Combined Effect of Spent Mushroom Substrate and Agro-Industrial Residues on Pleurotus columbinus Production and Intra-Cellular Polysaccharide Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Spawn Preparation
2.2. Substrates and Fermentation Parameters
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. Raw Materials, Substrate Analysis and Mycelium Growth Rate
3.2. Analysis of Substrates After P. columbinus Colonization
3.3. Laccase and Total Cellulases Production
3.4. Carbohydrate Production
3.5. Evaluation of Substrates for P. columbinus Cultivation
3.6. Intra-Cellullar Polysaccharide (ICP) Synthesis in Mushrooms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for Future foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Maftoun, P.; Johari, H.; Soltani, M.; Malik, R.; Othman, N.Z.; El Enshasy, H.A. The edible mushroom Pleurotus spp.: I. Biodiversity and nutritional values. Int. J. Biotechnol. Wellness Ind. 2015, 4, 67. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, X.; Xia, R.; Hou, Z.; Li, Y.; Feng, Y.; Pan, S.; Wang, Y.; Xu, H.; Huang, Z. Effect of mushroom root fermentation broth on the umami taste and nutrients Flammulina velutipes. J. Future Foods 2023, 3, 67–74. [Google Scholar] [CrossRef]
- Zisopoulos, F.K.; Ramírez, H.A.B.; van der Goot, A.J.; Boom, R.M. A resource efficiency assessment of the industrial mushroom production chain: The influence of data variability. J. Clean. Prod. 2016, 126, 394–408. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, R.; Zicari, S. Integrated Processing Technologies for Food and Agricultural by-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–438. [Google Scholar]
- Reis, N.; Botelho, B.G.; Franca, A.S.; Oliveira, L.S. Simultaneous detection of multiple adulterants in ground roasted coffee by ATR-FTIR spectroscopy and data fusion. Food Anal. Methods 2017, 10, 2700–2709. [Google Scholar] [CrossRef]
- Mefleh, M.; Pasqualone, A.; Caponio, F.; Faccia, M. Legumes as basic ingredients in the production of dairy-free cheese alternatives: A Review. J. Sci. Food Agric. 2022, 102, 8–18. [Google Scholar] [CrossRef]
- Brijbhooshan, B.; Singh, V.K.; Shalini, S. Response of field pea (Pisum sativum L. var arvense) to various planting methods, irrigation schedule and weed management practices. Legume Res. 2017, 40, 132–137. [Google Scholar] [CrossRef]
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.N.; Iliadis, K. Differences in chemical composition of field pea (Pisum sativum) cultivars: Effects of cultivation area and year. Food Chem. 2007, 103, 847–852. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Life cycle analysis of pistachio production in Greece. Sci. Total Environ. 2017, 595, 13–24. [Google Scholar] [CrossRef]
- Diamantis, I.; Dedousi, M.; Melanouri, E.-M.; Dalaka, E.; Antonopoulou, P.; Adelfopoulou, A.; Papanikolaou, S.; Politis, I.; Theodorou, G.; Diamantopoulou, P. Impact of spent mushroom substrate combined with hydroponic leafy vegetable roots on Pleurotus citrinopileatus productivity and fruit bodies biological properties. Microorganisms 2024, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Diamantopoulou, P.A.; Philippoussis, A.N. Valorization of spent oyster mushroom substrate and laccase recovery through successive solid state cultivation of Pleurotus, Ganoderma, and Lentinula strains. Appl. Microbiol. Biotechnol. 2017, 101, 5213–5222. [Google Scholar] [CrossRef] [PubMed]
- Hoa, H.T.; Wang, C.-L.; Wang, C.-H. The Effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.S.; Bento, H.B.; de Oliveira, F.; Picheli, F.P.; Dias, L.M.; Masarin, F.; Santos-Ebinuma, V.C. Xylanase production by Talaromyces amestolkiae valuing agroindustrial byproducts. BioTech 2022, 11, 15. [Google Scholar] [CrossRef]
- Chowdhary, P.; More, N.; Yadav, A.; Bharagava, R.N. Ligninolytic enzymes: An introduction and applications in the food industry. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–195. [Google Scholar] [CrossRef]
- Lavelli, V.; Proserpio, C.; Gallotti, F.; Laureati, M.; Pagliarini, E. Circular reuse of bio-resources: The role of Pleurotus spp. in the development of functional foods. Food Funct. 2018, 9, 1353–1372. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; EL-Ghwas, D.E.; Daba, G.M. Mushrooms as efficient enzymatic machinery. J. Biomed. Res. Environ. Sci. 2022, 3, 423–428. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Nassef, D.M.; Kotb, A.M.A.M.; Waly, E.A. Earliness, biological efficiency and basidiocarp yield of Pleurotus ostreatus and P. columbinus oyster mushrooms in response to different sole and mixed substrates. Assiut J. Agric. Sci. 2012, 43, 113–136. [Google Scholar] [CrossRef]
- Mandeel, Q.A.; Al-Laith, A.A.; Mohamed, S.A. Cultivation of οyster mushrooms (Pleurotus spp.) on various lignocellulosic wastes. World J. Microbiol. Biotechnol. 2005, 21, 601–607. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.; Aboshanab, K.M.; Yassien, M.A. Antiviral, cytotoxic, and antioxidant activities of three edible agaricomycetes mushrooms: Pleurotus columbinus, Pleurotus sajor-caju and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef]
- Irshad, A.; Tahir, A.; Sharif, S.; Khalid, A.; Ali, S.; Naz, A.; Sadia, H.; Ameen, A. Determination of nutritional and biochemical composition of selected Pleurotus spps. BioMed Res. Int. 2023, 2023, 8150909. [Google Scholar] [CrossRef]
- Amira, A.; El-Diasty, G.G. Evaluation of some white–rot fungi from Egypt for their ability to produce ligninolytic enzymes and decolorization of poly R-478. Bull. Fac. Sci. Elmenia Univ. 2006, 17, 1–44. [Google Scholar]
- Angelini, P.; Pellegrino, R.M.; Tirillini, B.; Flores, G.A.; Alabed, H.B.; Ianni, F.; Blasi, F.; Cossignani, L.; Venanzoni, R.; Orlando, G. Metabolomic profiling and biological activities of Pleurotus columbinus Quél. cultivated on different agri-food byproducts. Antibiotics 2021, 10, 1245. [Google Scholar] [CrossRef]
- Philippoussis, A.; Zervakis, G.; Diamantopoulou, P. Bioconversion of agricultural lignocellulosic wastes through the cultivation of the edible mushrooms Agrocybe aegerita, Volvariella volvacea and Pleurotus spp. World J. Microbiol. Biotechnol. 2001, 17, 191–200. [Google Scholar] [CrossRef]
- Sparks, D.L.; Fendorf, S.E.; Toner, C.V.; Carski, T.H. Kinetic methods and measurements. In SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 1275–1307. [Google Scholar] [CrossRef]
- American Public Health Association, American Water Works Association and Water Environmental Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association and Water Environmental Federation: Washington, DC, USA, 1998. [Google Scholar]
- Crocker, L.M.; DePeters, E.J.; Fadel, J.G.; Essex, S.E.; Perez-Monti, H.; Taylor, S.J. Ash content of detergent fibers in feeds, digesta and feces and its relevance in fiber digestibility calculations. J. Dairy Sci. 1998, 81, 1010–1014. [Google Scholar] [CrossRef]
- Goering, H.K. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970.
- Van Soest, P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Švagelj, M.; Berovič, M.; Boh, B.; Menard, A.; Simčič, S.; Wraber, B. Solid-state cultivation of Grifola frondosa [Dicks: Fr] SF Gray biomass and immunostimulatory effects of fungal intra-and extracellular β-Polysaccharides. New Biotechnol. 2008, 25, 150–156. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Patterns of major metabolites biosynthesis by different mushroom fungi grown on glucose-based submerged cultures. Bioprocess Biosyst. Eng. 2014, 37, 1385–1400. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y.; Blackburn, J.W. Batch stage study of lipid production from crude glycerol derived from yellow grease or animal fats through microalgal fermentation. Bioresour. Technol. 2010, 101, 6745–6750. [Google Scholar] [CrossRef]
- Atila, F. Evaluation of suitability of various agro-wastes for productivity of Pleurotus djamor, Pleurotus citrinopileatus and Pleurotus eryngii mushrooms. J. Exp. Agric. Int. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; El Sebaaly, Z.; Alsanad, M.A.; Najjar, R.; Böhme, M.; Yordanova, M.H.; Sassine, Y.N. Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition and nutritional value. PLoS ONE 2021, 16, e0255794. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Diamantis, I.; Dedousi, M.; Dalaka, E.; Antonopoulou, P.; Papanikolaou, S.; Politis, I.; Theodorou, G.; Diamantopoulou, P. Pleurotus ostreatus: Nutritional enhancement and antioxidant activity improvement through cultivation on spent mushroom substrate and roots of leafy vegetables. Fermentation 2025, 11, 20. [Google Scholar] [CrossRef]
- Dedousi, M.; Melanouri, E.-M.; Karayannis, D.; Kaminarides, E.-I.; Diamantopoulou, P. Utilization of spent substrates and waste products of mushroom cultivation to produce new crops of Pleurotus ostreatus, Pleurotus eryngii and Agaricus bisporus. Carbon Resour. Convers. 2024, 7, 100196. [Google Scholar] [CrossRef]
- Wang, S.; Xu, F.; Li, Z.; Zhao, S.; Song, S.; Rong, C.; Geng, X.; Liu, Y. The spent mushroom substrates of Hypsizigus marmoreus can be an effective component for growing the oyster mushroom Pleurotus ostreatus. Sci. Hortic. 2015, 186, 217–222. [Google Scholar] [CrossRef]
- Alsanad, M.; Sassine, Y.N.; El Sebaaly, Z.; Abou Fayssal, S. Spent coffee grounds influence on Pleurotus ostreatus production, composition, fatty acid profile, and lignocellulose biodegradation capacity. CyTA J. Food 2021, 19, 11–20. [Google Scholar] [CrossRef]
- Diamantis, I.; Papanikolaou, S.; Michou, S.; Anastasopoulos, V.; Diamantopoulou, P. Yeast lipids from crude glycerol media and utilization of lipid fermentation wastewater as maceration water in cultures of edible and medicinal mushrooms. Processes 2023, 11, 3178. [Google Scholar] [CrossRef]
- Zervakis, G.; Philippoussis, A.; Ioannidou, S.; Diamantopoulou, P. Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiol. 2001, 46, 231–234. [Google Scholar] [CrossRef]
- Atila, F. Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of Shiitake mushroom. Sci. Hortic. 2019, 245, 263–268. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Markou, G.; Kremmyda, A.; Haroutounian, S.A.; Arapoglou, D. Enrichment of pistachio shell with olive mill waste or Lathyrus clymenum pericarp mixtures via solid state fermentation with Pleurotus ostreatus. Fermentation 2022, 8, 59. [Google Scholar] [CrossRef]
- Parani, K.; Eyini, M. Biodegradation of coffee pulp waste by different fungal associations. Biosci. Discov. 2012, 3, 222–228. [Google Scholar]
- Akinfemi, A.; Adu, O.A.; Doherty, F. Conversion of sorghum stover into animal feed with white-rot fungi: Pleurotus ostreatus and Pleurotus pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Papadopoulou, K.; Lakhtar, H.; Roussos, S.; Parissopoulos, G.; Papanikolaou, S. Biomass, laccase and endoglucanase production by Lentinula edodes during solid state fermentation of reed grass, bean stalks and wheat straw residues. World J. Microbiol. Biotechnol. 2011, 27, 285–297. [Google Scholar] [CrossRef]
- Dedousi, M.; Melanouri, E.-M.; Diamantopoulou, P. Carposome productivity of Pleurotus ostreatus and Pleurotus eryngii growing on agro-industrial residues enriched with nitrogen, calcium salts and oils. Carbon Resour. Convers. 2023, 6, 150–165. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Penninckx, M.J.; Tsiklauri, N.; Elisashvili, V. Effect of nitrogen source on lignocellulolytic enzyme production by white-rot basidiomycetes under solid-state cultivation. World J. Microbiol. Biotechnol. 2006, 22, 391–397. [Google Scholar] [CrossRef]
- Economou, C.N.; Philippoussis, A.N.; Diamantopoulou, P.A. Spent mushroom substrate for a second cultivation cycle of Pleurotus mushrooms and dephenolization of agro-industrial wastewaters. FEMS Microbiol. Lett. 2020, 367, fnaa060. [Google Scholar] [CrossRef]
- Khalil, M.I.; Hoque, M.; Basunia, M.A.; Alam, N.; Khan, M.A. Production of cellulase by Pleurotus ostreatus and Pleurotus sajor-caju in solid state fermentation of lignocellulosic biomass. Turk. J. Agric. For. 2011, 35, 333–341. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Penninckx, M. Effect of growth substrate, method of fermentation and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J. Ind. Microbiol. Biotechnol. 2008, 35, 1531–1538. [Google Scholar] [CrossRef]
- Pandit, N.P.; Maheshwari, S.K. Optimization of cellulase enzyme production from sugarcane pressmud using oyster mushroom-Pleurotus sajor-caju by solid state fermentation. J. Bioremediation Biodegrad. 2012, 3, 1000140. [Google Scholar] [CrossRef]
- Zamora Zamora, H.D.; Silva, T.A.; Varao, L.H.; Baffi, M.A.; Pasquini, D. Simultaneous production of cellulases, hemicellulases and reducing sugars by Pleurotus ostreatus growth in one-pot solid state fermentation using Alstroemeria Sp. waste. Biomass Convers. Biorefinery 2021, 13, 4879–4892. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Papanikolaou, S.; Diamantopoulou, P. Mortierella ramanniana lipid fermentation wastewater as an innovative maceration liquid medium for sustainable solid-state cultivation of higher fungi. Waste Biomass Valorization 2024, 15, 6903–6925. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Olsen, L.M.; Ruthes, A.C.; Czelusniak, P.A.; Santana-Filho, A.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Exopolysaccharides, proteins and lipids in Pleurotus pulmonarius submerged culture using different carbon sources. Carbohydr. Polym. 2012, 87, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Ozturk Urek, R.; Ilgin, S. Production and partial characterization of the exopolysaccharide from Pleurotus sajor caju. Ann. Microbiol. 2019, 69, 1201–1210. [Google Scholar] [CrossRef]
- Silveira, M.L.; Smiderle, F.R.; Agostini, F.; Pereira, E.M.; Bonatti-Chaves, M.; Wisbeck, E.; Ruthes, A.C.; Sassaki, G.L.; Cipriani, T.R.; Furlan, S.A. Exopolysaccharide produced by Pleurotus sajor-caju: Its chemical structure and anti-inflammatory activity. Int. J. Biol. Macromol. 2015, 75, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sheng, K.; Yan, E.; Qiao, J.; Lv, F. Extraction, purification and antibacterial activities of a polysaccharide from spent mushroom substrate. Int. J. Biol. Macromol. 2012, 50, 840–843. [Google Scholar] [CrossRef]
- EL-Sayed, S.S.; Anwar, D.A.; Hassan, H.A. Comparison between peels of prickly pear and rice husk as additives to rice straw substrate for oyster mushroom (Pleurotus columbinus) production. Sci. J. Agric. Sci. 2023, 5, 19–37. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Refaei, E.F.S.; Abdalla, M.M.A.; Abdelgalil, S.H. Fruiting bodies yield of oyster mushroom (Pleurotus columbinus) as affected by different portions of compost in the substrate. Int. J. Recycl. Org. Waste Agric. 2016, 5, 281–288. [Google Scholar] [CrossRef]
- Lisiecka, J.; Prasad, R.; Jasinska, A. The Utilisation of Pholiota nameko, Hypsizygus marmoreus, and Hericium erinaceus spent mushroom substrates in Pleurotus ostreatus cultivation. Horticulturae 2021, 7, 396. [Google Scholar] [CrossRef]
- Lesa, K.N.; Khandaker, M.U.; Mohammad Rashed Iqbal, F.; Sharma, R.; Islam, F.; Mitra, S.; Emran, T.B. Nutritional value, medicinal importance, and health-promoting effects of dietary mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 2454180. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P.; Gkatzionis, K.; Ioannou, Z.; Sarris, D. Submerged cultivation of selected macro-fungi to produce mycelia rich in β-glucans and other bioactive compounds, valorizing side streams of the food industry. Carbon Resour. Convers. 2024, 7, 100198. [Google Scholar] [CrossRef]
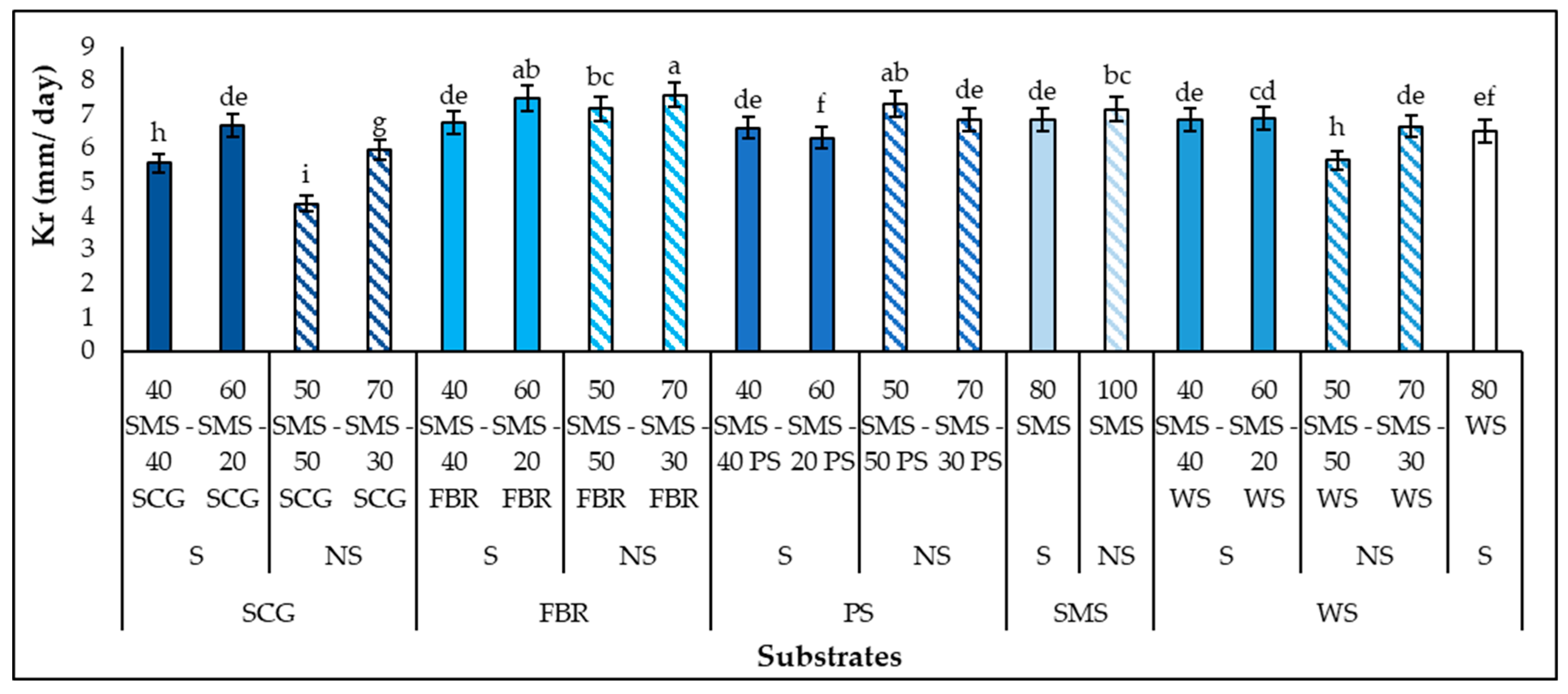

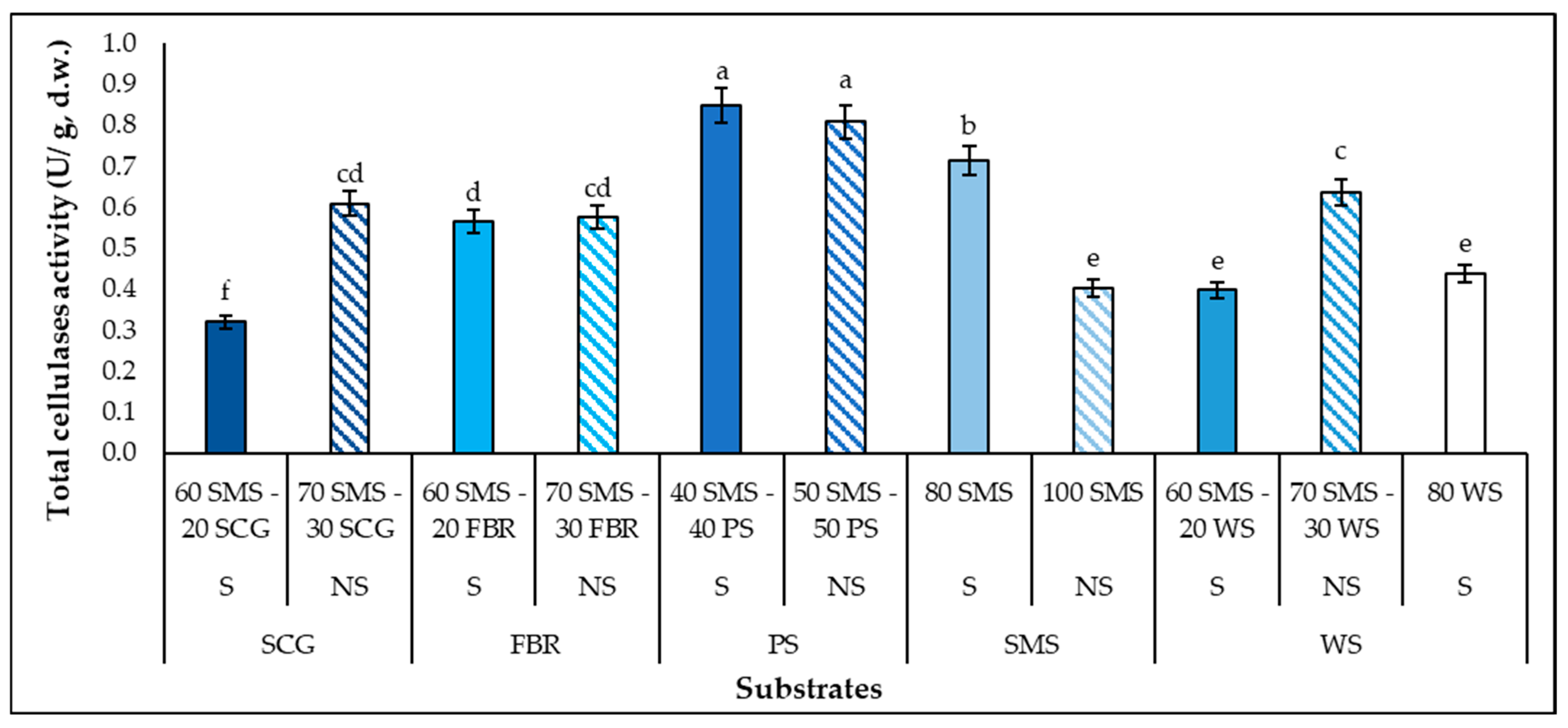
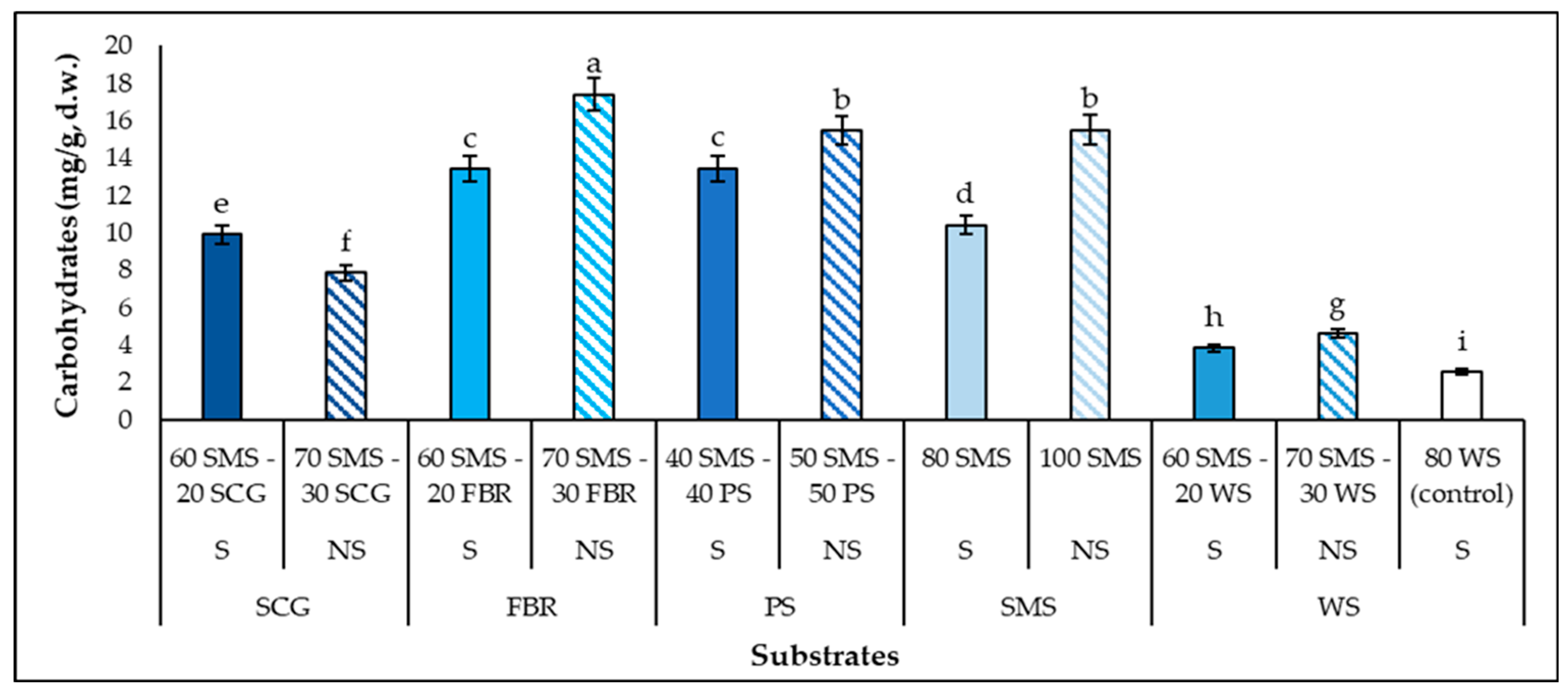
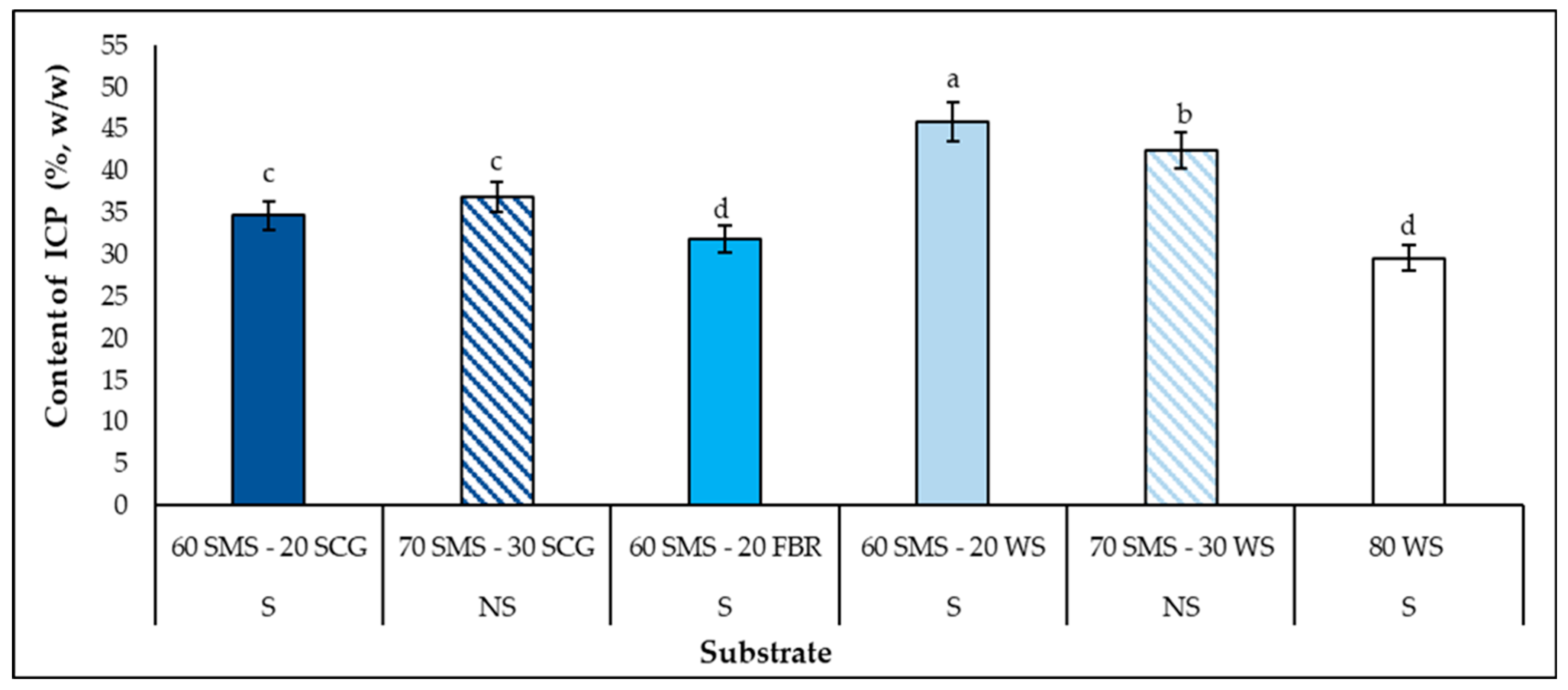
| Parameters (%) | SMS * | SCG | FBR | PS | WS | WB | SF |
|---|---|---|---|---|---|---|---|
| C | 29.0 ± 0.1 e ** | 32.9 ± 0.0 a | 29.7 ± 0.6 d | 31.1 ± 0.4 c | 31.5 ± 0.2 bc | 31.7 ± 0.2 b | 31.3 ± 0.1 bc |
| N | 0.8 ± 0.0 d | 1.2 ± 0.1 c | 0.6 ± 0.0 e | 0.5 ± 0.0 e | 0.5 ± 0.0 e | 1.9 ± 0.1 b | 6.6 ± 0.1 a |
| Protein | 5.0 ± 0.2 d | 7.5 ± 0.4 c | 3.6 ± 0.2 e | 3.3 ± 0.2 e | 3.0 ± 0.0 e | 11.6 ± 0.4 b | 41.0 ± 0.6 a |
| Cellulose | 37.1 ± 1.0 b | 22.6 ± 0.2 d | 38.1 ± 0.0 b | 33.3 ± 0.7 c | 41.2 ± 1.9 a | 10.4 ± 0.0 f | 13.9 ± 0.1 e |
| Lignin | 7.0 ± 0.3 c | 12.0 ± 0.2 b | 7.0 ± 0.2 c | 14.8 ± 0.5 a | 5.7 ± 0.0 d | 3.0 ± 0.1 e | 7.6 ± 0.2 c |
| Supplemented Substrates | C (% d.w.) | N (% d.w.) | C/N | Cellulose (%, w/w) | Lignin (%, w/w) |
|---|---|---|---|---|---|
| 40 SMS-40 SCG * | 30.5 ± 0.9 b ** | 1.3 ± 0.1 a | 22.7 ± 0.5 e | 26.5 ± 0.4 e | 9.9 ± 0.6 ab |
| 60 SMS-20 SCG | 30.2 ± 0.2 bcde | 1.3 ± 0.0 ab | 23.8 ± 0.9 de | 29.6 ± 0.4 d | 8.9 ± 0.6 bc |
| 40 SMS-40 FBR | 29.7 ± 0.1 cde | 1.1 ± 0.0 bc | 26.2 ± 1.0 cd | 32.3 ± 1.3 bc | 7.8 ± 0.7 cd |
| 60 SMS-20 FBR | 29.6 ± 0.1 de | 1.2 ± 0.1 bc | 25.5 ± 1.2 d | 32.5 ± 1.3 bc | 7.9 ± 0.7 cd |
| 40 SMS-40 PS | 30.3 ± 0.1 bcd | 1.1 ± 0.0 cd | 28.2 ± 1.1 bc | 30.8 ± 0.9 cd | 11.0 ± 0.9 a |
| 60 SMS-20 PS | 29.9 ± 0.1 bcde | 1.0 ± 0.0 cd | 29.0 ± 1.2 bc | 31.7 ± 0.9 bcd | 9.4 ± 0.9 b |
| 80 SMS | 29.5 ± 0.1 e | 1.0 ± 1.0 cd | 28.3 ± 1.5 bc | 32.7 ± 0.2 abc | 6.8 ± 0.6 de |
| 40 SMS-40 WS | 30.4 ± 0.2 bc | 1.1 ± 0.1 cd | 28.8 ± 1.7 bc | 33.9 ± 2.1 ab | 7.3 ± 0.5 d |
| 60 SMS-20 WS | 29.9 ± 0.2 bcde | 1.0 ± 0.0 cd | 29.2 ± 0.9 b | 33.3 ± 2.1 abc | 7.6 ± 0.5 cd |
| 80 WS | 31.4 ± 0.1 a | 0.9 ± 0.0 d | 34.0 ± 1.2 a | 35.2 ± 2.1 a | 5.7 ± 0.6 e |
| Non-Supplemented Substrates | C (% d.w.) | N (% d.w.) | C/N | Cellulose (%, w/w) | Lignin (%, w/w) |
|---|---|---|---|---|---|
| 50 SMS-50 SCG * | 30.4 ± 0.8 b ** | 1.0 ± 0.1 a | 32.0 ± 1.5 e | 30.3 ± 0.2 e | 9.5 ± 0.4 b |
| 70 SMS-30 SCG | 30.1 ± 0.1 bc | 0.9 ± 0.0 b | 34.5 ± 1.6 e | 33.4 ± 0.2 e | 8.5 ± 0.4 c |
| 50 SMS-50 FBR | 29.2 ± 0.2 de | 0.7 ± 0.0 c | 41.1 ± 0.7 d | 37.6 ± 1.1 bcd | 7.0 ± 0.5 d |
| 70 SMS-30 FBR | 29.1 ± 0.2 de | 0.7 ± 0.0 c | 43.8 ± 1.1 d | 37.8 ± 1.1 bcd | 7.0 ± 0.5 d |
| 50 SMS-50 PS | 30.0 ± 0.1 bc | 0.6 ± 0.0 d | 52.2 ± 1 bc | 35.7 ± 0.7 d | 10.9 ± 0.7 a |
| 70 SMS-30 PS | 29.5 ± 0.2 cde | 0.6 ± 0.0 d | 49.0 ± 2.2 c | 36.6 ± 0.7 cd | 9.4 ± 0.7 b |
| 100 SMS | 28.9 ± 0.2 e | 0.6 ± 0.0 d | 48.8 ± 2.4 c | 38.1 ± 0.0 bc | 7.0 ± 0.2 d |
| 50 SMS-50 WS | 30.2 ± 0.1 bc | 0.6 ± 0.0 de | 54.8 ± 2.8 b | 39.6 ± 1.9 ab | 6.4 ± 0.2 de |
| 70 SMS-30 WS | 29.7 ± 0.1 bcd | 0.6 ± 0.0 d | 51.4 ± 1.7 bc | 39.0 ± 1.9 b | 6.6 ± 0.2 d |
| Substrates | Incubation Period (Days) | Biomass (mg/g d.w.) | Crem (%, w/w) | Nrem (%, w/w) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supplemented | 60 SMS-20 SCG * | 12 ± 0 | f ** | C *** | 700.0 ± 17.2 | c | B | 29.6 ± 0.9 | a | A | 1.7 ± 0.1 | ab | AB |
| 60 SMS-20 FBR | 13 ± 1 | de | B | 279.2 ± 12.8 | h | D | 29.1 ± 0.4 | b | B | 1.6 ± 0.1 | bc | B | |
| 40 SMS-40 PS | 10 ± 0 | g | D | 558.2 ± 17.2 | d | C | 28.8 ± 0.5 | c | C | 1.7 ± 0.0 | a | A | |
| 80 SMS | 14 ± 0 | c | A | 746.0 ± 21.0 | b | A | 27.4 ± 0.2 | e | D | 1.4 ± 0.0 | de | C | |
| 60 SMS-20 WS | 13 ± 0 | d | B | 313.9 ± 12.8 | gh | D | 29.0 ± 0.0 | bc | BC | 1.3 ± 0.0 | ef | C | |
| Non-supplemented | 70 SMS-30 SCG | 12 ± 1 | ef | C | 803.7 ± 22.9 | a | A | 29.1 ± 0.1 | b | B | 1.5 ± 0.1 | cd | A |
| 70 SMS-30 FBR | 16 ± 0 | a | A | 494.7 ± 12.2 | e | C | 27.5 ± 0.2 | de | C | 1.3 ± 0.1 | ef | B | |
| 50 SMS-50 PS | 9 ± 0 | h | D | 554.9 ± 17.2 | d | B | 29.6 ± 1.6 | a | A | 1.0 ± 0.1 | g | C | |
| 100 SMS | 16 ± 1 | a | A | 756.0 ± 24.8 | b | A | 27.7 ± 0.2 | d | C | 0.8 ± 0.0 | f | D | |
| 70 SMS-30 WS | 15 ± 0 | b | B | 424.0 ± 19.9 | f | D | 28.9 ± 0.7 | bc | B | 0.8 ± 0.1 | h | D | |
| 80 WS (control) | 16 ± 0 | a | 345.2 ± 14.7 | g | 29.5 ± 0.3 | a | 1.3 ± 0.0 | h | |||||
| Substrates | Earliness (Days) | B.E. (%) | |||||
|---|---|---|---|---|---|---|---|
| Supplemented | 60 SMS-20 SCG * | 19 ± 0 | f ** | C *** | 87.1 ± 1.5 | a | A |
| 60 SMS-20 FBR | 21 ± 1 | de | B | 69.6 ± 2.1 | b | B | |
| 40 SMS-40 PS | 19 ± 1 | f | C | 28.5 ± 0.7 | h | E | |
| 80 SMS | 20 ± 0 | ef | BC | 49.7 ± 1.9 | f | D | |
| 60 SMS-20 WS | 24 ± 1 | c | A | 59.3 ± 2.3 | de | C | |
| Non-supplemented | 70 SMS-30 SCG | 20 ± 1 | f | C | 63.4 ± 1.2 | c | A |
| 70 SMS-30 FBR | 27 ± 1 | a | A | 23.4 ± 0.6 | i | D | |
| 50 SMS-50 PS | 19 ± 1 | f | C | 57.1 ± 1.6 | e | B | |
| 100 SMS | 22 ± 1 | cd | B | 34.6 ± 0.6 | g | C | |
| 70 SMS-30 WS | 25 ± 1 | b | A | 63.9 ± 1.7 | c | A | |
| 80 WS | 27 ± 1 | ab | 61.6 ± 2.4 | cd | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedousi, M.; Gardeli, C.; Papanikolaou, S.; Diamantopoulou, P. Combined Effect of Spent Mushroom Substrate and Agro-Industrial Residues on Pleurotus columbinus Production and Intra-Cellular Polysaccharide Synthesis. BioTech 2025, 14, 34. https://doi.org/10.3390/biotech14020034
Dedousi M, Gardeli C, Papanikolaou S, Diamantopoulou P. Combined Effect of Spent Mushroom Substrate and Agro-Industrial Residues on Pleurotus columbinus Production and Intra-Cellular Polysaccharide Synthesis. BioTech. 2025; 14(2):34. https://doi.org/10.3390/biotech14020034
Chicago/Turabian StyleDedousi, Marianna, Chrysavgi Gardeli, Seraphim Papanikolaou, and Panagiota Diamantopoulou. 2025. "Combined Effect of Spent Mushroom Substrate and Agro-Industrial Residues on Pleurotus columbinus Production and Intra-Cellular Polysaccharide Synthesis" BioTech 14, no. 2: 34. https://doi.org/10.3390/biotech14020034
APA StyleDedousi, M., Gardeli, C., Papanikolaou, S., & Diamantopoulou, P. (2025). Combined Effect of Spent Mushroom Substrate and Agro-Industrial Residues on Pleurotus columbinus Production and Intra-Cellular Polysaccharide Synthesis. BioTech, 14(2), 34. https://doi.org/10.3390/biotech14020034








