Comparative Potential of Chitinase and Chitosanase from the Strain Bacillus thuringiensis B-387 for the Production of Antifungal Chitosan Oligomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Bacterial Strain, Its Maintenance, and Cultivation Media
2.2.2. Purification of Chitinase/Chitosanase and Protein Measurement
2.2.3. Enzyme Activity Assay
2.2.4. Analysis of Biochemical and Catalytic Properties of the Enzymes
2.2.5. TLC- and MS-HPLC-Analyses of Chitin/Chitosan Breakdown Products by Chitinase and Chitosanase
2.2.6. Partial Depolymerization of Chitosan by Chitinase/Chitosanase and Molecular Mass Characteristics of Low-Molecular Weight Chitosan’s Fractions
2.2.7. NMR Analysis of Chitosan Oligomers’ Structure
2.2.8. Comparative Antifungal Assay of the Oligomers Generated by Partial Hydrolysis of Chitosan by Chitinase and Chitosanase
2.2.9. Microscopic Examination of the COS Effect on Fungal Hyphae Morphology
2.3. Statistical Analysis
3. Results
3.1. Bacterial Growth and Chitinase and Chitosanase Production
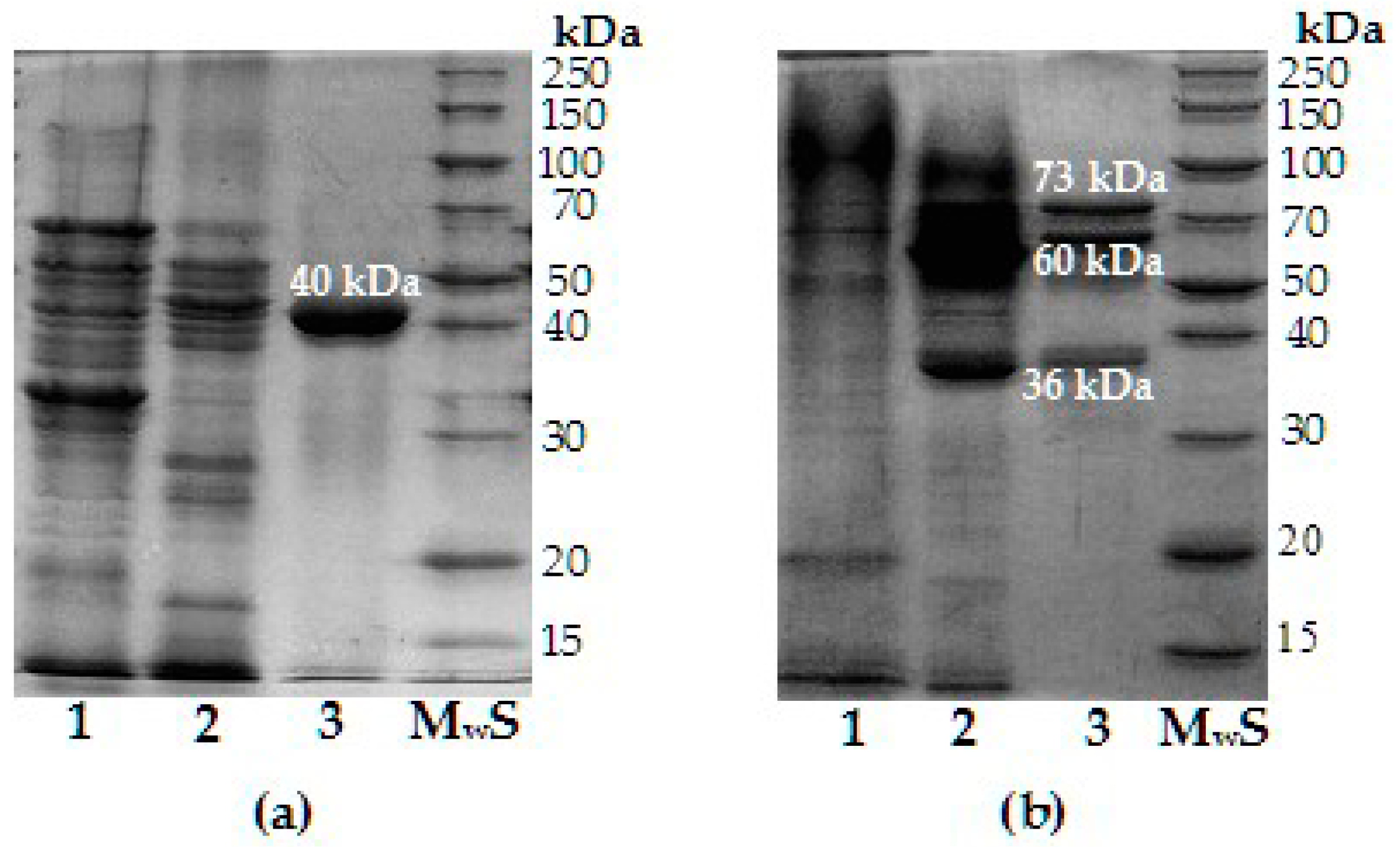
3.2. Purification Specifics and Main Characteristics of Chitinase and Chitosanase
3.3. Comparative Biochemical and Catalytic Characteristics of Purified Chitinase and Chitosanase
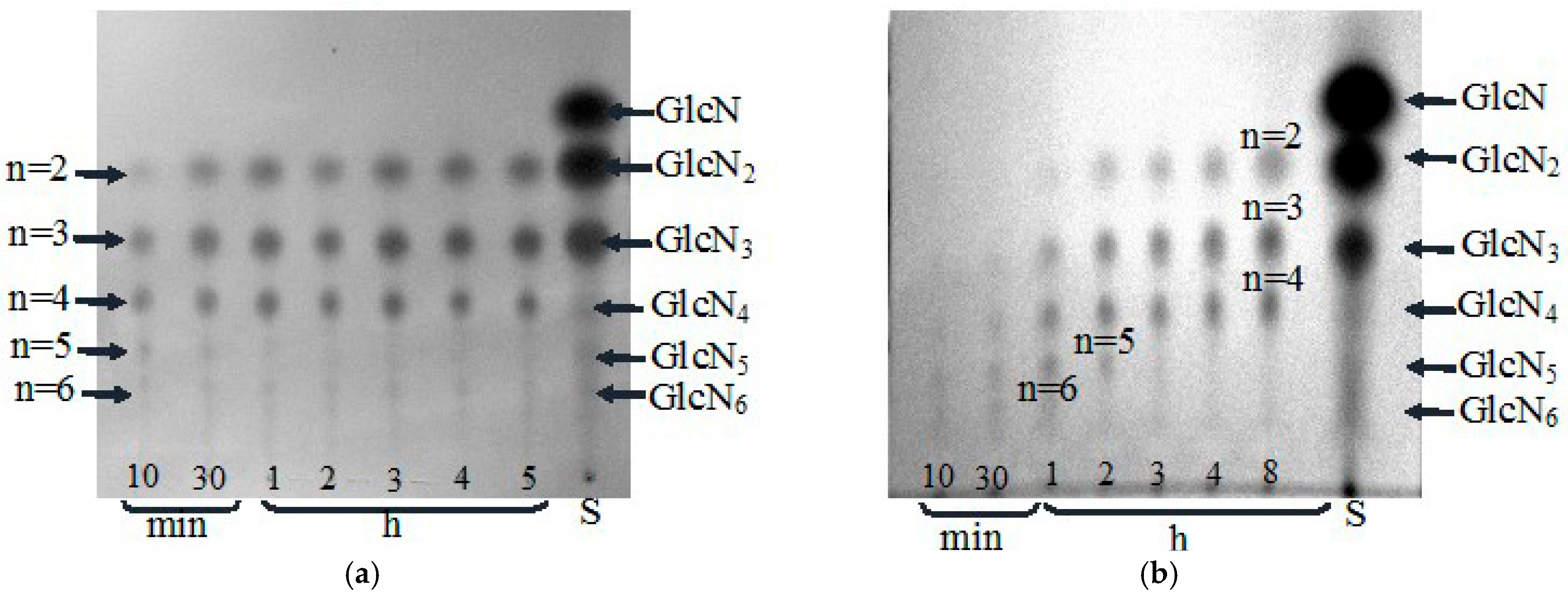
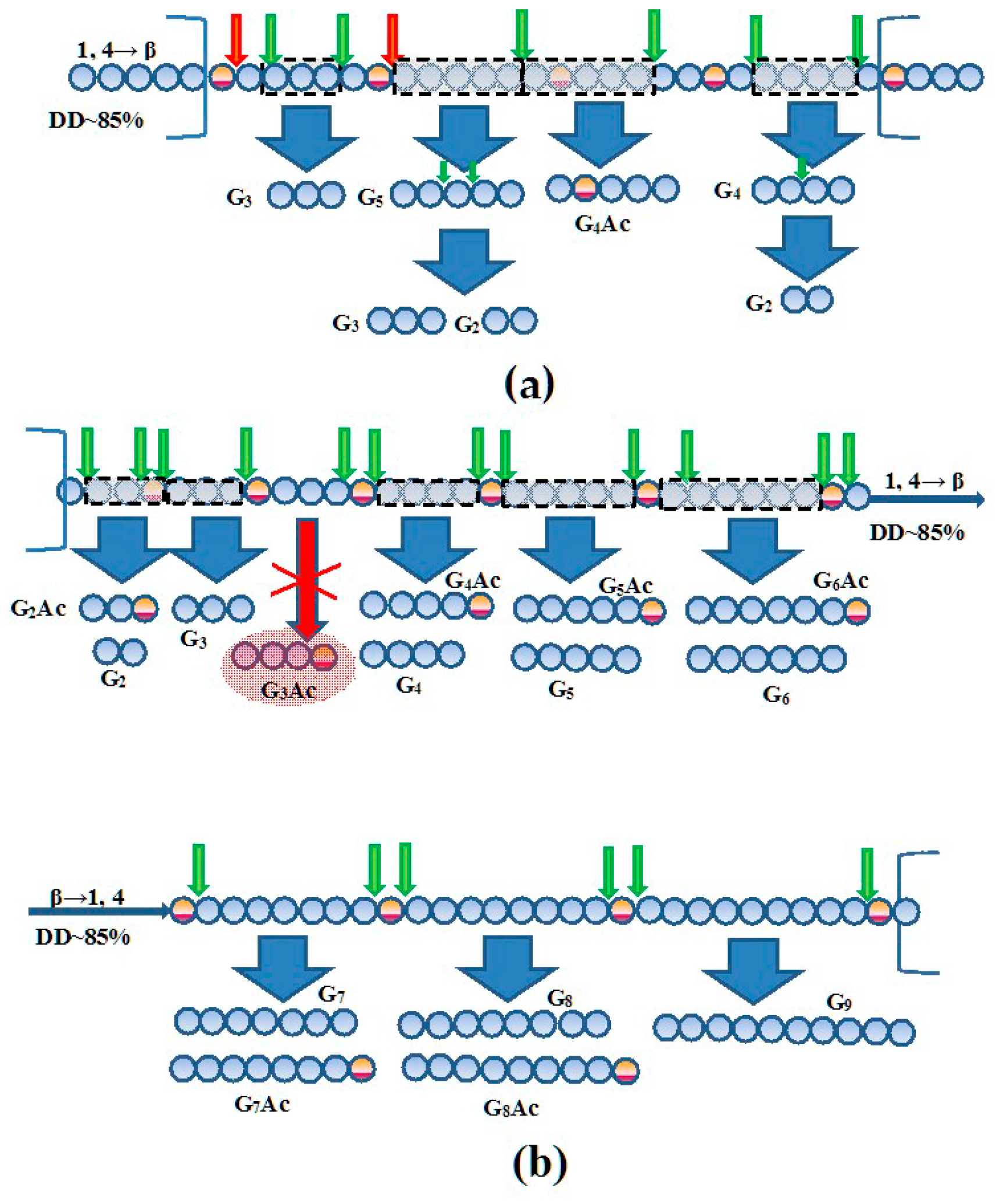
3.4. HPLC-MS Analysis of the COS Generated During Extensive Hydrolysis of Chitosan by Chitinase and Chitosanase
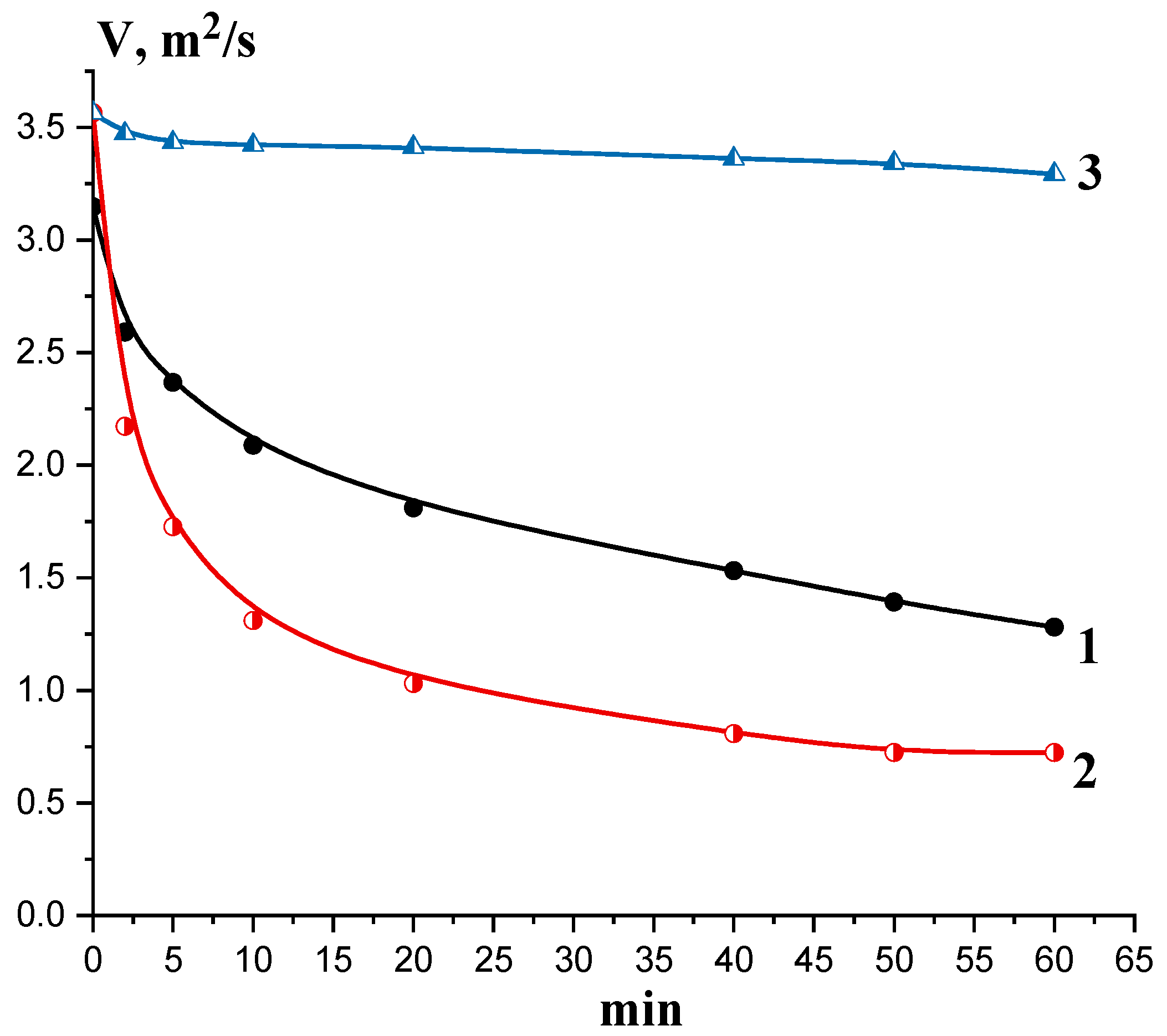
3.5. Molecular-Mass Characteristics, Solubility, and Antifungal Potential of the Oligomers Generated at Partial Depolymerization of Chitosan by Chitinase and Chitosanase
3.6. NMR Spectral Analysis of the Partially Hydrolyzed Chitosan Oligomers Produced by Chitinase and Chitosanase
3.7. Antifungal Effect of Oligomers Generated by Chitosan Hydrolysis Using Chitosanase and Chitinase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, S.; Zhou, S.; Tan, Y.; Feng, J.; Ba, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Biodegradation and prospect of polysaccharide from crustaceans. Mar. Drugs 2022, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and chitosan as polymers of the future—Obtaining, modification, life cycle assessment and main directions of application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; El Seoud, O.A. Sustainable biomaterials based on cellulose, chitin and chitosan composites—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Martinez Garcia, J.P.; Falomir, M.P.; Gozalbo, D. Chitin: A structural biopolysaccharide with multiple applications. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 1–10. [Google Scholar] [CrossRef]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. Technol. Appl. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 103–1051. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Iber, B.T.; Kazan, N.A.; Torsabo, D.; Omuwa, J.W. A review of various sources of chitin and chitosan in nature. JRM 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- do Amaral Sobral, P.J.; Gebremariam, G.; Drudi, F.; De Aguiar Saldanha Pinheiro, A.C.; Romani, S.; Rocculi, P.; Dalla Rosa, M. Rheological and viscoelastic properties of chitosan solutions prepared with different chitosan or acetic acid concentrations. Foods 2022, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Rosca, I.; Ursu, L.; Dascalu, A. Chitosan oligomers—Synthesis, characterization and properties. Cellulose Chem. Technol. 2022, 56, 767–776. [Google Scholar] [CrossRef]
- Anil, S. Potential medical applications of chitooligosaccharides. Polymers 2022, 14, 3558. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Le Folle, M.D. Advances in chitooligosaccharides chemical modifications. Biopolymers 2021, 112, e23461. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in research of chitosan chemical modification technologies and their applications. Mar. Drugs. 2022, 20, 536. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Hasan, N.; Kashif Ali, S.; Shin, J.; Gopal, J.; Muthu, M.; Oh, J.-W. Novel chitosan derivatives and their multifaceted biological applications. Appl. Sci. 2022, 22, 3267. [Google Scholar] [CrossRef]
- Leal, M.R.S.; Lima, L.R.A.; Rodrigues, N.E.R.; Soares, P.A.G.; Carneiro-da-Cunha, M.G.; Albuquerque, P.B.S. A review on the biological activities and the nutraceutical potential of chitooligosaccharides. Carbohydr. Res. 2025, 548, 109336. [Google Scholar] [CrossRef]
- Guan, Z.; Feng, Q. Chitosan and chitooligosaccharide: The promising non-plant-derived prebiotics with multiple biological activities. Int. J. Mol. Sci. 2022, 23, 6761. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of low molecular weight chitosan and chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Aam, B.; Heggset, E.; Norberg, A.; Sǿrlie, M.; Vårum, K.; Ejsink, V. Production of chitooligosaccharides and their potential application in medicine. Review. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Sørlie, M.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Using chitosan to understand chitinases and the role of processivity in the degradation of recalcitrant polysaccharides. React. Funct. Polym. 2020, 148, 104488. [Google Scholar] [CrossRef]
- Zhang, L.L.; Jiang, X.H.; Xiao, X.F.; Zhang, W.X.; Shi, Y.Q.; Wang, Z.P.; Zhou, H.X. Expression and characterization of a novel cold-adapted chitosanase from marine Renibacterium sp. suitable for chitooligosaccharides preparation. Mar. Drugs 2021, 19, 596. [Google Scholar] [CrossRef]
- Cui, D.; Yang, J.; Lu, B.; Shen, H. Efficient preparation of chitooligosaccharide with a potential chitosanase Csn-SH and its application for fungi disease protection. Front. Microbiol. 2021, 12, 682829. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, H.; Zhu, B.; Yao, Z.; Jiang, L. Purification and biochemical characterization of a novel chitosanase cloned from the gene of Kitasatospora setae KM-6054 and its application in the production of chitooligosaccharides. World J. Microbiol. Biotechnol. 2023, 39, 111. [Google Scholar] [CrossRef]
- Kendra, D.F.; Hadwiger, L.A. Characterization of the smallest chitosan oligomer that is maximally antifungal to Fusarium solani and elicits pisatin formation in Pisum sativum. Exp. Mycol. 1984, 8, 276–281. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Blagodatskikh, I.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of Candida albicans: Molecular weight–activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef]
- Park, Y.; Kim, M.H.; Park, S.C.; Cheong, H.; Jang, M.K.; Nah, J.W.; Hahm, K.S. Investigation of the antifungal activity and mechanism of action of LMWS-chitosan. J. Microbiol. Biotechnol. 2008, 18, 1729–1734. [Google Scholar]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef]
- Wu, C.; Yu, X.; Zheng, P.; Chen, P.; Wu, D. Rational redesign of chitosanase to enhance thermostability and catalytic activity to produce chitooligosaccharides with a relatively high degree of polymerization. J. Agric. Food Chem. 2023, 71, 15213–15223. [Google Scholar] [CrossRef]
- Jung, W.J.; Park, R.D. Bioproduction of chitooligosaccharides: Present and perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Zhu, M.; Chen, W.; Yu, S.; Zhong, B. Overexpression and biochemical properties of a GH46 chitosanase from marine Streptomyces hygroscopicus R1 suitable for chitosan oligosaccharides preparation. Front. Microbiol. 2022, 12, 816845. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, L.; Mao, X.; Cao, R.; Liu, Q. Identification of a key loop for tuning transglycosylation activity in the substrate-binding region of a chitosanase. J. Agric. Food Chem. 2023, 71, 5585–5591. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.B.; Lin, Y.C.; Chen, H.H. Low molecular weight chitosan prepared with the aid of cellulase, lysozyme and chitinase: Characterization and antibacterial activity. Food Chem. 2009, 116, 47–53. [Google Scholar] [CrossRef]
- Juárez-Hernández, E.O.; Casados-Vázquez, L.E.; Brieba, L.G.; Torres-Larios, A.; Jimenez-Sandoval, P.; Barboza-Corona, J.E. The crystal structure of the chitinase ChiA74 of Bacillus thuringiensis has a multidomain assembly. Sci. Rep. 2019, 9, 2591. [Google Scholar] [CrossRef]
- Kobayashi, T.; Koide, O.; Deguchi, S.; Horikoshi, K. Characterization of chitosanase of a deep biosphere Bacillus strain. Biosci. Biotechnol. Biochem. 2011, 75, 669–673. [Google Scholar] [CrossRef]
- Lee, H.S.; Jang, J.S.; Choi, S.K.; Lee, D.W.; Kim, E.J.; Jung, H.C.; Pan, J.G. Identification and expression of GH-8 family chitosanases from several Bacillus thuringiensis subspecies. FEMS Microbiol. Lett. 2007, 277, 133–141. [Google Scholar] [CrossRef]
- Martínez-Zavala, S.A.; Barboza-Pérez, U.E.; Hernández-Guzmán, G.; Bideshi, D.K.; Barboza-Corona, J.E. Chitinases of Bacillus thuringiensis: Phylogeny, modular structure, and applied potentials. Front. Microbiol. 2020, 10, 3032. [Google Scholar] [CrossRef]
- Aktuganov, G.E.; Safina, V.R.; Galimzianova, N.F.; Gilvanova, E.A.; Kuzmina, L.Y.; Melent’ev, A.I.; Baymiev, A.H.; Lopatin, S.A. Constitutive chitosanase from Bacillus thuringiensis B-387 and its potential for preparation of antimicrobial chitooligomers. World J. Microbiol. Biotechnol. 2022, 38, 167. [Google Scholar] [CrossRef]
- Rodriguez-Kabana, R.; Godoy, G.; Morgan-Jones, G.; Shelby, R.A. The determination of soil chitinase activity: Conditions for assay and ecological studies. Plant Soil. 1983, 75, 95–106. [Google Scholar] [CrossRef]
- Li, Z.; Cho, S.; Kwon, I.C.; Janát-Amsbury, M.M.; Huh, K.M. Preparation and characterization of glycol chitin as a new thermogelling polymer for biomedical applications. Carbohydr. Polym. 2013, 92, 2267–2275. [Google Scholar] [CrossRef]
- Helistö, P.; Aktuganov, G.; Galimzianova, N.; Melentjev, A.; Korpela, T. Lytic enzyme complex of an antagonistic Bacillus sp. X-b: Isolation and purification of components. J. Chromatogr. B Biomed. App. 2001, 758, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Aktuganov, G.E.; Galimzianova, N.F.; Gilvanova, E.A.; Pudova, E.A.; Kuzmina, L.Y.; Melentiev, A.I.; Safina, V.R. Purification and characterization of exo-β-1,4-glucosaminidase produced by chitosan-degrading fungus, Penicillium sp. IB-37-A. World J. Microbiol. Biotechnol. 2019, 35, 18. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Measuring protein concentration in the presence of nucleic acids by a280/a260: The method of Warburg and Christian. CHS Protoc. 2006, 1, pdb.prot4252. [CrossRef]
- Imoto, T.; Yagishita, K. A simple activity measurement of lysozyme. Agric. Biol. Chem. 1971, 35, 1154–1156. [Google Scholar] [CrossRef]
- Haab, D.; Hagspiel, K.; Szakmary, K.; Kubicek, P. Formation of the extracellular proteases from Trichoderma reesi QM 9414 involved in cellulase degradation. J. Biotechnol. 1990, 16, 187–198. [Google Scholar] [CrossRef]
- Aktuganov, G.E.; Galimzianova, N.F.; Gilvanova, E.A.; Kuzmina, L.Y.; Boyko, T.F.; Safina, V.R.; Melentiev, A.I. Characterization of chitinase produced by the alkaliphilic Bacillus mannanilyticus IB-OR17 B1 strain. Appl. Biochem. Microbiol. 2018, 54, 505–511. [Google Scholar] [CrossRef]
- Kulig, D.; Król-Kilińska, Ż.; Bobak, Ł.; Żarowska, B.; Jarmoluk, A.; Zimoch-Korzycka, A. Functional properties of chitosan oligomers obtained by enzymatic hydrolysis. Polymers 2023, 15, 3801. [Google Scholar] [CrossRef]
- Seo, D.-J.; Lee, J.-H.; Song, Y.-S.; Park, R.-D.; Jung, W.-J. Expression patterns of chitinase and chitosanase produced from Bacillus cereus in suppression of phytopathogen. Microb. Pathog. 2014, 73, 31–36. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Lacombe-Harvey, M.-E.; Brzezinski, R.; Beaulieu, C. Chitinolytic functions in actinobacteria: Ecology, enzymes, and evolution. Appl. Microbiol. Biotechnol. 2018, 102, 7219–7230. [Google Scholar] [CrossRef]
- Poria, V.; Rana, A.; Kumari, A.; Grewal, J.; Pranaw, K.; Singh, S. Current perspectives on chitinolytic enzymes and their agro-industrial applications. Biology 2021, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Hoell, I.A.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 331–366. [Google Scholar] [CrossRef]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, M.; Viens, P.; Ghinet, M.G.; Brzezinski, R. Diversity of family GH46 chitosanases in Kitasatospora setae KM-6054. Appl. Microbiol. Biotechnol. 2017, 101, 7877–7888. [Google Scholar] [CrossRef]
- Oyeleye, A.; Normi, Y.M. Chitinase: Diversity, limitations, and trends in engineering for suitable applications. Biosci. Rep. 2018, 38, BSR2018032300. [Google Scholar] [CrossRef]
- Mitsutomi, M.; Isono, M.; Uchiyama, A.; Nikaidou, N.; Ikegami, T.; Watanabe, T. Chitosanase activity of the enzyme previously reported as β-1,3-1,4-glucanase from Bacillus circulans WL-12. Biosci. Biotechnol. Biochem. 1998, 62, 2107–2114. [Google Scholar] [CrossRef]
- Gao, X.A.; Ju, W.T.; Jung, W.J.; Park, R.D. Purification and characterization of chitosanase from Bacillus cereus D-11. Carbohydr. Polym. 2008, 72, 513–520. [Google Scholar] [CrossRef]
- Zitouni, M.; Fortin, M.; Scheerle, R.K.; Letzel, T.; Matteau, D.; Rodrigue, S.; Brzezinski, R. Biochemical and molecular characterization of a thermostable chitosanase produced by the strain Paenibacillus sp. 1794 newly isolated from compost. Appl. Microbiol. Biotechnol. 2013, 97, 5801–5813. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, S.; Wang, S.; Li, X.; Su, L.; Ma, Y.; Li, J.; Song, J. Extracellular overexpression of chitosanase from Bacillus sp. TS in Escherichia coli. Appl. Biochem. Biotechnol. 2015, 175, 3271–3286. [Google Scholar] [CrossRef] [PubMed]
- Cheba, B.A.; Zaghloul, T.I. Bacillus Sp. R2 chitinase: Substrate specificity, shelf-life stability, and antifungal activity. Procedia Manuf. 2020, 46, 879–884. [Google Scholar] [CrossRef]
- Chen, L.; Wei, Y.; Shi, M.; Li, Z.; Zhang, S.H. An archaeal chitinase with a secondary capacity for catalyzing cellulose and its biotechnological applications in shell and straw degradation. Front. Microbiol. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Hardt, M.; Laine, R.A. Mutation of active site residues in the chitin-binding domain ChBDChiA1 from chitinase A1 of Bacillus circulans alters substrate specificity: Use of a green fluorescent protein binding assay. Arch. Biochem. Biophys. 2004, 426, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shao, S.; Li, L.; Cheng, Z.; Tian, L.; Gao, P.; Wang, L. Substrate-binding specificity of chitinase and chitosanase as revealed by active-site architecture analysis. Carbohydr. Res. 2015, 418, 50–56. [Google Scholar] [CrossRef]
- Singh, R.; Weikert, T.; Basa, S.; Moerschbacher, B.M. Structural and biochemical insight into mode of action and subsite specificity of a chitosan degrading enzyme from Bacillus spec. MN. Sci. Rep. 2019, 9, 1132. [Google Scholar] [CrossRef]
- Liu, Y.L.; Jiang, S.; Ke, Z.M.; Wu, H.S.; Chi, C.W.; Guo, Z.Y. Recombinant expression of a chitosanase and its application in chitosan oligosaccharide production. Carbohydr. Res. 2009, 344, 815–819. [Google Scholar] [CrossRef] [PubMed]
- de Assis, C.F.; Araújo, N.K.; Pagnoncelli, M.G.; da Silva Pedrini, M.R.; de Macedo, G.R.; dos Santos, E.S. Chitooligosaccharides enzymatic production by Metarhizium anisopliae. Bioprocess. Biosyst. Eng. 2010, 33, 893–899. [Google Scholar] [CrossRef]
- Su, H.; Zhao, H.; Jia, Z.; Guo, C.; Sun, J.; Mao, X. Biochemical characterization of a GH46 chitosanase provides insights into the novel digestion specificity. J. Agric. Food Chem. 2023, 71, 2038–2048. [Google Scholar] [CrossRef]
- Li, J.; Du, Y.; Liang, H. Low molecular weight water-soluble chitosans: Preparation with the aid of cellulase, characterization, and solubility. J. Appl. Polym. Sci. 2006, 102, 1098–1105. [Google Scholar] [CrossRef]
- Yin, H.; Du, Y.; Zhang, J. Low molecular weight and oligomeric chitosans and their bioactivities. Curr. Top. Med. Chem. 2009, 9, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.C.; Hoa, N.V.; Trung, T.S. Chapter 15—Preparation, properties, and application of low-molecular-weight chitosan. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 453–471. [Google Scholar] [CrossRef]
- Suryani, S.; Chaerunisaa, A.Y.; Joni, I.M.; Ruslin, R.; Ramadhan, O.A.N.; Wardhana, Y.W.; Sabarwati, S.H. Production of low molecular weight chitosan using a combination of weak acid and ultrasonication methods. Polymers 2022, 14, 3417. [Google Scholar] [CrossRef] [PubMed]
- Roncal, T.; Oviedo, A.; López de Armentia, I.; Fernández, L.; Villarán, M.C. High yield production of monomer-free chitosan oligosaccharides by pepsin catalyzed hydrolysis of a high deacetylation degree chitosan. Carbohydr. Res. 2007, 342, 2750–2756. [Google Scholar] [CrossRef]
- Hirano, S.; Watanabe, M.; Seki, K.; Ando, A.; Saito, A.; Mitsutomi, M. Classification of chitosanases by hydrolytic specificity toward N1, N4-diacetylchitohexaose. Biosci. Biotechnol. Biochem. 2012, 76, 1932–1937. [Google Scholar] [CrossRef]
- Kohlhoff, M.; Niehues, A.; Wattjes, J.; Bénéteau, J.; Cord-Landwehr, S.; El Gueddari, N.E.; Bernard, F.; Rivera-Rodriguez, G.R.; Moerschbacher, B.M. Chitinosanase: A fungal chitosan hydrolyzing enzyme with a new and unusually specific cleavage pattern. Carbohydr. Polym. 2017, 174, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Alburquenque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hjeljord, L.G.; Aam, B.B.; Sørlie, M.; Tronsmo, A. Antifungal effect of chito-oligosaccharides with different degrees of polymerization. Eur. J. Plant Pathol. 2015, 141, 147–158. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Melhem, M.S.; Bonfietti, L.X.; Rodriguez-Tudela, J.L. Susceptibility test for fungi: Clinical and laboratorial correlations in medical mycology. Rev. Inst. Med. Trop. Sao Paulo 2015, 57 (Suppl. S19), 57–64. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; Guedes, G.M.M.; da Silva, M.L.Q.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; Cordeiro, R.A.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Effect of the molecular weight of chitosan on its antifungal activity against Candida spp. in planktonic cells and biofilm. Carbohydr. Polym. 2018, 195, 662–669. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the assessment of in vitro and in vivo antifungal combinations. J. Fungi 2021, 7, 113. [Google Scholar] [CrossRef]
- de Azevedo, M.I.G.; Souza, P.F.N.; Monteiro Júnior, J.E.; Grangeiro, T.B. Chitosan and chitooligosaccharides: Antifungal potential and structural insights. Chem. Biodivers. 2024, 21, e202400044. [Google Scholar] [CrossRef] [PubMed]
- Stepnova, E.A.; Tikhonov, V.E.; Lopatin, S.A.; Varlamov, V.P.; Yamskov, I.A. Molecular-weight-dependent fungicidal activity of chitosan. Moscow Univ. Chem. Bull. 2007, 62, 257–258. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ganan, M.; Lorentzen, S.B.; Agger, J.W.; Heyward, C.A.; Bakke, O.; Knutsen, S.H.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sørlie, M. Antifungal activity of well-defined chito-oligosaccharide preparations against medically relevant yeasts. PLoS ONE 2019, 14, e0210208. [Google Scholar] [CrossRef]
- Viegas de Souza, R.H.; Takaki, M.; de Oliveira Pedro, R.; dos Santos Gabriel, J.; Tiera, M.J.; de Oliveira Tiera, V.A. Hydrophobic effect of amphiphilic derivatives of chitosan on the antifungal activity against Aspergillus flavus and Aspergillus parasiticus. Molecules 2013, 18, 4437–4450. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef]

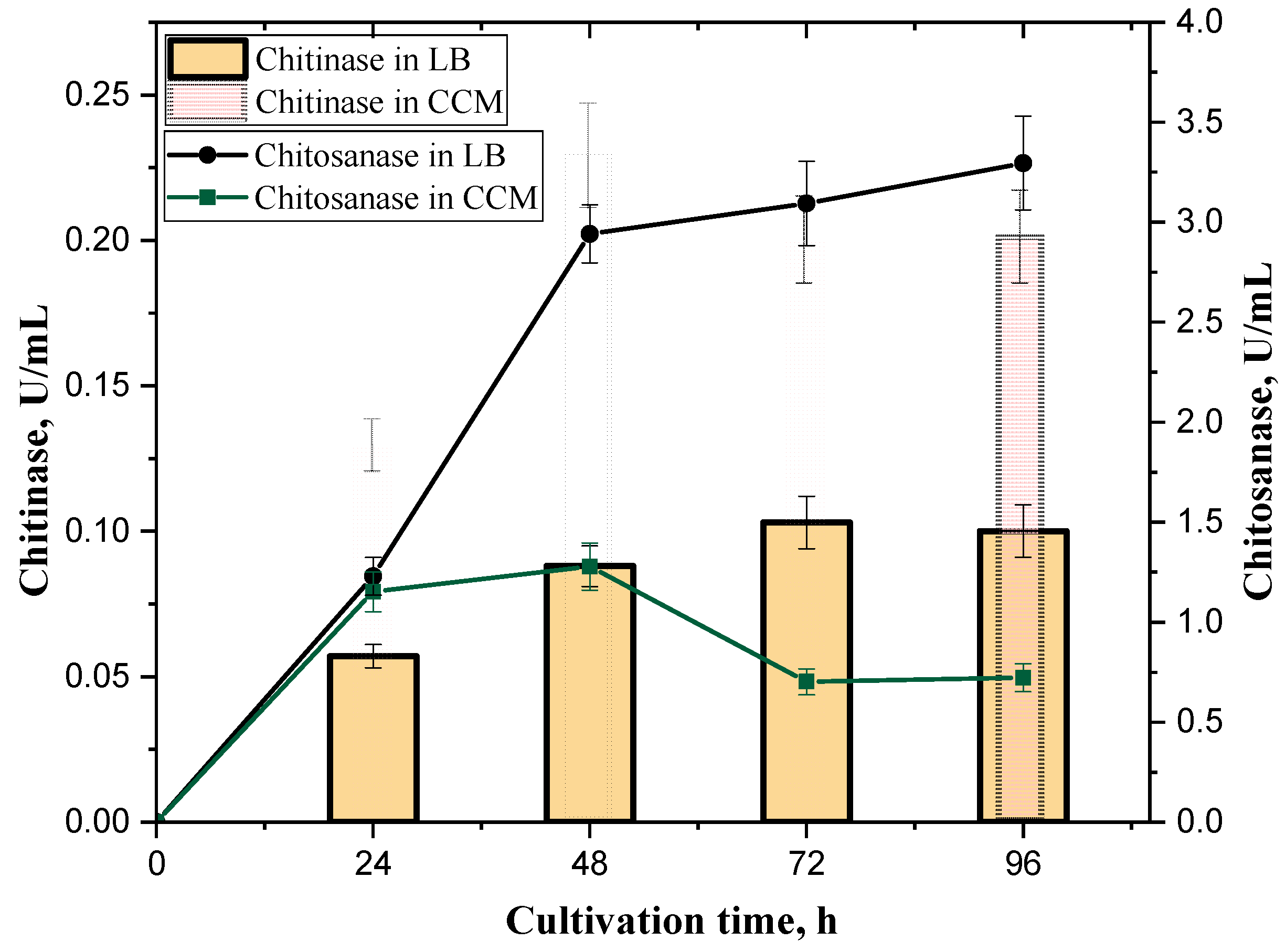




| Cultivation Period | LB Broth | ||||
|---|---|---|---|---|---|
| Growth Characteristics | Protein in CS a, mg/mL | Protease, AU b/mL | pH of CS | ||
| OD600 | ×107 CFU/mL | ||||
| 0 h | 0.05 ± 0.01 | 1.77 ± 0.76 c | – | 0 | 6.81 |
| 24 h | 6.01 ± 0.14 | 66 ± 8 | 6.87 ± 0.35 | 0.093 ± 0.010 | 8.01 |
| 48 h | 5.09 ± 0.30 | 14.8 ± 3 | 8.87 ± 0.86 | 0.587 ± 0.057 | 8.70 |
| 72 h | 3.16 ± 0.10 | 3.8 ± 0.60 | 8.93 ± 0.75 | 0.394 ± 0.029 | 8.92 |
| 96 h | 2.47 ± 0.10 | 0.02 ± 0.004 | 9.38 ± 0.60 | 0.115 ± 0.007 | 9.02 |
| The medium with 1% (w/v) of colloidal chitin (CCM) | |||||
| 0 h | – d | 1.77 ± 0.76 c | – | 0 | 6.53 |
| 24 h | – | 56 ± 15 | 5.66 ± 0.37 | 0.139 ± 0.012 | 5.90 |
| 48 h | – | 12 ± 2 | 5.35 ± 0.45 | 0.165 ± 0.014 | 8.30 |
| 72 h | – | 0.10 ± 0.02 | 3.73 ± 0.42 | 0.078 ± 0.008 | 8.80 |
| 96 h | – | 0.05 ± 0.001 | 3.85 ± 0.38 | 0.089 ± 0.014 | 8.99 |
| Step of Purification | Total Enzyme Activity, U | Total Protein, mg | Specific Activity, U/mg | Purification Degree, -Fold | Enzyme Yield, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| CF a, (96 h) | 1619.68 | 119.25 | 4722.3 | 1040 | 0.343 | 0.064 | 1 | 1 | 100 | 100 |
| VivaFlow 200 module (30 kDa) | 931.32 | 107.46 | 822.72 | 870.96 | 1.132 | 0.123 | 3.3 | 1.9 | 58 | 90 |
| Affinity adsorption b | 730.48 | 69.20 | 204.79 | 441.94 | 3.567 | 0.157 | 10.4 | 2.5 | 45 | 58 |
| Phenyl-Sepharose CL 4B | — | 34.79 | — | 22.79 | — | 1.527 | — | 24 | — | 29 |
| CM-Sepharose Fast Flow | 147.39 | — | 4.57 | — | 32.252 | — | 94 | — | 9 | — |
| Characteristics | Chitosanase | Chitinase |
|---|---|---|
| Temperature optimum, °C | 55 | 55 |
| Thermal stability, °C a | 60 | 60 |
| Optimal pH | 6.5 | 6.5 |
| pH stability b | 6–10 | 4–9 |
| pI | 7.5–8 | ND |
| Molecular weight | 40 | 73 |
| KM, mg/mL (toward chitosan DD 85%) | 0.22 | >5.4 |
| Vmax (toward chitosan DD 85%), μM × mL−1 × mg−1 | 56.52 | 0.124 |
| Vmax (toward colloidal chitin), μM × mL−1 × mg−1 | ND c | 14.1 |
| Action mechanism to a specific substrate | Endo- d | Exo- e |
| Final products of chitosan (DD 85%) hydrolysis | (GlcN)2-4 f | (GlcN) 2-4 f |
| Major products of colloidal chitin hydrolysis | NA g | (GlcNAc)2 and GlcNAc d |
| Catalytic activity, nkat (chitosan DD 85%) | 942.19 | ND a |
| kcat, s−1 | 5.84 × 103 | ND a |
| Inhibition by metal cations and detergents | 10 mM Zn2+, Hg+, Cd2+, Ag+ Fe+2 and 10 mM SDS | 1–10 mM Hg+, Cd2+, Ag+, Fe+2, and 10 mM SDS |
| Activation by metal cations and surfactants | 10 mM tween-80 | 10 mM tween-80 |
| No. | Main Ion Peaks, m/z (Proposed Adducts and Charges) | COS Product | Retention Time, min | Rel. Content a, % |
|---|---|---|---|---|
| Chitosan DD 85% degradation products by chitosanase 40 kDa b | ||||
| 1 | 363.14 (Na+), 341.14+, 342.17 (H+) | (GlcN)2 | 5.47 | 30 |
| 2 | 502.23, 503.23 (H+), 524.2 (Na+) | (GlcN)3 | 6.29 | 54.2 |
| 3 | 332.152+, 332.652+, 333.15 (2H2+) 663.29+, 663.79+, 664.29 (H+) | (GlcN)4 | 7.30 | 15 |
| 4 | 412.692+ | (GlcN)5 | 7.30 | 0.1 |
| 5 | 433.692+, 705.3+, 705.8+ | GlcNAc-(GlcN)4 | 7.30 | 0.7 |
| Chitosan DD 85% degradation products by chitinase 73 kDa c | ||||
| 1 | 341.15+, 342.15 (H+), 363.13 (Na+) | (GlcN)2 | 5.65 | 12.2 |
| 2 | 502.22+, 503.22 (H+), 524.2 (Na+) | (GlcN)3 | 5.70 | 12.1 |
| 3 | 544.2+, 545.22 (H+), 566.21(Na+) | (GlcN)2-GlcNAc | 5.70 | 1.6 |
| 4 | 663.29+, 663.79+, 664.28 (H+), 663.31+, 332.142+, 332.652+, 333.15 (H+) | (GlcN)4 | 6.80 | 7 |
| 5 | 412.682+, 413.182+, 413.68 (2H2+), 824.41+, 824.37+ | (GlcN)5 | 7.81 | 19.3 |
| 6 | 433.682+, 435.18 (2H2+) | (GlcN)4-GlcNAc | 7.81 | 2.7 |
| 7 | 493.212+, 493.712+, 494.21 (2H2+), 493.232+, 493.732+, 494.23 (2H2+) | (GlcN)6 | 7.98 | 19.2 |
| 8 | 514.222+, 514.722+, 515.21 (2H2+), 514.242+, 514.742+ | GlcNAc-(GlcN)5 | 8.00 | 6 |
| 9 | 573.772+, 574.272+, 574.77 (2H2+) | (GlcN)7 | 8.68 | 13.6 |
| 10 | 594.782+ | (GlcN)6-GlcNAc | 10.01 | 0.4 |
| 11 | 654.302+, 654.802+, 655.31 (2H2+) | (GlcN)8 | 10.05 | 3.4 |
| 12 | 755.852+ | (GlcN)8-GlcNAc | 10.57 | 0.1 |
| 13 | 734.832+, 735.332+ | (GlcN)9 | 11.16 | 0.2 |
| Chitosan DD 50% degradation products by chitinase 73 kDa d | ||||
| 1 | 222.10+, 222.23+, 222.32+, 222.39+, 222.55+, 223.10 (H+) | GlcNAc | 4.11 | 12 |
| 2 | 204.09 (Na+) | GlcN | 4.09 | 7.7 |
| 3 | 343.18 (2H2+), 341.16+, 363.14 (Na+) | (GlcN)2 | 4.30 | 5.4 |
| 4 | 443.2+, 444.19 (H+), 447.17 (2H2+) | (GlcNAc)2 | 4.35 | 4.8 |
| 5 | 405.15 (Na+), 383.17+, 383.35+, 383.55+, 383.76+, 384.17 (H+), 406.16 (H+ Na+), 405.15 (Na+) | GlcN-GlcNAc | 4.92 | 22.7 |
| 6 | 502.23+, 503.24 (H+), 524.22 (Na+) | (GlcN)3 | 6.24 | 8.4 |
| 7 | 544.24+, 545.24 (H+), 566.23 (Na+) | (GlcN)2-GlcNAc | 6.25 | 12.9 |
| 8 | 608.22+ | GlcNAc-GlcN-GlcNAc (presumably) | 6.25 | 0.7 |
| 9 | 663.27+ | (GlcN)4 | 7.16 | 0.8 |
| 10 | 705.28+, 706.28 (H+) | (GlcN)3-GlcNAc | 7.17 | 0.8 |
| 11 | 747.30+, 748.27 (H+) | GlcNAc-(GlcN)2-GlcNAc | 7.20 | 0.8 |
| 12 | 433.692+, 434.20 (2H2+), 434.7 (2H2+) | (GlcN)4-GlcNAc | 7.67 | 22.5 |
| 13 | 412.692+ | (GlcN)5 | 7.92 | 0.4 |
| Incubation Time and Temperature | Fraction | Mn, a kDa | Mw, b kDa | PDI c | Solubility d, g/L | Yield, % | Inhibition of B. sorokiniana, ED50, mg/mL e |
|---|---|---|---|---|---|---|---|
| – | Initial polymeric chitosan | 128.5 | 369.2 | 2.87 | – | – | 0.10 |
| Depolymerization by chitosanase (~2.5–11 U/g substrate) | |||||||
| 1 h at 50 °C | The precipitated fraction after adding 0.5 M NaOH | 22.5 | 66.5 | 2.96 | 48.1 | 48.1 | 0.29 |
| 2 h at 50 °C | 23.25 | 45.34 | 1.95 | 57.7 | 29.8 | 0.45 | |
| 2 h at 50 °C | Supernatant after adding 0.5 M NaOH | 1.96 | 2.19 | 1.12 | 63.0 | 8 | 2.50 |
| 4 h at 50 °C | Total hydrolysate | 1.92 | 2.41 | 1.26 | 64.9 | 68 | 1.29 |
| Depolymerization by chitinase (~8–14 U/g substrate) | |||||||
| 1 h at 50 °C | The precipitated fraction after adding 0.5 M NaOH | 27.43 | 71.74 | 2.62 | 24.4 | 43.4 | 0.69 |
| 1 h at 50 °C | Supernatant after adding 0.5 M NaOH | 2.52 | 2.89 | 1.15 | 42.8 | <6 | 3.00 |
| 24 h at 37 °C f | Total hydrolysate | 7.32 | 15.38 | 2.10 | 21.6 | 73 | 1.22 |
| Tested Fungal Strain | MIC, μg × mL−1 | ||||
|---|---|---|---|---|---|
| Chitosanase Products/Hydrolysis Time | Chitinase Products/Hydrolysis Time | ||||
| 2 h | 4 h | 1 h | 24 h | ||
| 45.3 kDa a | 2.19 kDa b | 2.4 kDa c | 71.7 kDa a | 15.4 kDa c | |
| A. alternata VKM F-3047 | 200 ± 15 | 2250 ± 250 | 750 ± 55 | 310 ± 30 | 575 ± 50 |
| B. sorokiniana IB G-12 | 240 ± 20 | 1850 ± 200 | 675 ± 45 | 320 ± 35 | 590 ± 55 |
| F. culmorum VKM F-844 | <21 | 1520 ± 140 | 230 ± 20 | 38 ± 5 | 200 ± 20 |
| F. gibbosum VKM F-848 | <19 | NI d | 665 ± 55 | 55 ± 5 | 540 ± 45 |
| F. graminearum VKM F-1668 | <20 | NI | 485 ± 35 | 90 ± 10 | 390 ± 35 |
| F. oxysporum VKM F-137 | <21 | 2500 ± 200 | 150 ± 20 | 75 ± 5 | 145 ± 10 |
| F. solani VKM F-142 | <20 | 2500 ± 170 | 295 ± 25 | 65 ± 10 | 280 ± 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktuganov, G.; Lobov, A.; Galimzianova, N.; Gilvanova, E.; Kuzmina, L.; Milman, P.; Ryabova, A.; Melentiev, A.; Chetverikov, S.; Starikov, S.; et al. Comparative Potential of Chitinase and Chitosanase from the Strain Bacillus thuringiensis B-387 for the Production of Antifungal Chitosan Oligomers. BioTech 2025, 14, 35. https://doi.org/10.3390/biotech14020035
Aktuganov G, Lobov A, Galimzianova N, Gilvanova E, Kuzmina L, Milman P, Ryabova A, Melentiev A, Chetverikov S, Starikov S, et al. Comparative Potential of Chitinase and Chitosanase from the Strain Bacillus thuringiensis B-387 for the Production of Antifungal Chitosan Oligomers. BioTech. 2025; 14(2):35. https://doi.org/10.3390/biotech14020035
Chicago/Turabian StyleAktuganov, Gleb, Alexander Lobov, Nailya Galimzianova, Elena Gilvanova, Lyudmila Kuzmina, Polina Milman, Alena Ryabova, Alexander Melentiev, Sergey Chetverikov, Sergey Starikov, and et al. 2025. "Comparative Potential of Chitinase and Chitosanase from the Strain Bacillus thuringiensis B-387 for the Production of Antifungal Chitosan Oligomers" BioTech 14, no. 2: 35. https://doi.org/10.3390/biotech14020035
APA StyleAktuganov, G., Lobov, A., Galimzianova, N., Gilvanova, E., Kuzmina, L., Milman, P., Ryabova, A., Melentiev, A., Chetverikov, S., Starikov, S., & Lopatin, S. (2025). Comparative Potential of Chitinase and Chitosanase from the Strain Bacillus thuringiensis B-387 for the Production of Antifungal Chitosan Oligomers. BioTech, 14(2), 35. https://doi.org/10.3390/biotech14020035








