and other parasites cause serious health conditions that are difficult to manage, presenting a significant challenge to the scientific community [
15].
One of the greatest advances in medicine was the creation of antibiotics to treat microbial infections, but the resistance of these bacteria has become a threat to human health [
16]. To combat multi-resistant bacteria, natural products are a promising strategy against these pathogenic microorganisms. In this context, the use of rosemary-based extracts, for instance, can slow down antimicrobial resistance and act as a potential pharmacotherapeutic agent in combating these bacteria [
17].
Rosmarinus officinalis L. or
Salvia rosmarinus Spenn, popularly called rosemary, is an aromatic shrub native to the Mediterranean Basin [
18]. Rosemary is traditionally used as tea and as a seasoning for food, but it is also utilized in the cosmetic and pharmaceutical industries [
19,
20]. The antimicrobial action of rosemary has been widely reported in the literature against various types of bacteria and fungi, such as
Escherichia coli,
Klebsiella pneumoniae,
Streptococcus mutans, and
Pseudomonas aeruginosa [
21],
Candida albicans,
C. dubliniensis,
C. glabrata,
C. krusei, and
C. tropicalis [
13]. This antimicrobial action is associated with the presence of phytocompounds such as caffeic acid, carnosic acid, chlorogenic acid, oleanolic acid, rosmarinic acid, ursolic acid, alpha-pinene, camphor, carnosol, eucalyptol, rosmadial, and rosmanol [
22,
23].
Another method that has been studied is the combination of rosemary essential oil with conventional antibiotics. This combination has demonstrated synergistic results against strains of multidrug-resistant bacteria, offering a potential way to combat antimicrobial resistance [
21]. In addition to its antimicrobial and antifungal properties, rosemary also exhibits several other biological benefits, including antioxidant activity, hepatoprotective effects, and even insecticidal uses [
24]. One particularly noteworthy property of rosemary is its neuroprotective action, which can combat oxidative and neurotoxic damage. This effect is attributed to the presence of rosmarinic acid in its composition [
25].
Given the therapeutic properties of rosemary extract reported in the literature, it is very important to verify its potential effectiveness against polymicrobial biofilms formed by P. aeruginosa, A. baumannii, K. pneumoniae, and C. albicans, which could provide a possible alternative to conventional biofilm control methods. Therefore, the aim of this study was to evaluate the rosemary extract regarding its phytochemical composition, antioxidant activity, and antibacterial effects against polymicrobial biofilms associating multidrug-resistant bacterial strains with C. albicans. The null hypothesis asserts that the extract does not possess antioxidant, antibacterial, or anti-biofilm properties.
4. Discussion
Given the significant challenge of finding new alternatives to combat antimicrobial-resistant pathogens, this study evaluated the phytochemical composition and potential of rosemary (Rosmarinus officinalis L.) extract in eliminating polymicrobial biofilms composed of C. albicans and A. baumannii; C. albicans and K. pneumoniae; and C. albicans and P. aeruginosa. The phytochemical profile of the plant extract was outlined, revealing antimicrobial effectiveness at different concentrations against planktonic cultures and polymicrobial biofilms of fungi and multidrug-resistant bacteria. As a result, the null hypothesis of this study was dismissed.
In 2017, the World Health Organization (WHO) released a priority list of pathogens for antimicrobial drug development, placing
A. baumannii at the highest priority within the critical category [
26]. By 2019, antimicrobial resistance was linked to an estimated 4.95 million deaths globally, with 1.27 million of those directly caused by bacterial infections from antibiotic-resistant pathogens [
4].
Due to the growing global concern regarding multidrug-resistant bacteria, the medicinal properties of plants have gained attention as promising alternatives to conventional antibiotics, particularly in the form of phytotherapeutics [
27,
28].
The presence of phytochemical constituents in natural plant extracts—such as flavonoids, phenols, alkaloids, tannins, glycosides, saponins, and terpenoids—determines their diverse therapeutic properties [
29]. Flavonoids and other phenolic compounds, for instance, are secondary metabolites found in various plant species and play crucial roles in their biological activities [
30].
In this study,
R. officinalis extract was found to contain flavonoids (5.12 mg/mL) and total phenols (7.70 mg/mL). A study by Megateli et al. (2018) [
31] quantified phenolic compounds in
R. officinalis using electromagnetic induction heating to enhance extraction. Under optimal conditions, the process yielded a maximum phenolic content of 127.87 ± 2.1 mg gallic acid equivalents per gram of dry weight and a total flavonoid content of 14.48 ± 1.5 mg quercetin equivalents per gram of dry weight.
Similarly, Bellumori et al. (2021) [
32] conducted a phytochemical analysis using high-performance liquid chromatography (HPLC-DAD) and identified various phenolic compounds in
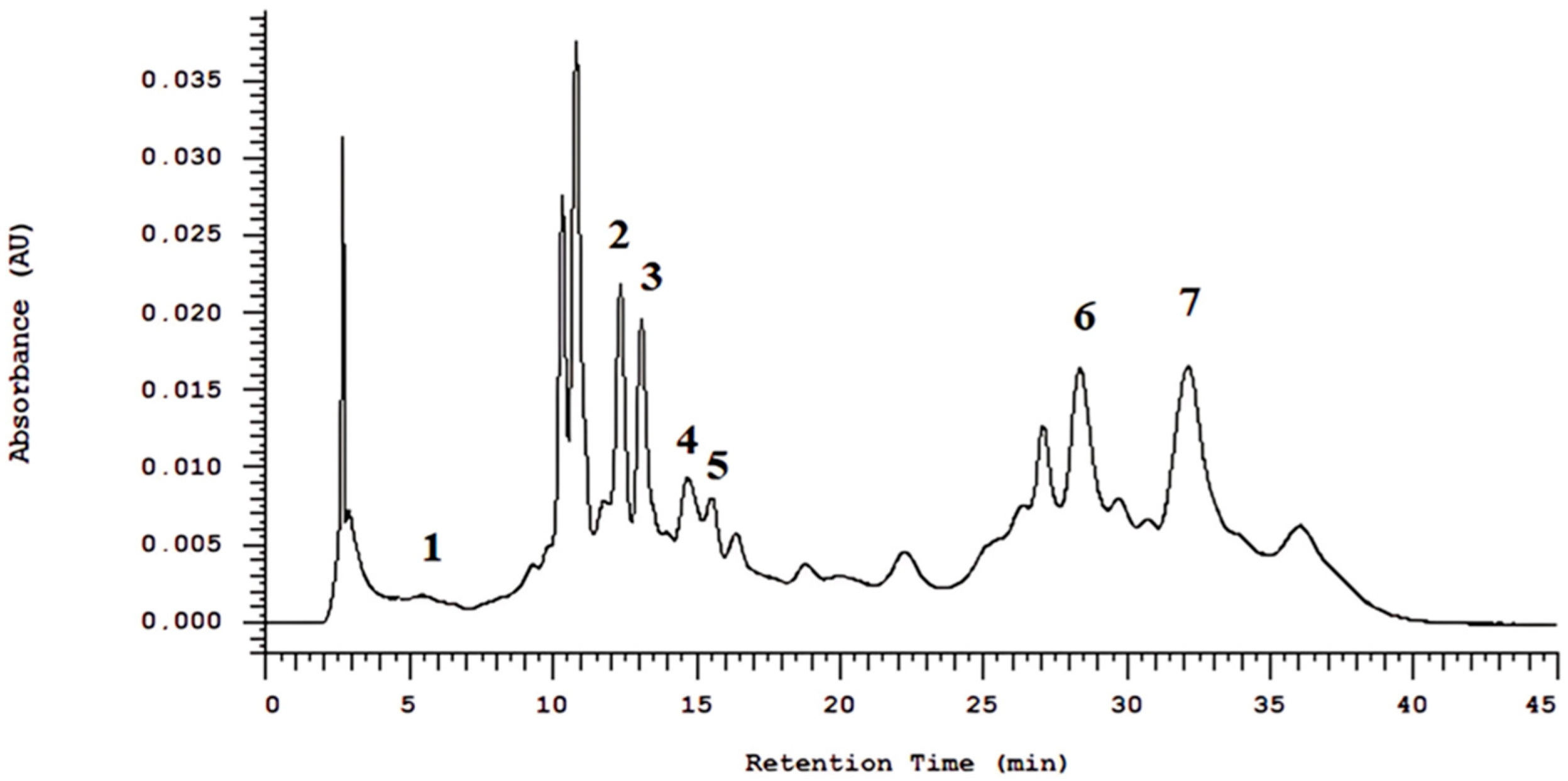
R. officinalis, including rosmarinic acid, vanillic acid, cinnamic acid, rutin, kaempferol, trans-chalcone, and quercetin. In the present study, HPLC-DAD analysis of the hydroalcoholic extract of
R. officinalis detected peaks corresponding to 5-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, caffeoylquinic acid, gallotannin, rosmarinic acid, and carnosic acid.
Moreover, rosmarinic acid has the ability to integrate into the plasma membrane of microorganisms, compromising its structural integrity. This interaction leads to the leakage of ions, proteins, and other essential intracellular components, ultimately resulting in cell death. Additionally, contact between rosmarinic acid and microbial cells induces the generation of reactive oxygen species (ROS), which cause oxidative damage to critical biomolecules such as proteins, membrane lipids, and nucleic acids, thereby enhancing the compound’s antimicrobial effect [
33,
34,
35].
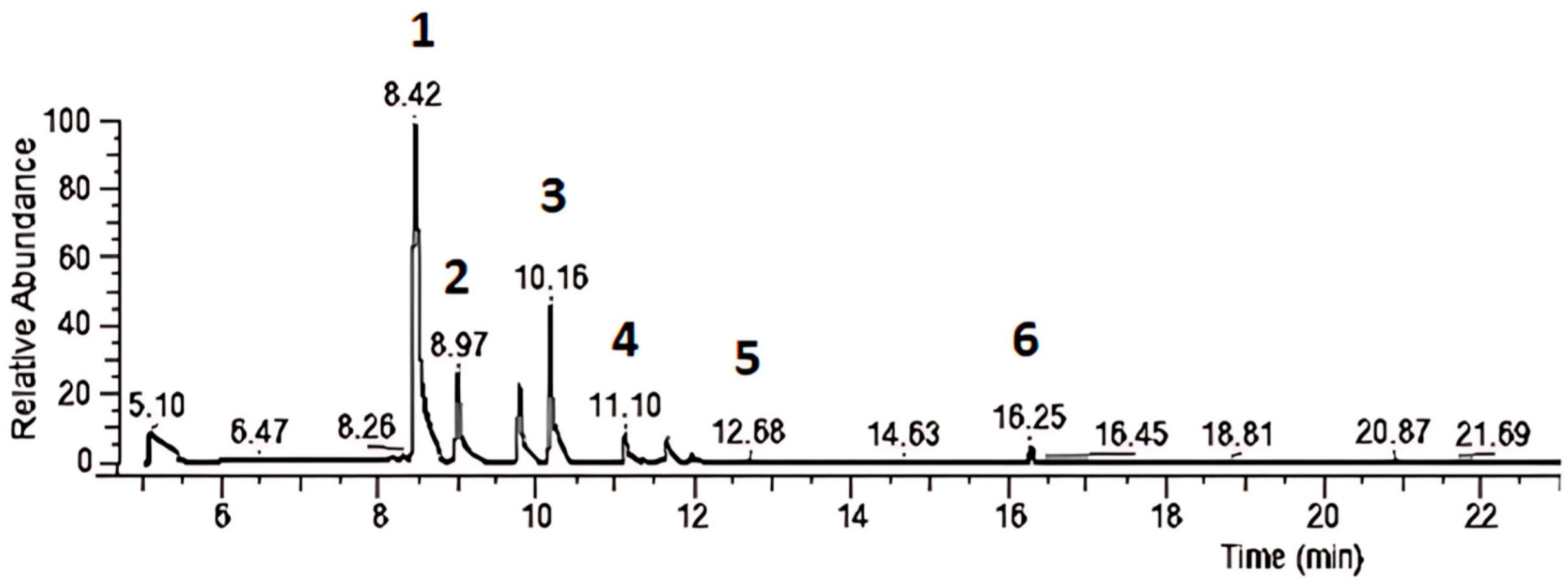
For gas chromatography/mass spectrometry (GC/MS) analysis, the hydroalcoholic extract of
R. officinalis in this study exhibited peaks for α-pinene, 2,2-dimethyl-5-methylene norbornane, β-myrcene, D-limonene, cyclohexene, 1-methyl-4-(1-methylethylidene), and camphor. In comparison, Naqvi et al. (2024) used reverse-phase high-performance liquid chromatography (RP-HPLC) to evaluate the active constituents of hydroethanolic
R. officinalis extract, identifying diosmetin, rutin, and apigenin [
36]. Hashemi et al. (2023) analyzed
R. officinalis essential oil via GC/MS and found that the main components were α-pinene (24.6%), 1,8-cineole (14.1%), camphor (13.5%), camphene (8.1%), and limonene (6.1%) [
37]. These variations across studies may be attributed to differences in extraction methods, solvent selection, and analytical technique parameters, which can influence the final composition of the extracts.
Antioxidants play a crucial role in preventing and treating diseases associated with oxidative damage, such as cancer, cardiovascular diseases, and neurodegenerative disorders [
38,
39]. Regarding the antioxidant activity of
R. officinalis, the DPPH radical scavenging assay was employed, a widely used technique for assessing antioxidant capacity. The hydroalcoholic extract of
R. officinalis exhibited an EC
50 (effective concentration required to neutralize 50% of free radicals) of 19.53 µg/mL.
Another study evaluating
R. officinalis essential oil reported an average yield of 0.92% and an antioxidant activity of 68.73 µg trolox g
−1 ± 0.61, with a 63.5% reduction in DPPH radical levels. In a moisturizing fluid containing 0.5%
R. officinalis essential oil, the antioxidant activity was measured at 37.3 µg trolox g
−1 ± 8.21, with a 36% DPPH radical reduction [
40]. Thomazini et al. (2021) also observed similar results, reporting an IC
50 of 40.28 μg/mL for the aqueous extract of
R. officinalis [
41].
α-Pinene is a bicyclic monoterpene widely found in the essential oils of various plants and is one of the main constituents of rosemary (
Rosmarinus officinalis L.) extract [
42,
43]. Studies have shown that α-pinene can modulate cellular pathways related to oxidative stress and inflammatory responses, highlighting its relevance in the phytotherapeutic use of rosemary [
44]. α-Pinene exhibits significant antioxidant activity, attributed to its ability to scavenge free radicals and inhibit lipid peroxidation. This activity is associated with the presence of conjugated double bonds in its structure, which allow the stabilization of ROS. These mechanisms suggest that α-pinene may play a key role in cellular protection against oxidative damage, making it a promising compound for the development of natural antioxidant therapeutic agents.
Barros et al. (2023) evaluated its anti-biofilm activity against
Candida albicans, and in vitro results indicated that the antifungal effect of α-pinene may be related to its interaction with ergosterol in the fungal cytoplasmic membrane. In the time-kill assay, the antifungal activity was not time-dependent and also inhibited biofilm formation, disrupting up to 88% of the existing biofilm [
45].
In regard of microbiological analyses, the R. officinalis extract demonstrated bactericidal and fungicidal activity against all evaluated strains of A. baumannii (MMC of 1.88 mg/mL), P. aeruginosa (MMC of 1.88 mg/mL), and C. albicans (MMC 1.88 to 3.75 mg/mL). However, for K. pneumoniae, only the clinical strain (K.p. CL) exhibited inhibition (MMC of 3.75 mg/mL). The Minimum Inhibitory Concentration (MIC) values could not be determined due to extract-induced turbidity, which hindered visual reading. Therefore, MMC values were considered in this study.
Several studies have explored the antimicrobial potential of
R. officinalis [
13,
21]. Kafa et al. (2022) investigated the antibacterial and antibiofilm activities of essential oils from
R. officinalis,
Pelargonium graveolens, and
Mentha piperita against drug-resistant clinical strains of
A. baumannii. The most potent antibacterial effects were observed with peppermint essential oil (2.5–5 μL/mL), followed by geranium (5–20 μL/mL) and rosemary (5–20 μL/mL) [
46].
Another study evaluated the efficacy of
R. officinalis essential oil against multidrug-resistant clinical bacterial pathogens. The results showed promising antibacterial activity against seven bacteria:
Escherichia coli,
Enterobacter cloacae,
Staphylococcus aureus,
Serratia odorifera,
K. pneumoniae,
Klebsiella oxytoca, and
Aeromonas hydrophila, with MIC values of 35.7, 17.85, 71.4, 8.9, 17.8, 285.7, and 35.7 µg/mL, respectively, and MBC values of 142.8, 71.4, 285.7, 35.7, 71.4, 571.5, and 71.4 µg/mL, respectively. This study suggested that
R. officinalis essential oil could be used as a therapeutic agent in combating a wide range of multidrug-resistant bacteria [
47].
The antifungal effect of glycolic extract of
R. officinalis was observed in the study by Meccatti et al. (2021) [
13]. The minimum fungicidal concentration for
C. albicans, C. glabrata, C. krusei, and
C. tropicalis ranged from 25 to 50 mg/mL. Additionally, the authors assessed the antibiofilm potential of
R. officinalis glycolic extract. Among the various forms of antimicrobial resistance, biofilm formation is one of the main resistance mechanisms. Biofilm is a structure composed of self-generated extracellular polymeric substances that encase bacteria and fungi on surfaces, with one of its functions being to protect them from environmental stress, preventing phagocytosis and promoting colonization [
48]. The study cited earlier demonstrated that the glycolic extract of
R. officinalis was effective in disrupting
C. albicans biofilms after 30 min of exposure and
C. dubliniensis biofilms after 24 h at a concentration of 50 mg/mL [
13].
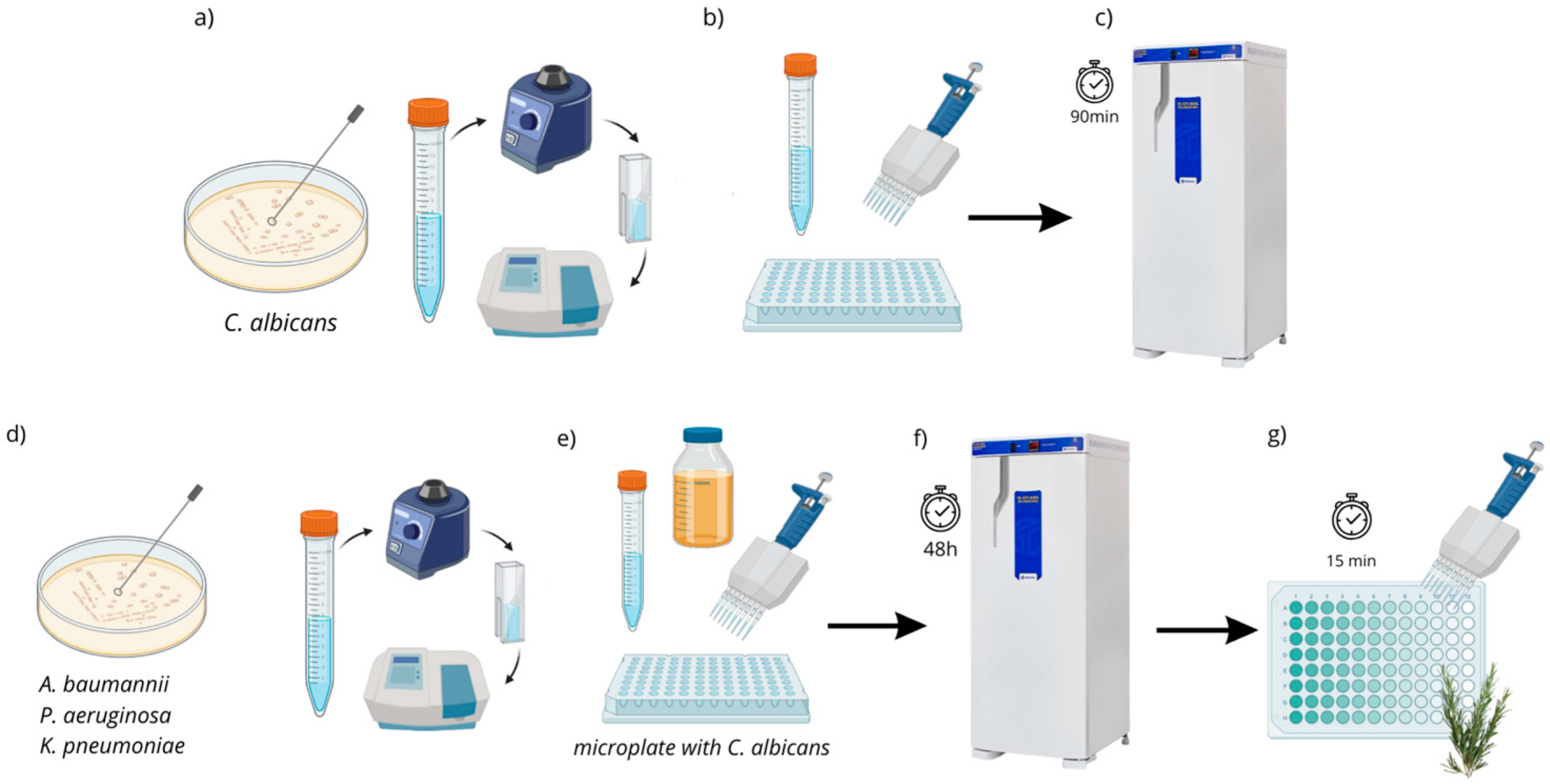
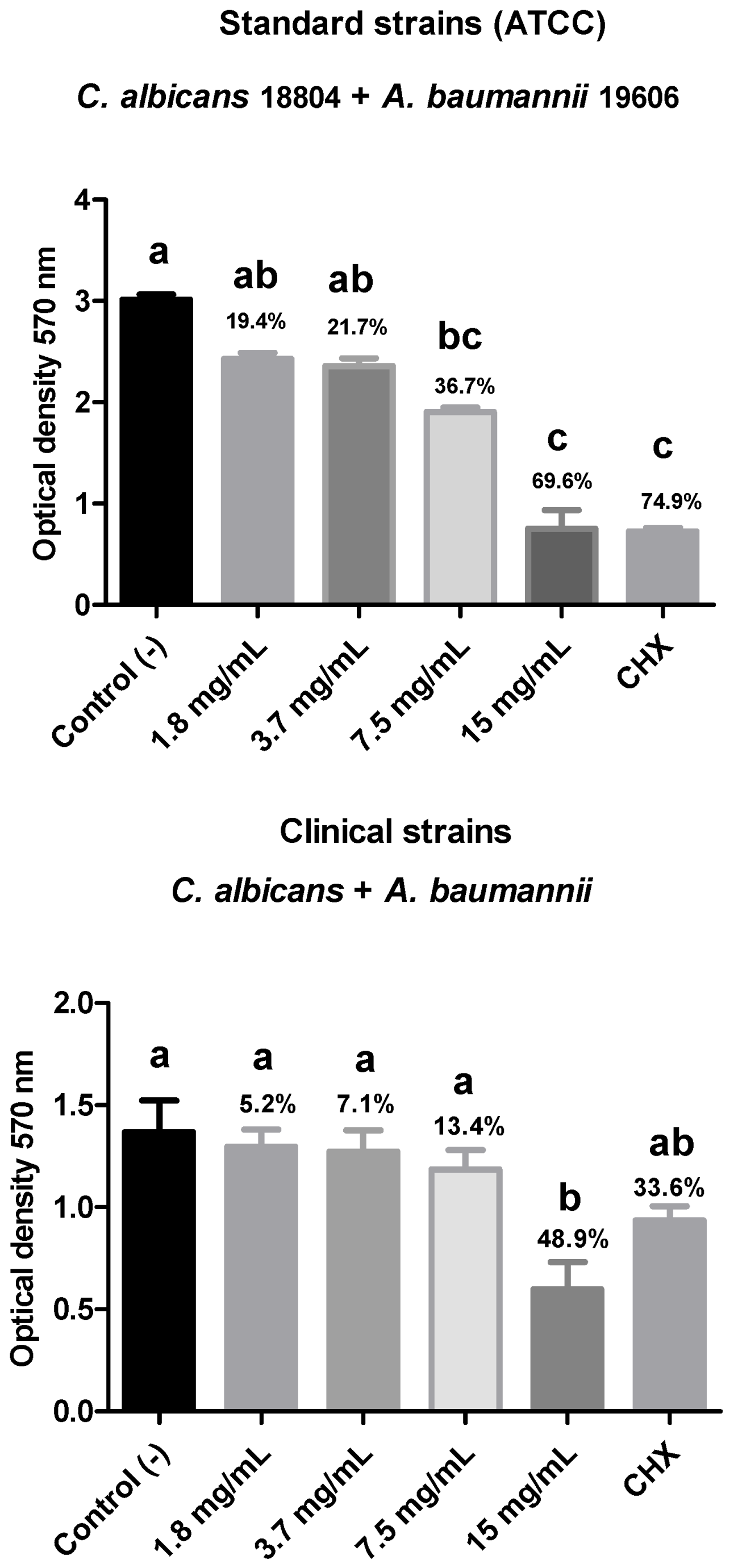
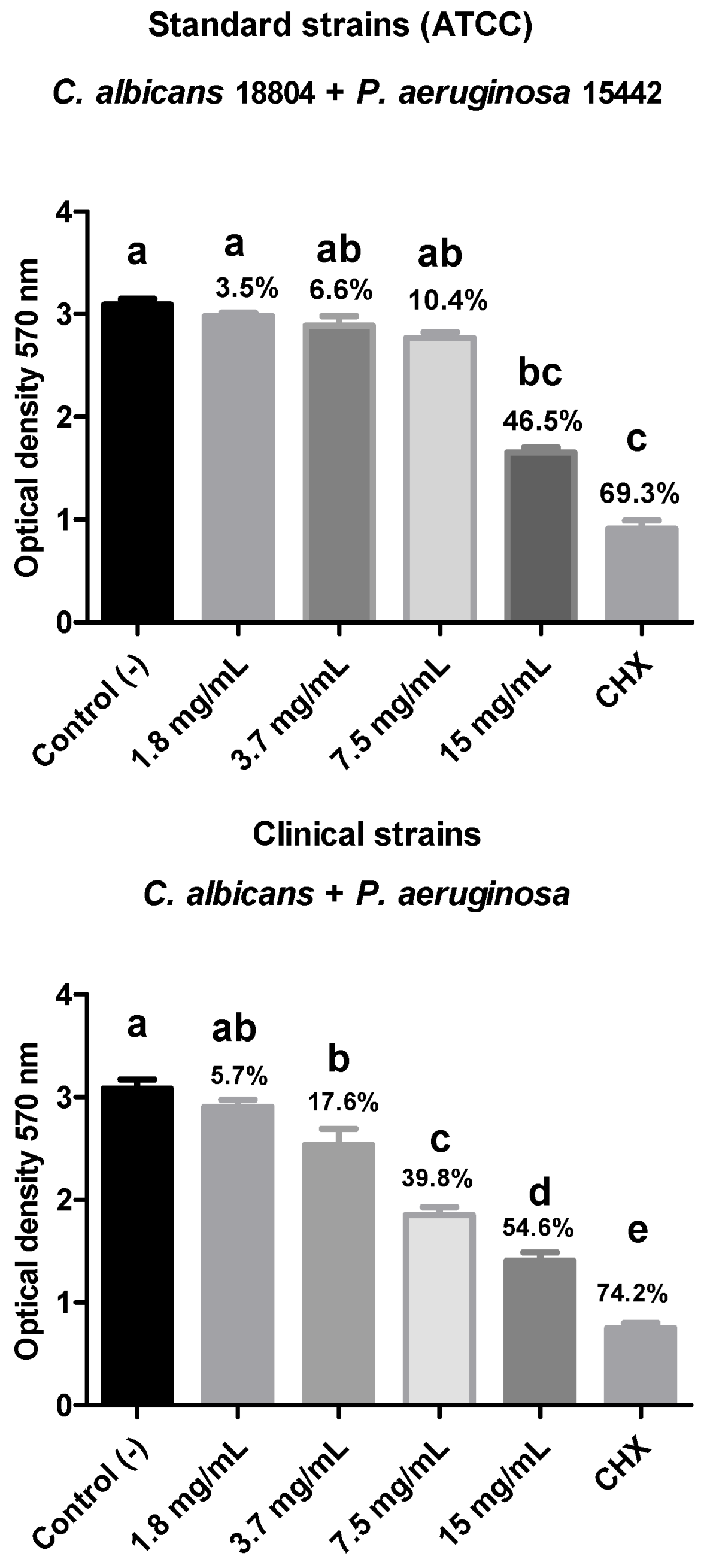
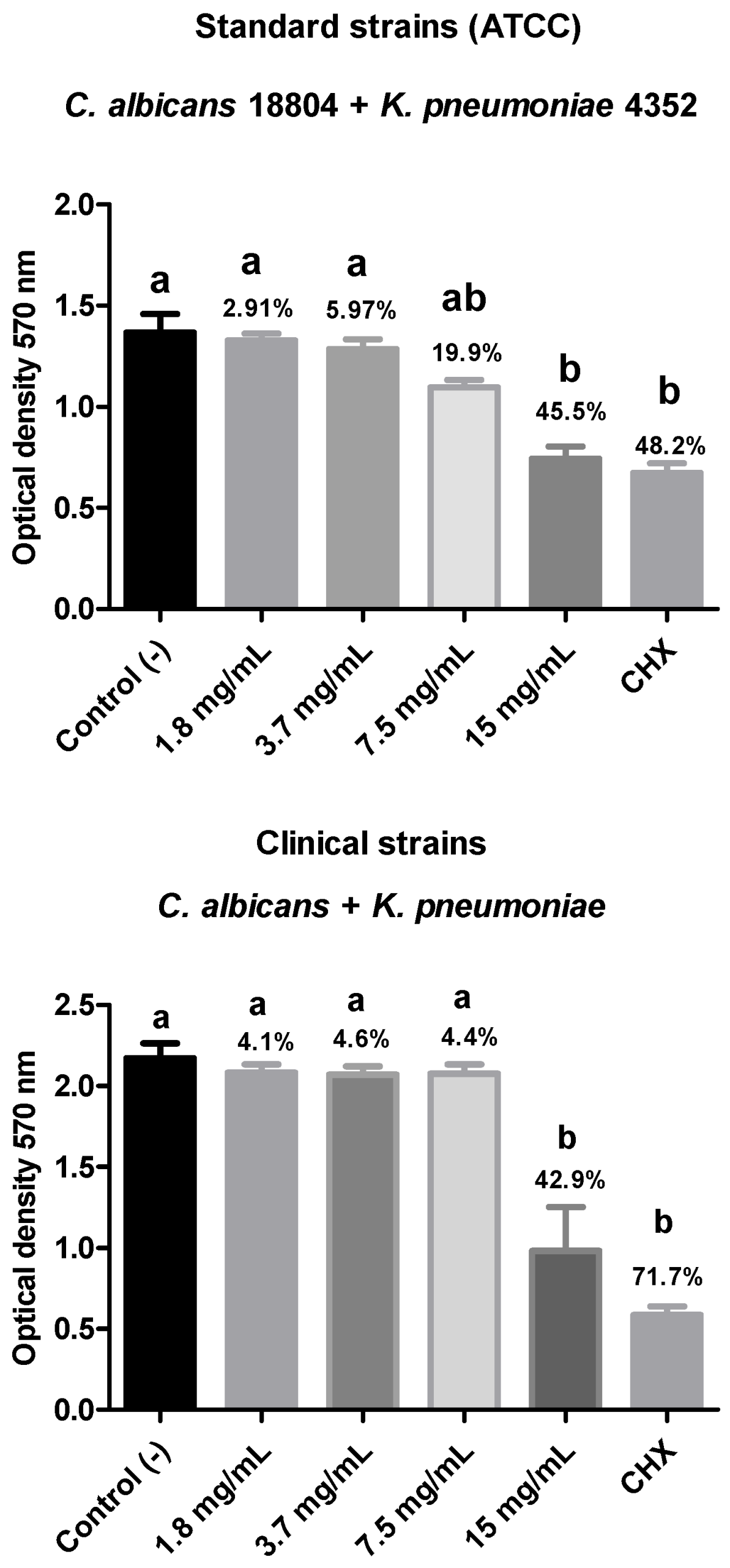
In the present study, the antibiofilm action of R. officinalis extract was evaluated against polymicrobial biofilms of C. albicans and multidrug-resistant bacteria (C. albicans + A. baumannii; C. albicans + K. pneumoniae; C. albicans + P. aeruginosa) after 15 min of contact using the MTT cell viability test. A reduction in biofilm viability was observed for all analyzed strains, with the polymicrobial biofilm composed of ATCC strains of C. albicans (18804) and A. baumannii (19606) showing the highest reduction in viable microorganisms (69.6%), demonstrating statistical equivalence to the positive control and statistical difference (p < 0.05) from the negative control.
According to the study by de Oliveira JR et al. (2017),
R. officinalis extract was shown to be effective in reducing mono- and polymicrobial biofilms, effectively contributing to the in vitro control of significant microbial species responsible for various infections in the oral cavity and other body regions, such as
C. albicans,
S. aureus,
Enterococcus faecalis,
Streptococcus mutans, and
P. aeruginosa [
49]. Another study evaluated
R. officinalis oil, which showed biofilm inhibition potential of
S. epidermidis that was greater than 57% at a concentration of 25 μL ml
−1, and a biofilm eradication of 67% was observed at a concentration of 50 μL ml
−1 [
50]. In another study on the in vivo prophylactic potential of
R. officinalis glycolic extract, it was found that the extract is an excellent prophylactic antifungal agent at 6.25 mg/mL for 72 h and 12.5 mg/mL for 24 h in a
Galleria mellonella larval model [
51].
The reviewed literature revealed no studies addressing the action of R. officinalis extract against polymicrobial biofilms of the species combinations tested in this study. The present work presented important findings regarding the antimicrobial, antibiofilm, and chemical composition of the plant extract, revealing an exploitable potential for the formulation of new compounds. Future analyses may explore the development of a complementary antiseptic agent aimed at combating biofilms linked to hospital-acquired infections. Thus, this extract may be a promising therapeutic agent for controlling pathogenic microorganisms and biofilms.