Investigation of the Cytotoxicity of Cu(II), Au(III), and Pd(II) Complexes with 2,4-Dithiouracil and 6-Propyl-2-thiouracil Derivatives
Abstract
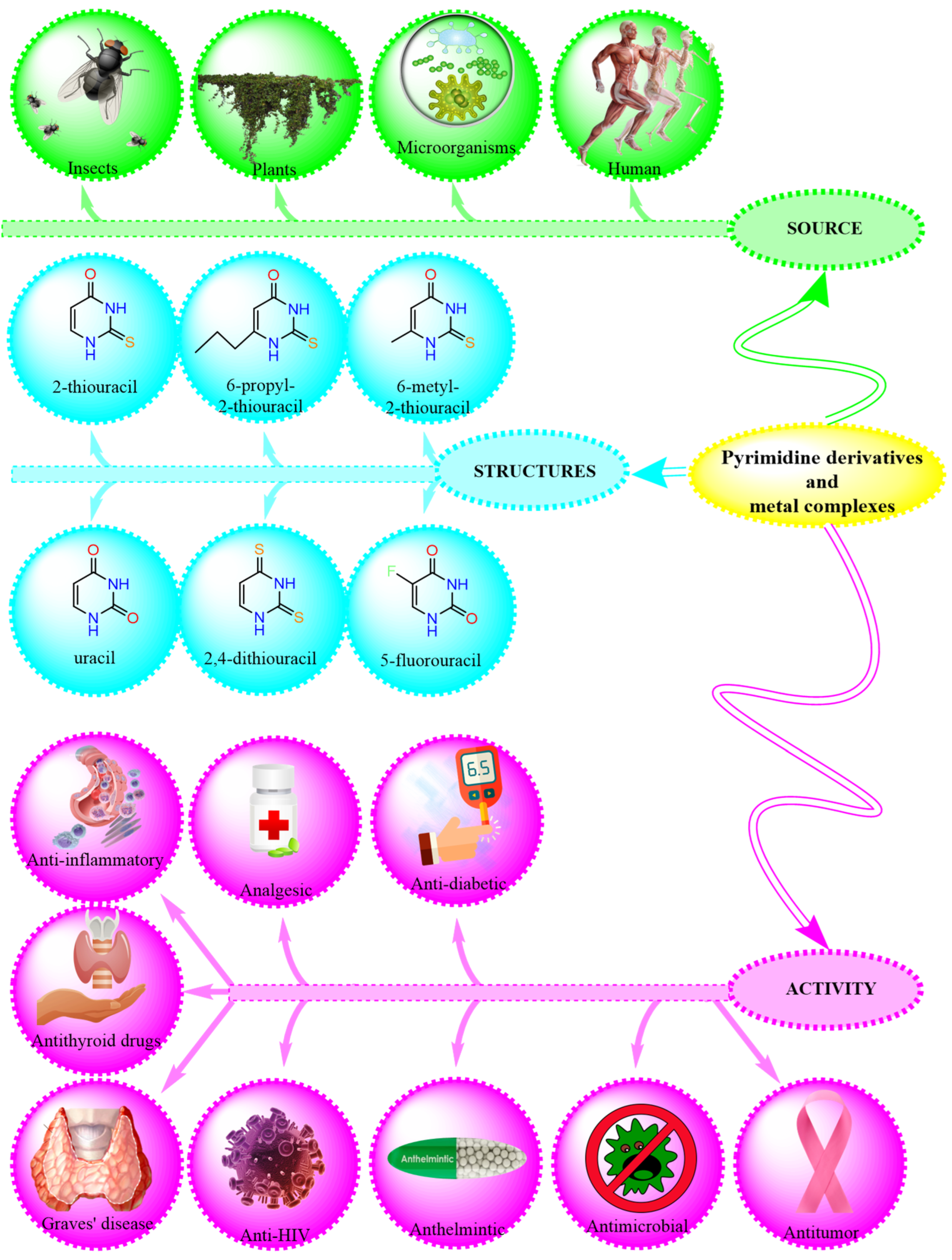
1. Introduction
2. Materials and Methods
2.1. Cytotoxicity Assay
2.2. Cell Viability Assessment
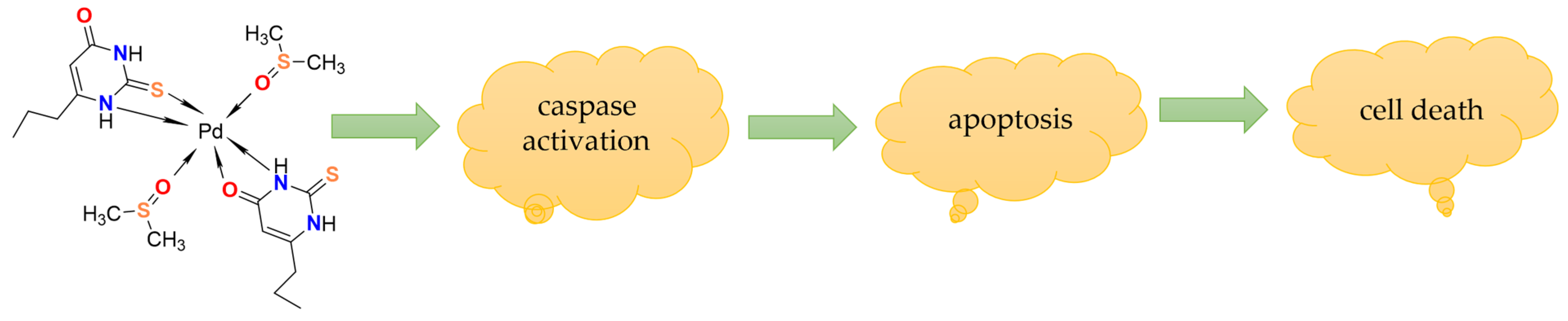
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Undare, S.S.; Valekar, N.J.; Patravale, A.A.; Jamale, D.K.; Vibhute, S.S.; Walekar, L.S.; Kolekar, G.B.; Deshmukh, M.B.; Anbhule, P.V. One-pot synthesis and in vivo biological evaluation of new pyrimidine privileged scaffolds as potent anti-inflammatory agents. Res. Chem. Intermed. 2016, 42, 4373–4386. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Fatahala, S.S.; El-Hameed, R.H.A.; El Saeed Mohamed, M. Pyrimidine as a naturally occurring bioactive ring and its importance in different arenas. Egypt. J. Chem. 2025, 68, 71–98. [Google Scholar] [CrossRef]
- Shi, W.-X.; Lv, J.-Y.; Xiao, W.-J.; Xie, H.-D.; Li, J.-J.; Jiang, Y.-L.; Su, X.-C.; Xue, J.-Y.; Li, C.-X.; Zou, Y.; et al. Pyrimidines to Pyridines: Two Atom Swap Skeletal Editing. CCS Chem. 2025. ahead of print. [Google Scholar] [CrossRef]
- Tantawya, E.S.; Nafie, M.S.; Morsyd, H.A.; El-Sayed, H.A.; Moustafaa, A.H.; Mohammed, S.M. Synthesis of novel bioactive pyrido[2,3-d]pyrimidine derivatives with potent cytotoxicity through apoptosis as PIM-1 kinase inhibitors. RSC Adv. 2024, 14, 11098–11111. [Google Scholar] [CrossRef] [PubMed]
- Mohite, M.; Sheokand, S.; Maravanji, B. Synthesis of Pyrimidines and Quinazolines via Acceptorless Dehydrogenative Coupling Catalyzed by PNN-Pd Complex. Asian J. Org. Chem. 2025, 14, e202500165. [Google Scholar] [CrossRef]
- Sakhare, D.T. Green Synthesis, Characterization and Biological Evaluation of Divalent Transition Metal Complexes of Substituted Aminopyrimidine Novel Schiff Base Ligand. Sci. J. Chem. 2025, 13, 1–10. [Google Scholar]
- Zhang, W.; Chen, J.; Du, X. Design, Synthesis of Novel Pyrimidine Derivatives Containing Alkenyl Moieties With Herbicidal Activities. J. Heterocycl. Chem. 2025, 62, 5–12. [Google Scholar] [CrossRef]
- Pant, S.; Kumar K, R.; Rana, P.; Anthwal, T.; Ali, S.M.; Gupta, M.; Chauhan, M.; Nain, S. Novel Substituted Pyrimidine Derivatives as Potential Anti-Alzheimer’s Agents: Synthesis, Biological, and Molecular Docking Studies. ACS Chem. Neurosci. 2024, 15, 783–797. [Google Scholar] [CrossRef]
- Abolibda, T.Z.; El-Sayed, A.-A.A.A.; Farag, B.; Zaki, M.E.A.; Alrehaily, A.; Elbadawy, H.M.; Al-Shahri, A.A.; Alsenani, S.R.; Gomha, S.M. Novel thiazolyl-pyrimidine derivatives as potential anticancer agents: Synthesis, biological evaluation, and molecular docking studies. Results Chem. 2025, 13, 102008. [Google Scholar] [CrossRef]
- Abdelrahman, A.H.; Azab, M.E.; Hegazy, M.A.; Labena, A.; Ramadan, S.K. Design, Synthesis, Antiproliferative Screening, and In Silico Studies of Some Pyridinyl-Pyrimidine Candidates. J. Heterocycl. Chem. 2025, 62, 303–315. [Google Scholar] [CrossRef]
- Golubyatnikova, L.G.; Khisamutdinov, R.А.; Grabovskii, S.А.; Kabal’nova, N.N.; Murinov, Y.I. Complexes of Palladium(II) and Platinum(II) with 6-tert-Butyl-2-thiouracil. Russ. J. Gen. Chem. 2017, 87, 117–121. [Google Scholar] [CrossRef]
- Vetter, C.; Kaluđerović, G.N.; Paschke, R.; Kluge, R.; Schmidt, J.; Steinborn, D. Synthesis, characterization and in vitro cytotoxicity studies of platinum(IV) complexes with thiouracil ligands. Inorg. Chim. Acta 2010, 363, 2452–2460. [Google Scholar] [CrossRef]
- Carvalho, D.E.L.; Oliveira, K.M.; Bomfim, L.M.; Soares, M.B.P.; Bezerra, D.P.; Batista, A.A.; Correa, R.S. Nucleobase Derivatives as Building Blocks to Form Ru(II)-BasedComplexes with High Cytotoxicity. ACS Omega 2020, 5, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.S.; Bomfim, L.M.; Oliveira, K.M.; Moreira, D.R.M.; Soares, M.B.P.; Ellena, J.; Bezerra, D.P.; Batista, A.A. Ru(II) complexes containing uracil nucleobase analogs with cytotoxicity against tumor cells. J. Inorg. Biochem. 2019, 198, 110751. [Google Scholar] [CrossRef]
- Bomfim, L.M.; de Araujo, F.A.; Dias, R.B.; Sales, C.B.S.; Gurgel Rocha, C.A.; Correa, R.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. Ruthenium(II) complexes with 6-methyl-2-thiouracil selectively reduce cell proliferation, cause DNA double-strand break and trigger caspase-mediated apoptosis through JNK/p38 pathways in human acute promyelocytic leukemia cells. Sci. Rep. 2019, 9, 11483. [Google Scholar] [CrossRef]
- Oladipo, M.A.; Isola, K.T. Coordination Possibility of Uracil and Applications of Some of Its Complexes: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 386–394. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Worachartcheewan, A.; Pingaew, R.; Suksrichavalit, T.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Metal Complexes of Uracil Derivatives with Cytotoxicity and Superoxide Scavenging Activity. Lett. Drug Des. Discov. 2012, 9, 282–287. [Google Scholar] [CrossRef]
- Al-Halbosy, A.T.F.; Hamada, A.A.; Faihan, A.S.; Saleh, A.M.; Yousef, T.A.; Abou-Krisha, M.M.; Alhalafi, M.H.; Al-Janabi, A.S.M. Thiourea Derivative Metal Complexes: Spectroscopic, Anti-Microbial Evaluation, ADMET, Toxicity, and Molecular Docking Studies. Inorganics 2023, 11, 390. [Google Scholar] [CrossRef]
- Palma, G.; D’Aiuto, M.; Rea, D.; Bimonte, S.; Lappano, R.; Sinicropi, M.S.; Maggiolini, M.; Longo, P.; Arra, C.; Saturnino1, C. Organo-metallic compounds: Novel molecules in cancer therapy. Biochem. Pharmacol. Open Access 2014, 13, 1603–1615. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Khalid, H.; Hanif, M.; Hashmi, M.A.; Mahmood, T.; Ayub, K.; Monim-Ul-Mehboob, M. Copper complexes of bioactive ligands with superoxide dismutase activity. Mini Rev. Med. Chem. 2013, 13, 1944–1956. [Google Scholar] [CrossRef]
- Shokohi-Pour, Z.; Chiniforoshan, H.; Momtazi-Borojeni, A.A.; Notash, B. A novel Schiff base derived from the gabapentin drug and copper (II) complex: Synthesis, characterization, interaction with DNA/protein and cytotoxic activity. J. Photochem. Photobiol. B 2015, 162, 34–44. [Google Scholar] [CrossRef]
- Zou, T.; Ching, A.; Lum, T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical biology of anticancer gold(III) and gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef]
- da Silva Maia, P.I.; Deflon, V.M.; Abram, U. Gold(III) complexes in medicinal chemistry. Future Med. Chem. 2014, 6, 1515–1536. [Google Scholar] [CrossRef]
- Ferna, J.; Elie, B.T.; Sulzmaier, F.J.; Sanau, M.; Ramos, J.W.; Contel, M. Organometallic titanocene-gold compounds as potential chemotherapeutics in renal cancer. Study of their protein kinase inhibitory properties. Organometallics 2014, 33, 6669–6681. [Google Scholar] [CrossRef]
- Rana, B.K.; Nandy, A.; Bertolasi, V.; Bielawski, C.W.; Saha, K.D.; Dinda, J. Novel gold(I)– and gold(III)–N-heterocyclic carbene complexes: Synthesis and evaluation of their anticancer properties. Organometallics 2014, 33, 2544–2548. [Google Scholar] [CrossRef]
- Abou-Melha, K.S. Elaborated studies for the ligitional behavior of thiouracil derivative towards Ni(II), Pd(II), Pt(IV), Cu(II) and UO22 2 ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 6–16. [Google Scholar] [CrossRef]
- Masoud, M.S.; Amira, M.F.; Ramadan, A.M.; El-Ashry, G.M. Synthesis and characterization of some pyrimidine, purine, amino acid and mixed ligand complexes. Spectrochim. Acta Part A 2008, 69, 230–238. [Google Scholar] [CrossRef]
- Singh, U.P.; Ghose, R.; Ghose, A.K.; Sodhi, A.; Singh, S.M.; Singh, R.K. The effect of histidine on the structure and antitumoractivity of metal-5-halouracil complexes. J. Inorg. Biochem. 1989, 37, 325–329. [Google Scholar] [CrossRef]
- El-Morsy, F.A.; Jean-Claude, B.J.; Butler, I.S.; El- Sayed, S.A.; Mostafa, S.I. Synthesis, characterization and anticancer activity of new zinc(II), molybdate(II), palladium(II), silver(I), rhodium(III), ruthenium(II) and platinum(II) complexes of 5,6-diamino-4-hydroxy2-mercaptopyrimidine. Inorg. Chim. Acta 2014, 423, 144–155. [Google Scholar] [CrossRef]
- Abás, E.; Pena-Martínez, R.; Aguirre-Ramírez, D.; Rodríguez-Diéguez, A.; Laguna, M.; Grasa, L. New selective thiolate gold(I) complexes inhibit proliferation of different human cancer cells and induce apoptosis in primary cultures of mouse colon tumors. Dalton Trans. 2020, 49, 1915–1927. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, S.; Singh, S.M. Synthesis, characterization and antitumour activity of metal complexes of 5-carboxy-2-thiouraci. Met.-Based Drugs 1998, 5, 35–39. [Google Scholar] [CrossRef]
- Papazoglou, I.; Cox, P.J.; Hatzidimitriou, A.G.; Kokotidou, C.; Choli-Papadopoulou, T.; Aslanidis, P. Copper(I) halide complexes of 5-carbethoxy-2-thiouracil: Synthesis, structure and in vitro cytotoxicity. Eur. J. Med. Chem. 2014, 78, 383–391. [Google Scholar] [CrossRef]
- Hoeschele, J.D.; Piscataway, N.J. Ethylenediamineplatinum(II) 2,4-Dioxopyrimidine Complexes. U.S. Patent 4,207,416, 10 June 1980. [Google Scholar]
- Illán-Cabeza, N.A.; García-García, A.R.; Moreno-Carretero, M.N.; Martínez-Martos, J.M.; Ramírez-Expósito, M.J. Synthesis, characterization and antiproliferative behavior of tricarbonyl complexes of rhenium(I) with some 6-amino-5-nitrosouracil derivatives: Crystal structure of fac-[ReCl(CO)3(DANU-N5,O4)] (DANU = 6-amino-1,3-dimethyl-5-nitrosouracil). J. Inorg. Biochem. 2005, 99, 1637–1645. [Google Scholar] [CrossRef]
- Marinova, P.; Tsoneva, S.; Frenkeva, M.; Blazheva, D.; Slavchev, A.; Penchev, P. New Cu(II), Pd(II) and Au(III) complexes with 2-thiouracil: Synthesis, Characteration and Antibacterial Studies. Russ. J. Gen. Chem. 2022, 92, 1578–1584. [Google Scholar] [CrossRef]
- Marinova, P.; Hristov, M.; Tsoneva, S.; Burdzhiev, N.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Synthesis, Characterization, and Antibacterial Studies of New Cu(II) and Pd(II) Complexes with 6-Methyl-2-Thiouracil and 6-Propyl-2-Thiouracil. Appl. Sci. 2023, 13, 13150. [Google Scholar] [CrossRef]
- Marinova, P.; Stoitsov, D.; Burdzhiev, N.; Tsoneva, S.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Investigation of the Complexation Activity of 2,4-Dithiouracil with Au(III) and Cu(II) and Biological Activity of the Newly Formed Complexes. Appl. Sci. 2024, 14, 6601. [Google Scholar] [CrossRef]
- Marinova, P.; Burdzhiev, N.; Blazheva, D.; Slavchev, A. Synthesis and Antibacterial Studies of a New Au(III) Complex with 6-Methyl-2-Thioxo-2,3-Dihydropyrimidin-4(1H)-One. Molbank 2024, 2024, M1827. [Google Scholar] [CrossRef]
- Marinova, P.E.; Tamahkyarova, K.D. Synthesis and Biological Activities of Some Metal Complexes of 2-Thiouracil and Its Derivatives: A Review. Compounds 2024, 4, 186–213. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A review on metal complexes and its anti-cancer activities: Recent updates from in vivo studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent development of gold(I) and gold(III)complexes as therapeutic agents for cancer diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef]
- Kim, J.H.; Ofori, S.; Parkin, S.; Vekaria, H.; Sullivan, P.G.; Awuah, S.G. Anticancer gold(III)-bisphosphine complex alters the mitochondrial electron transport chain to induce in vivo tumor inhibition. Chem. Sci. 2021, 12, 7467–7479. [Google Scholar] [CrossRef]
- Arojojoye, A.S.; Kim, J.H.; Olelewe, C.; Parkin, S.; Awuah, S.G. Chiral gold(III) complexes: Speciation, in vitro, and in vivo anticancer profile. Chem. Commun. 2022, 58, 10237–10240. [Google Scholar] [CrossRef]
- Mirzadeh, N.; Telukutla, S.R.; Luwor, R.; Privér, S.; Velma, G.R.; Jakku, R.K.; Andrew N, S.; Plebanski, M.; Christian, H.; Bhargava, S. Dinuclear orthometallatedgold(I)-gold(III) anticancer complexes with potent in vivo activity through an ROS-dependent mechanism. Metallomics 2021, 13, mfab039h. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Gan, Q.; Liu, Y.X.; Xiong, Y.H.; Mao, Z.W.; Le, X.Y. Two new Cu(II) dipeptide complexes based on 5-methyl-2-(2’-pyridyl)benzimidazole as potential antimicrobial and anticancer drugs: Special exploration of their possible anticancer mechanism. Eur. J. Med. Chem. 2018, 154, 220–232. [Google Scholar] [CrossRef]
- Reddy, T.S.; Privér, S.H.; Mirzadeh, N.; Bhargava, S.K. Synthesis of gold(I) phosphine complexes containing the 2-BrC6F4PPh2 ligand: Evaluation of anticancer activity in 2D and 3D spheroidal models of HeLa cancer cells. Eur. J. Med. Chem. 2018, 145, 291–301. [Google Scholar] [CrossRef]
- Fei, B.L.; Tu, S.; Wei, Z.; Wang, P.; Qiao, C.; Chen, Z.F. Optically pure chiral copper(II) complexes of rosin derivative as attractive anticancer agents with potential anti-metastatic and anti-angiogenic activities. Eur. J. Med. Chem. 2019, 176, 175–186. [Google Scholar] [CrossRef]
- Khan, T.M.; Gul, N.S.; Lu, X.; Wei, J.H.; Liu, Y.C.; Sun, H.; Liang, H.; Orvig, C.; Chen, Z.F. In vitro and in vivo anti-tumor activity of two gold(III) complexes with isoquinoline derivatives as ligands. Eur. J. Med. Chem. 2019, 163, 333–343. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Matadamas-Martínez, F.; Yépez-Mulia, L.; Pérez-Koldenkova, V.; Leyte-Lugo, M.; Rodríguez-Villar, K.; Cortés-Benítez, F.; Macías-Jiménez, A.P.; González-Sánchez, I.; Romero-Velásquez, A.; et al. Synthesis and Cytotoxic Activity of Combretastatin A-4 and 2,3-Diphenyl-2H-indazole Hybrids. Pharmaceuticals 2021, 14, 815. [Google Scholar] [CrossRef]
- Zeng, Z.-F.; Huang, Q.-P.; Cai, J.-H.; Zheng, G.-J.; Huang, Q.-C.; Liu, Z.-L.; Chen, Z.-L.; Wei, Y.-H. Synthesis, Characterization, DNA/HSA Interactions, and Anticancer Activity of Two Novel Copper(II) Complexes with 4-Chloro-3-Nitrobenzoic Acid Ligand. Molecules 2021, 26, 4028. [Google Scholar] [CrossRef]
- Becit, M.; Aydın Dilsiz, S.; Başaran, N. Interaction of curcumin on cisplatin cytotoxicity in HeLa and HepG2 carcinoma cells. Istanb. J. Pharm. 2020, 50, 202–210. [Google Scholar] [CrossRef]
- Ganesan, B.S.; Prabhakaran, P. Effect of HeLa Cell Density Towards Cisplatin Treatment. Proc. Sci. Math. 2022, 12, 58–65. Available online: https://science.utm.my/procscimath/wp-content/uploads/sites/605/2022/11/07_Barthi-S-Ganesen_58-65new-1.pdf (accessed on 8 November 2024).
- Aborehab, N.M.; Osama, N. Effect of Gallic acid in potentiating chemotherapeutic effect of Paclitaxel in HeLa cervical cancer cells. Cancer Cell Int. 2019, 19, 154. [Google Scholar] [CrossRef]
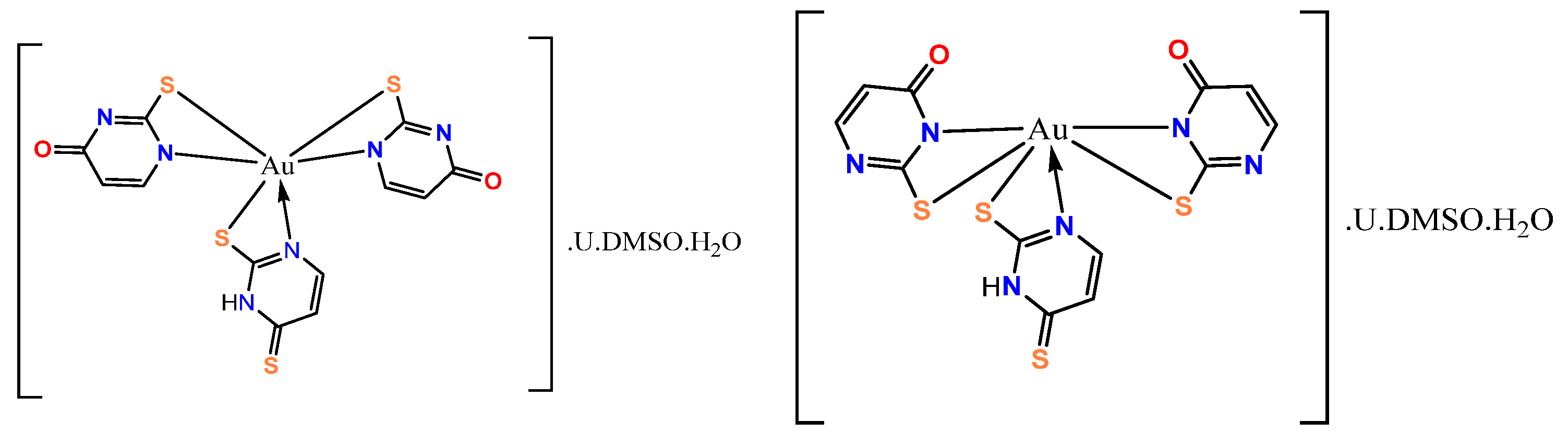
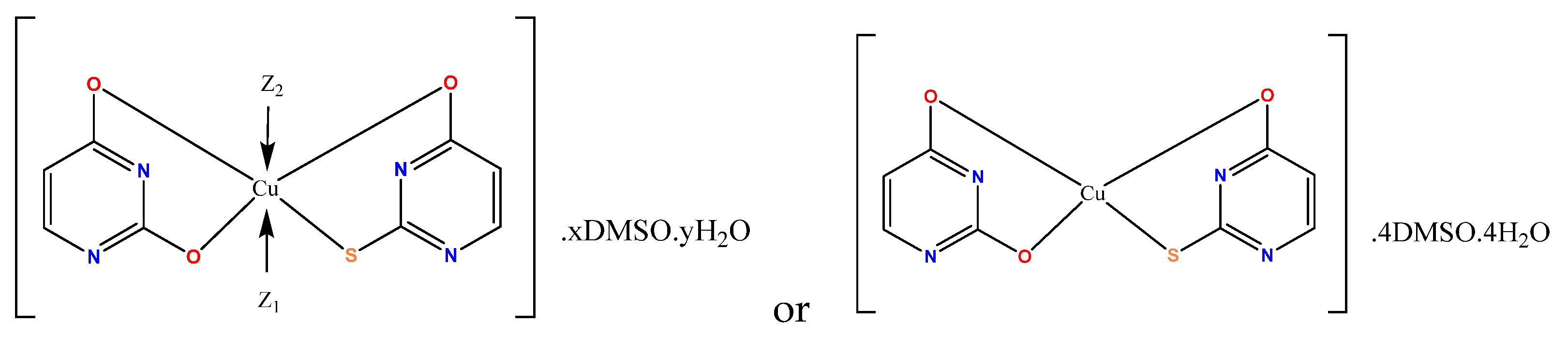
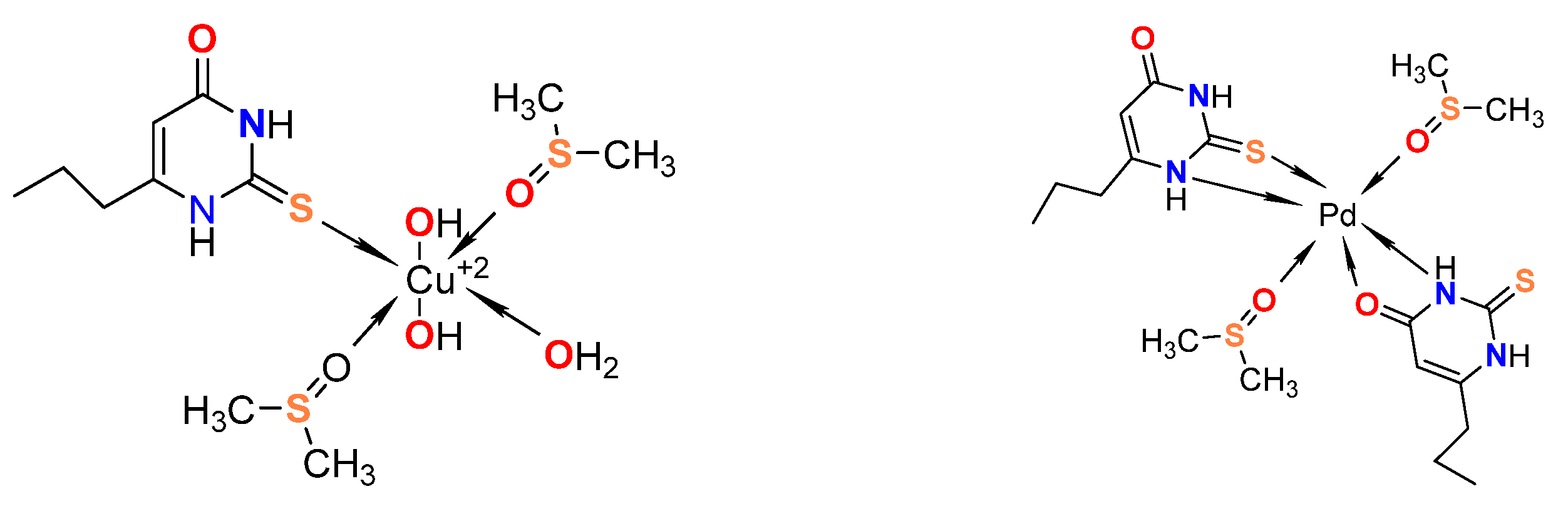
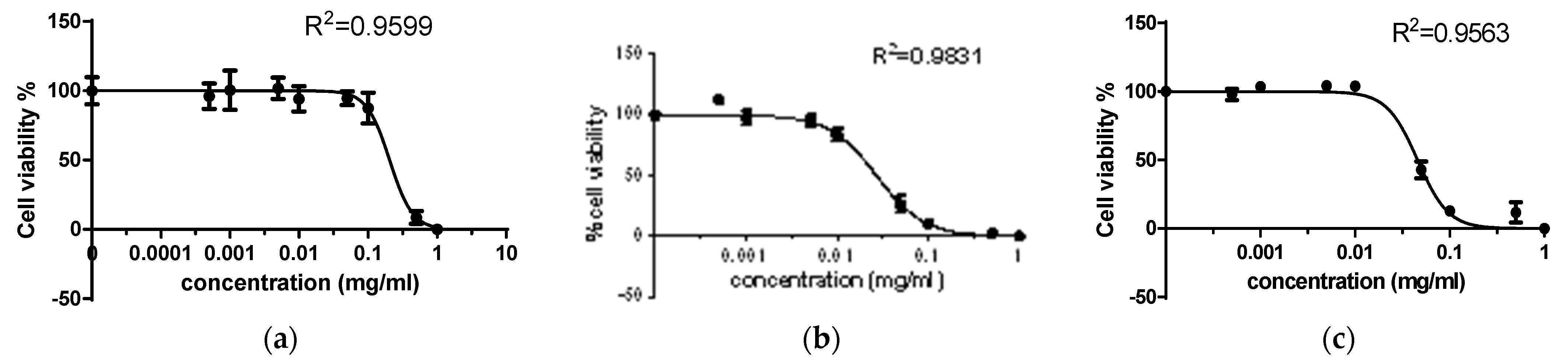
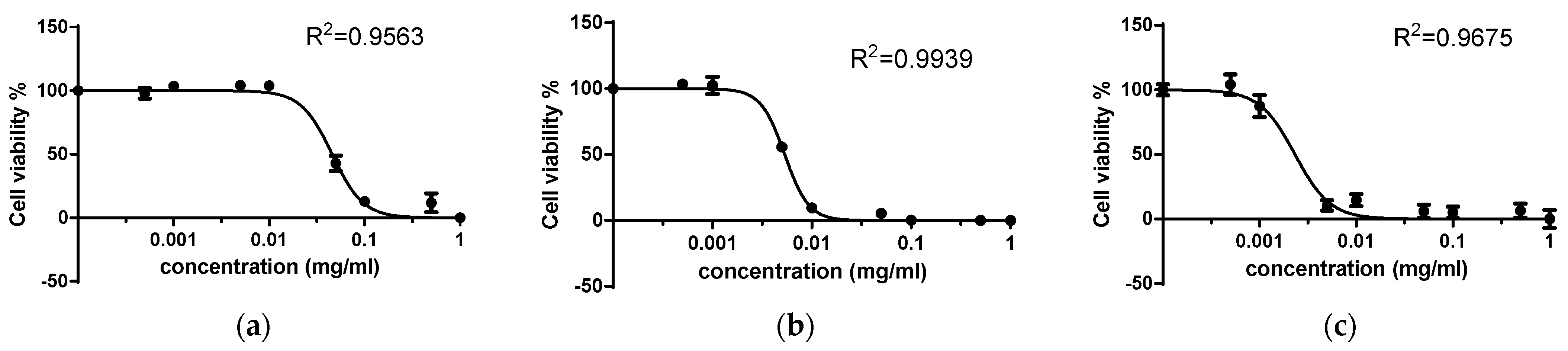
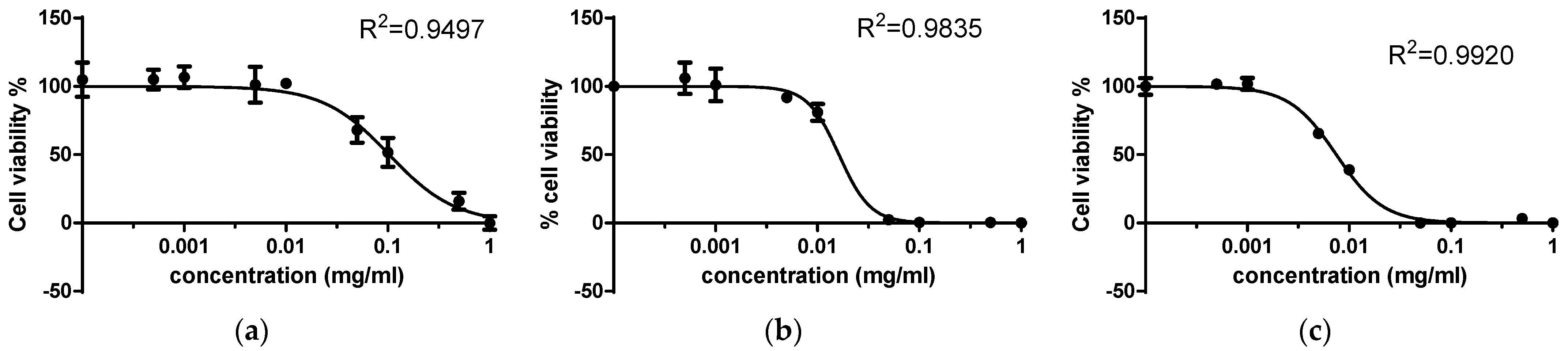
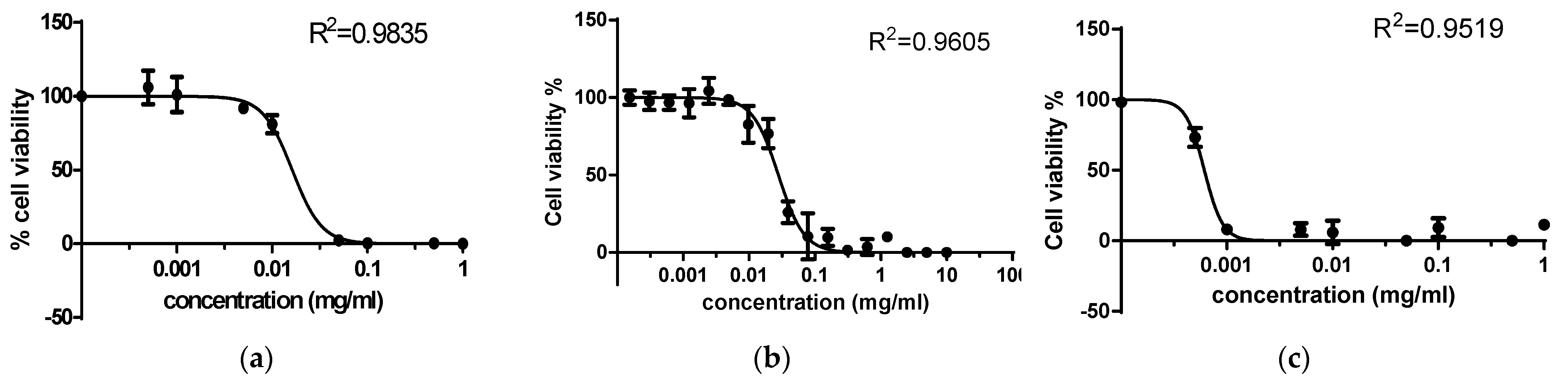
| Compound | Cytotoxic Concentration (CD50), mM | |
|---|---|---|
| Normal Cell Line | Tumor Cell Line | |
| Vero Cell Line (Kidney Cells from African Green Monkey) | HeLa Cell Line (Human Cervical Carcinoma) | |
| 2,4-dithiouracil | 1.42075 | 0.31674 |
| Cu(II) complex | 0.0396 | 0.0078 |
| Au(III) complex | 0.0385 | 0.00196 |
| Order | Au(III) complex > Cu(II) complex > 2,4-DTu | Au(III) complex > Cu(II) complex > 2,4-DTu |
| Compound | Cytotoxic Concentration (CD50), mM | |
|---|---|---|
| Normal Cell Line | Tumor Cell line | |
| Vero Cell Line (Kidney Cells from African Green Monkey) | HeLa Cell Line (Human Cervical Carcinoma) | |
| 6-propyl-2-thiouracil | 0.60683 | 0.0955 |
| Cu(II) complex | 0.0368 | 0.0113 |
| Pd(II) complex | 0.00779 | 0.00064 |
| Order | Pd(II) complex > Cu(II) complex > 6-Pro-2Tu | Pd(II) complex > Cu(II) complex > 6-Pro-2Tu |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinova, P.; Blazheva, D.; Slavchev, A.; Genova-Kalou, P. Investigation of the Cytotoxicity of Cu(II), Au(III), and Pd(II) Complexes with 2,4-Dithiouracil and 6-Propyl-2-thiouracil Derivatives. BioTech 2025, 14, 53. https://doi.org/10.3390/biotech14030053
Marinova P, Blazheva D, Slavchev A, Genova-Kalou P. Investigation of the Cytotoxicity of Cu(II), Au(III), and Pd(II) Complexes with 2,4-Dithiouracil and 6-Propyl-2-thiouracil Derivatives. BioTech. 2025; 14(3):53. https://doi.org/10.3390/biotech14030053
Chicago/Turabian StyleMarinova, Petya, Denica Blazheva, Aleksandar Slavchev, and Petia Genova-Kalou. 2025. "Investigation of the Cytotoxicity of Cu(II), Au(III), and Pd(II) Complexes with 2,4-Dithiouracil and 6-Propyl-2-thiouracil Derivatives" BioTech 14, no. 3: 53. https://doi.org/10.3390/biotech14030053
APA StyleMarinova, P., Blazheva, D., Slavchev, A., & Genova-Kalou, P. (2025). Investigation of the Cytotoxicity of Cu(II), Au(III), and Pd(II) Complexes with 2,4-Dithiouracil and 6-Propyl-2-thiouracil Derivatives. BioTech, 14(3), 53. https://doi.org/10.3390/biotech14030053







