Developments in Extracellular Matrix-Based Angiogenesis Therapy for Ischemic Heart Disease: A Review of Current Strategies, Methodologies and Future Directions
Abstract
1. Introduction
2. The ECM in Normal Cardiac Physiology
3. The ECM in the Chronically Ischemic Heart
4. The ECM in the Acutely Infarcted Heart
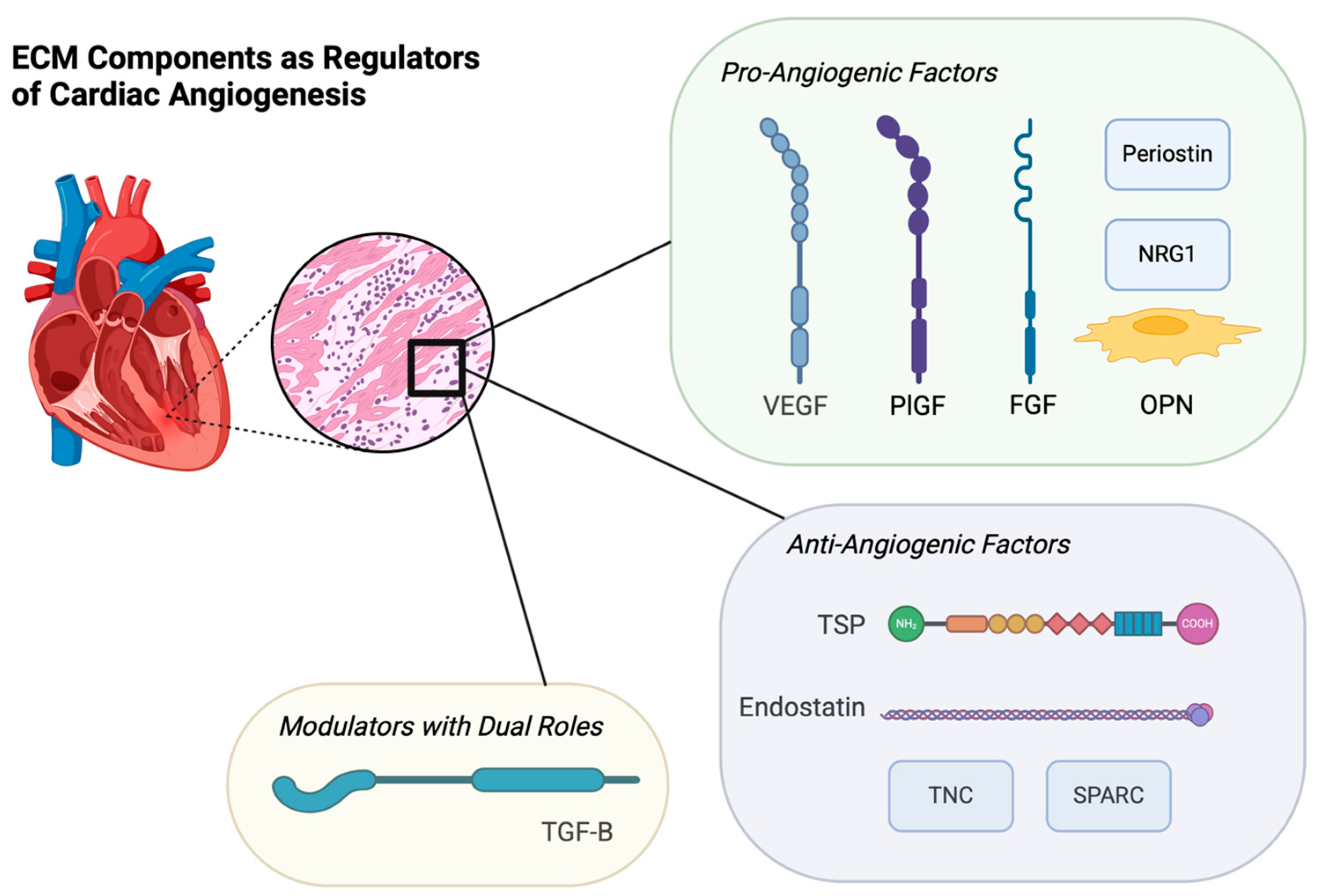
5. ECM Components as Regulators of Cardiac Angiogenesis
| ECM Component | Role in Cardiac Angiogenesis |
|---|---|
| Endostatin |
|
| Vascular Endothelial Growth Factor (VEGF) |
|
| Placental Growth Factor (PIGF) | |
| Neuregulin-1 (NRG1) | |
| Fibroblast Growth Factor (FGF) | |
| Transforming Growth Factor-β (TGF-β) | |
| Secreted Protein, Acidic and Rich in Cysteine (SPARC) | |
| Osteopontin (OPN) | |
| Thrombospondins (TSPs) | |
| Tenascins (TNC) | |
| Periostin |
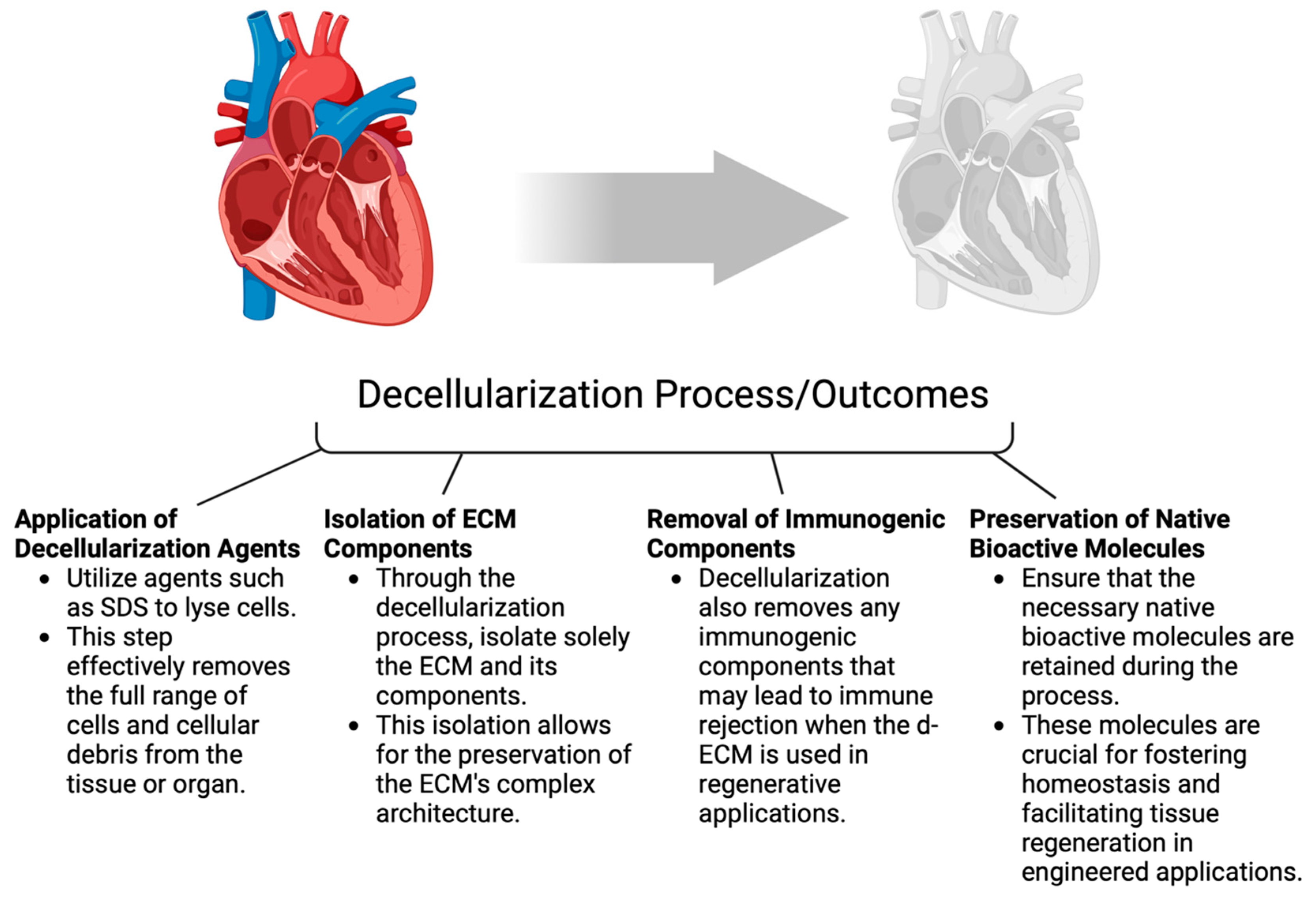
6. Exploring Various Types of ECM Biomaterials
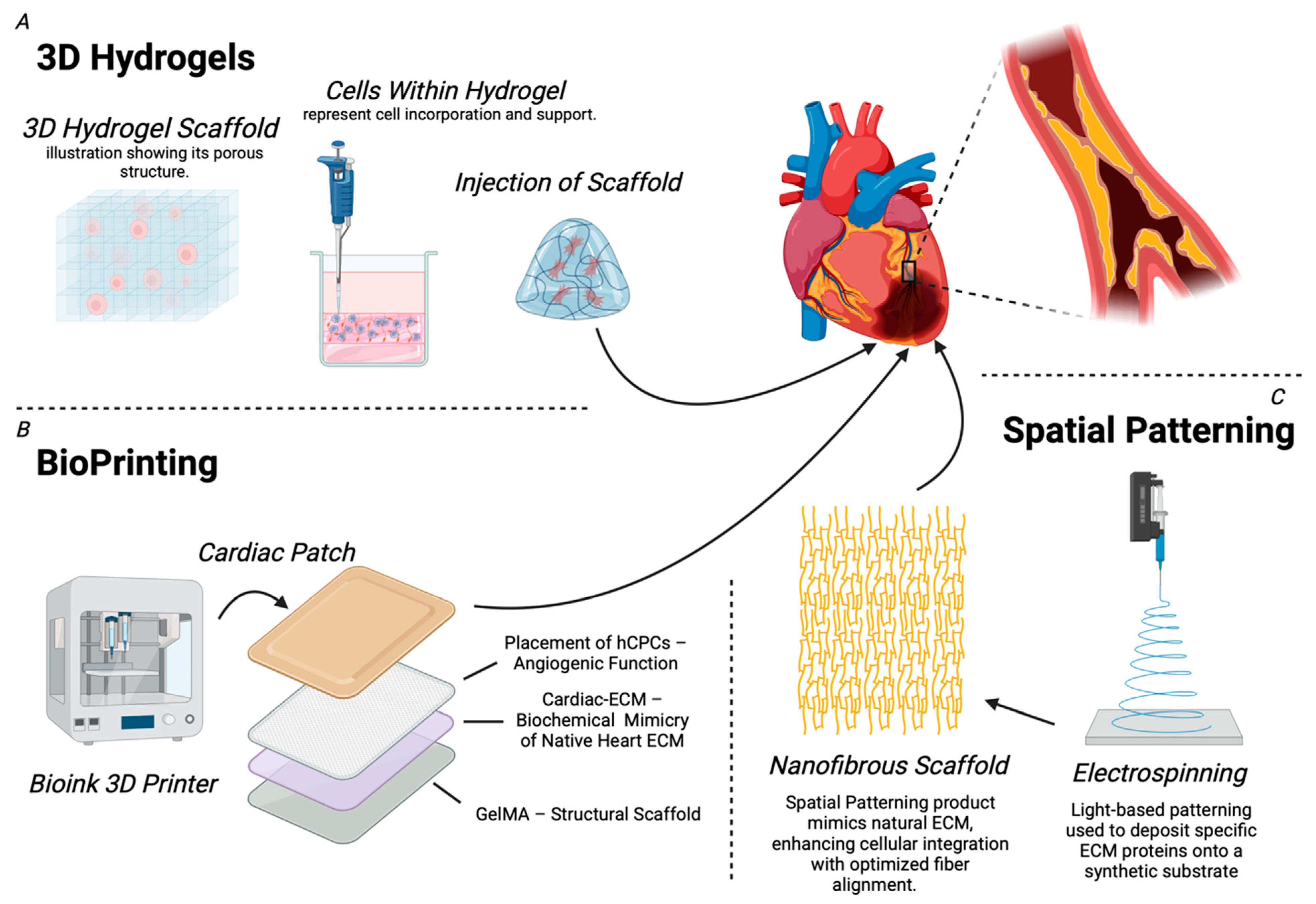
7. Bioengineered ECM Therapies: Applications in Cardiac Angiogenesis
| Component | Function | Advantages | Limitations | Applications/Outcomes |
|---|---|---|---|---|
| 3D Hydrogels |
| |||
| Bioprinting |
| |||
| Spatial Patterning |
|
8. Future Directions and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. WHO. 9 December 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 24 January 2025).
- Dababneh, E.; Goldstein, S. Chronic Ischemic Heart Disease Selection of Treatment Modality. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fitridge, R.; Thompson, M. (Eds.) Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 978-0-9871718-2-5. Available online: http://www.ncbi.nlm.nih.gov/books/NBK534260/ (accessed on 12 March 2024).
- Oikawa, F.T.C.; Hueb, W.; Nomura, C.H.; Hueb, A.C.; Villa, A.V.; da Costa, L.M.A.; de Melo, R.M.V.; Rezende, P.C.; Segre, C.A.W.; Garzillo, C.L.; et al. Abnormal elevation of myocardial necrosis biomarkers after coronary artery bypass grafting without established myocardial infarction assessed by cardiac magnetic resonance. J. Cardiothorac. Surg. 2017, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Keepers, B.; Liu, J.; Qian, L. What’s in a cardiomyocyte—And how do we make one through reprogramming? Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118464. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.P.; Forte, E.; Harvey, R.P.; Kovacic, J.C. Developmental origin and lineage plasticity of endogenous cardiac stem cells. Development 2016, 143, 1242–1258. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Kovacic, J.C. Extracellular Matrix in Ischemic Heart Disease, Part 4/4. J. Am. Coll. Cardiol. 2020, 75, 2219–2235. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the Inflammatory Response in Cardiac Repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Guo, Y.; Pu, W.T. Cardiomyocyte Maturation: New Phase in Development. Circ. Res. 2020, 126, 1086–1106. [Google Scholar] [CrossRef]
- Piersma, B.; Bank, R.A. Collagen cross-linking mediated by lysyl hydroxylase 2: An enzymatic battlefield to combat fibrosis. Essays Biochem. 2019, 63, 377–387. [Google Scholar] [CrossRef]
- Silva, A.C.; Pereira, C.; Fonseca, A.C.R.G.; Pinto-do-Ó, P.; Nascimento, D.S. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2020, 8, 621644. [Google Scholar] [CrossRef]
- Zeng, Q.; Guo, Y.; Liu, L.; Zhang, X.; Li, R.; Zhang, C.; Hao, Q.; Shi, C.; Wu, J.; Guan, J. Cardiac Fibroblast-Derived Extracellular Matrix Produced In Vitro Stimulates Growth and Metabolism of Cultured Ventricular Cells. Int. Heart J. 2013, 54, 40–44. [Google Scholar] [CrossRef]
- Weber, K.T. Cardiac interstitium in health and disease: The fibrillar collagen network. J. Am. Coll. Cardiol. 1989, 13, 1637–1652. [Google Scholar] [CrossRef]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Creemers, E.E.; Kassiri, Z. Matrix as an Interstitial Transport System. Circ. Res. 2014, 114, 889–902. [Google Scholar] [CrossRef]
- Chute, M.; Aujla, P.; Jana, S.; Kassiri, Z. The Non-Fibrillar Side of Fibrosis: Contribution of the Basement Membrane, Proteoglycans, and Glycoproteins to Myocardial Fibrosis. J. Cardiovasc. Dev. Dis. 2019, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Bellon, G.; Pasco, S.; Monboisse, J.C. Matrikines in the regulation of extracellular matrix degradation. Biochimie 2005, 87, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhang, L. Cardiac ECM: Its Epigenetic Regulation and Role in Heart Development and Repair. Int. J. Mol. Sci. 2020, 21, 8610. [Google Scholar] [CrossRef]
- Beltrami, C.A.; Finato, N.; Rocco, M.; Feruglio, G.A.; Puricelli, C.; Cigola, E.; Quaini, F.; Sonnenblick, E.H.; Olivetti, G.; Anversa, P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 1994, 89, 151–163. [Google Scholar] [CrossRef]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef]
- Mukherjee, D.; Sen, S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J. Clin. Investig. 1991, 88, 1141–1146. [Google Scholar] [CrossRef]
- Reinhardt, D. Cardiac remodelling in end stage heart failure: Upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart 2002, 88, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Coker, M.L.; Heung, L.J.; Bond, B.R.; Gunasinghe, H.R.; Etoh, T.; Goldberg, A.T.; Zellner, J.L.; Crumbley, A.J. A Matrix Metalloproteinase Induction/Activation System Exists in the Human Left Ventricular Myocardium and Is Upregulated in Heart Failure. Circulation 2000, 102, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Christian Danielsen, C.; Wiggers, H.; Rud Andersen, H. Increased Amounts of Collagenase and Gelatinase in Porcine Myocardium Following Ischemia and Reperfusion. J. Mol. Cell. Cardiol. 1998, 30, 1431–1442. [Google Scholar] [CrossRef]
- Santos, A.B.S.; Junges, M.; Silvello, D.; Macari, A.; Araújo, B.S.D.; Seligman, B.G.; Duncan, B.B.; Rohde, L.E.P.; Clausell, N.; Foppa, M. Early Change of Extracellular Matrix and Diastolic Parameters in Metabolic Syndrome. Arq. Bras. Cardiol. 2013, 101, 311–316. [Google Scholar] [CrossRef]
- Bornstein, P.; Sage, E.H. Matricellular proteins: Extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Matricellular Proteins in Cardiac Adaptation and Disease. Physiol. Rev. 2012, 92, 635–688. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Garza, M.A. Cardiac remodeling and physical training post myocardial infarction. World J. Cardiol. 2015, 7, 52. [Google Scholar] [CrossRef]
- Alfonso-Jaume, M.A.; Bergman, M.R.; Mahimkar, R.; Cheng, S.; Jin, Z.Q.; Karliner, J.S.; Lovett, D.H. Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1838–H1846. [Google Scholar] [CrossRef]
- Fang, W.; He, A.; Xiang, M.-X.; Lin, Y.; Wang, Y.; Li, J.; Yang, C.; Zhang, X.; Liu, C.-L.; Sukhova, G.K.; et al. Cathepsin K-deficiency impairs mouse cardiac function after myocardial infarction. J. Mol. Cell. Cardiol. 2019, 127, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.V.A.; Lorizio, D.; Vuerich, R.; Lippi, M.; Nascimento, D.S.; Zacchigna, S. Extracellular Matrix-Based Approaches in Cardiac Regeneration: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 15783. [Google Scholar] [CrossRef]
- Del Monte-Nieto, G.; Fischer, J.W.; Gorski, D.J.; Harvey, R.P.; Kovacic, J.C. Basic Biology of Extracellular Matrix in the Cardiovascular System, Part 1/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, A.; Sugimoto, H.; Yang, C.; Lively, J.; Zeisberg, M.; Kalluri, R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc. Natl. Acad. Sci. USA 2003, 100, 4766–4771. [Google Scholar] [CrossRef]
- Mitsuma, W.; Kodama, M.; Hanawa, H.; Ito, M.; Ramadan, M.M.; Hirono, S.; Obata, H.; Okada, S.; Sanada, F.; Yanagawa, T.; et al. Serum Endostatin in the Coronary Circulation of Patients With Coronary Heart Disease and Its Relation to Coronary Collateral Formation. Am. J. Cardiol. 2007, 99, 494–498. [Google Scholar] [CrossRef]
- Panchal, V.R.; Rehman, J.; Nguyen, A.T.; Brown, J.W.; Turrentine, M.W.; Mahomed, Y.; March, K.L. Reduced pericardial levels of endostatin correlate with collateral development in patients with ischemic heart disease. J. Am. Coll. Cardiol. 2004, 43, 1383–1387. [Google Scholar] [CrossRef]
- Johnson, T.; Zhao, L.; Manuel, G.; Taylor, H.; Liu, D. Approaches to therapeutic angiogenesis for ischemic heart disease. J. Mol. Med. 2019, 97, 141–151. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, X.; Li, X.; Hu, M.; Wan, W.; Wen, Y.; He, Y.; Zheng, X. Intramyocardial delivery of VEGF165 via a novel biodegradable hydrogel induces angiogenesis and improves cardiac function after rat myocardial infarction. Heart Vessel. 2016, 31, 963–975. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in Cardiomyocytes and Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef]
- Accornero, F.; Molkentin, J.D. Placental growth factor as a protective paracrine effector in the heart. Trends Cardiovasc. Med. 2011, 21, 220–224. [Google Scholar] [CrossRef]
- Stangret, A.; Dykacz, W.; Jabłoński, K.; Wesołowska, A.; Klimczak-Tomaniak, D.; Kochman, J.; Tomaniak, M. The cytokine trio—Visfatin, placental growth factor and fractalkine—And their role in myocardial infarction with non-obstructive coronary arteries (MINOCA). Cytokine Growth Factor Rev. 2023, 74, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Witman, N.; Zhou, C.; Häneke, T.; Xiao, Y.; Huang, X.; Rohner, E.; Sohlmér, J.; Grote Beverborg, N.; Lehtinen, M.L.; Chien, K.R.; et al. Placental growth factor exerts a dual function for cardiomyogenesis and vasculogenesis during heart development. Nat. Commun. 2023, 14, 5435. [Google Scholar] [CrossRef]
- Hemanthakumar, K.A.; Kivelä, R. Angiogenesis and angiocrines regulating heart growth. Vasc. Biol. 2020, 2, R93–R104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, J.; Zhang, P.; Zhang, X.; Wang, Y.; Chen, W.; Zhao, Y.; Cui, X. Neuregulin-1, a potential therapeutic target for cardiac repair. Front. Pharmacol. 2022, 13, 945206. [Google Scholar] [CrossRef]
- De Keulenaer, G.W.; Feyen, E.; Dugaucquier, L.; Shakeri, H.; Shchendrygina, A.; Belenkov, Y.N.; Brink, M.; Vermeulen, Z.; Segers, V.F.M. Mechanisms of the Multitasking Endothelial Protein NRG-1 as a Compensatory Factor During Chronic Heart Failure. Circ Heart Fail. 2019, 12, e006288. [Google Scholar] [CrossRef] [PubMed]
- Dugaucquier, L.; Feyen, E.; Mateiu, L.; Bruyns, T.A.M.; De Keulenaer, G.W.; Segers, V.F.M. The role of endothelial autocrine NRG1/ERBB4 signaling in cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H443–H455. [Google Scholar] [CrossRef]
- Rao, Z.; Shen, D.; Chen, J.; Jin, L.; Wu, X.; Chen, M.; Li, L.; Chu, M.; Lin, J. Basic Fibroblast Growth Factor Attenuates Injury in Myocardial Infarction by Enhancing Hypoxia-Inducible Factor-1 Alpha Accumulation. Front. Pharmacol. 2020, 11, 1193. [Google Scholar] [CrossRef]
- House, S.L.; Bolte, C.; Zhou, M.; Doetschman, T.; Klevitsky, R.; Newman, G.; Schultz, J.E.J. Cardiac-Specific Overexpression of Fibroblast Growth Factor-2 Protects Against Myocardial Dysfunction and Infarction in a Murine Model of Low-Flow Ischemia. Circulation 2003, 108, 3140–3148. [Google Scholar] [CrossRef]
- Lodyga, M.; Cambridge, E.; Karvonen, H.M.; Pakshir, P.; Wu, B.; Boo, S.; Kiebalo, M.; Kaarteenaho, R.; Glogauer, M.; Kapoor, M.; et al. Cadherin-11–mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-β. Sci. Signal. 2019, 12, eaao3469. [Google Scholar] [CrossRef]
- Lajiness, J.D.; Conway, S.J. Origin, development, and differentiation of cardiac fibroblasts. J. Mol. Cell. Cardiol. 2014, 70, 2–8. [Google Scholar] [CrossRef]
- Klingberg, F.; Chau, G.; Walraven, M.; Boo, S.; Koehler, A.; Chow, M.L.; Olsen, A.L.; Im, M.; Lodyga, M.; Wells, R.G.; et al. The ED-A domain enhances the capacity of fibronectin to store latent TGF-β binding protein-1 in the fibroblast matrix. J. Cell Sci. 2018, 131, jcs.201293. [Google Scholar] [CrossRef]
- Wijelath, E.S.; Rahman, S.; Namekata, M.; Murray, J.; Nishimura, T.; Mostafavi-Pour, Z.; Patel, Y.; Suda, Y.; Humphries, M.J.; Sobel, M. Heparin-II Domain of Fibronectin Is a Vascular Endothelial Growth Factor-Binding Domain: Enhancement of VEGF Biological Activity by a Singular Growth Factor/Matrix Protein Synergism. Circ. Res. 2006, 99, 853–860. [Google Scholar] [CrossRef]
- Korf-Klingebiel, M.; Reboll, M.R.; Grote, K.; Schleiner, H.; Wang, Y.; Wu, X.; Klede, S.; Mikhed, Y.; Nobre, A.; Bauersachs, J.; et al. Heparan Sulfate–Editing Extracellular Sulfatases Enhance VEGF Bioavailability for Ischemic Heart Repair. Circ. Res. 2019, 125, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Kupprion, C.; Motamed, K.; Sage, E.H. SPARC (BM-40, Osteonectin) Inhibits the Mitogenic Effect of Vascular Endothelial Growth Factor on Microvascular Endothelial Cells. J. Biol. Chem. 1998, 273, 29635–29640. [Google Scholar] [CrossRef]
- Zhao, X.; Johnson, J.N.; Singh, K.; Singh, M. Impairment of Myocardial Angiogenic Response in the Absence of Osteopontin. Microcirculation 2007, 14, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, M.; Yin, L.; Fu, G.; Liu, Z. Role of thrombospondin-1 and thrombospondin-2 in cardiovascular diseases (Review). Int. J. Mol. Med. 2020, 45, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Manzaneque, J.C.; Lane, T.F.; Ortega, M.A.; Hynes, R.O.; Lawler, J.; Iruela-Arispe, M.L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA 2001, 98, 12485–12490. [Google Scholar] [CrossRef]
- Dardik, R.; Solomon, A.; Loscalzo, J.; Eskaraev, R.; Bialik, A.; Goldberg, I.; Schiby, G.; Inbal, A. Novel Proangiogenic Effect of Factor XIII Associated with Suppression of Thrombospondin 1 Expression. Arter. Thromb. Vasc. Biol. 2003, 23, 1472–1477. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Nebgen, D.R.; Polverini, P.J. Downregulation of Endothelial Cell Thrombospondin 1 Enhances in vitro Angiogenesis. J. Vasc. Res. 1994, 31, 178–185. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Zhu, Y.-H.; Yang, Z.; Huynh, G.; Bornstein, P. Altered Extracellular Matrix Remodeling and Angiogenesis in Sponge Granulomas of Thrombospondin 2-Null Mice. Am. J. Pathol. 2001, 159, 1255–1262. [Google Scholar] [CrossRef]
- Tomii, Y.; Kamochi, J.; Yamazaki, H.; Sawa, N.; Tokunaga, T.; Ohnishi, Y.; Kijima, H.; Ueyama, Y.; Tamaoki, N.; Nakamura, M. Human thrombospondin 2 inhibits proliferation of microvascular endothelial cells. Int. J. Oncol. 2002, 20, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Torii, S.; Hara, S.; Maruyama, K.; Arai, T.; Imanaka-Yoshida, K. Tenascin-C in Tissue Repair After Myocardial Infarction in Humans. Int. J. Mol. Sci. 2023, 24, 10184. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Tenascin-C in Heart Diseases—The Role of Inflammation. Int. J. Mol. Sci. 2021, 22, 5828. [Google Scholar] [CrossRef] [PubMed]
- Ballard, V.L.T.; Sharma, A.; Duignan, I.; Holm, J.M.; Chin, A.; Choi, R.; Hajjar, K.A.; Wong, S.; Edelberg, J.M. Vascular tenascin-C regulates cardiac endothelial phenotype and neovascularization. FASEB J. 2006, 20, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, T.; Imanaka-Yoshida, K.; Shimojo, N.; Matsushima, S.; Taki, W.; Yoshida, T. Tenascin-C enhances crosstalk signaling of integrin αvβ3/PDGFR-β complex by SRC recruitment promoting PDGF-induced proliferation and migration in smooth muscle cells. J. Cell. Physiol. 2011, 226, 2617–2624. [Google Scholar] [CrossRef]
- Kudo, A.; Kii, I. Periostin function in communication with extracellular matrices. J. Cell Commun. Signal. 2018, 12, 301–308. [Google Scholar] [CrossRef]
- Sugiura, T.; Takamatsu, H.; Kudo, A.; Amann, E. Expression and Characterization of Murine Osteoblast-Specific Factor 2 (OSF-2) in a Baculovirus Expression System. Protein Expr. Purif. 1995, 6, 305–311. [Google Scholar] [CrossRef]
- Dixon, I.M.C.; Landry, N.M.; Rattan, S.G. Periostin Reexpression in Heart Disease Contributes to Cardiac Interstitial Remodeling by Supporting the Cardiac Myofibroblast Phenotype. In Periostin; Kudo, A., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1132, pp. 35–41. ISBN 9789811366567. [Google Scholar]
- Hakuno, D.; Kimura, N.; Yoshioka, M.; Mukai, M.; Kimura, T.; Okada, Y.; Yozu, R.; Shukunami, C.; Hiraki, Y.; Kudo, A.; et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2010, 120, 2292–2306. [Google Scholar] [CrossRef]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.-F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Shao, R.; Bao, S.; Bai, X.; Blanchette, C.; Anderson, R.M.; Dang, T.; Gishizky, M.L.; Marks, J.R.; Wang, X.-F. Acquired Expression of Periostin by Human Breast Cancers Promotes Tumor Angiogenesis through Up-Regulation of Vascular Endothelial Growth Factor Receptor 2 Expression. Mol. Cell. Biol. 2004, 24, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, B.S.M.S.; Kudo, Y.; Ogawa, I.; Kitagawa, M.; Kitajima, S.; Hatano, H.; Tilakaratne, W.M.; Miyauchi, M.; Takata, T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br. J. Cancer 2006, 95, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.A.; Richardson, W.J.; Holmes, J.W. Modifying the mechanics of healing infarcts: Is better the enemy of good? J. Mol. Cell. Cardiol. 2016, 93, 115–124. [Google Scholar] [CrossRef]
- Honda, S.; Asaumi, Y.; Yamane, T.; Nagai, T.; Miyagi, T.; Noguchi, T.; Anzai, T.; Goto, Y.; Ishihara, M.; Nishimura, K.; et al. Trends in the Clinical and Pathological Characteristics of Cardiac Rupture in Patients With Acute Myocardial Infarction Over 35 Years. J. Am. Heart Assoc. 2014, 3, e000984. [Google Scholar] [CrossRef]
- McInnes, A.D.; Moser, M.A.J.; Chen, X. Preparation and Use of Decellularized Extracellular Matrix for Tissue Engineering. J. Funct. Biomater. 2022, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Vilaça-Faria, H.; Noro, J.; Reis, R.L.; Pirraco, R.P. Extracellular matrix-derived materials for tissue engineering and regenerative medicine: A journey from isolation to characterization and application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar] [CrossRef]
- Cowling, R.T.; Kupsky, D.; Kahn, A.M.; Daniels, L.B.; Greenberg, B.H. Mechanisms of cardiac collagen deposition in experimental models and human disease. Transl. Res. 2019, 209, 138–155. [Google Scholar] [CrossRef]
- Kutty, J.K.; Cho, E.; Soo Lee, J.; Vyavahare, N.R.; Webb, K. The effect of hyaluronic acid incorporation on fibroblast spreading and proliferation within PEG-diacrylate based semi-interpenetrating networks. Biomaterials 2007, 28, 4928–4938. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Surya, V.N.; Gole, M.; Walker, T.W.; Yang, W.; Lai, E.S.; Ostrowski, M.A.; Fuller, G.G.; Dunn, A.R.; Huang, N.F. Nanoscale Patterning of Extracellular Matrix Alters Endothelial Function under Shear Stress. Nano Lett. 2016, 16, 410–419. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Joshi, P.A.; Lai, E.S.; Gujar, P.; Joubert, L.-M.; Chen, B.; Huang, N.F. Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. Regen. Med. 2015, 10, 745–755. [Google Scholar] [CrossRef]
- Ozguldez, H.O.; Cha, J.; Hong, Y.; Koh, I.; Kim, P. Nanoengineered, cell-derived extracellular matrix influences ECM-related gene expression of mesenchymal stem cells. Biomater. Res. 2018, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics. Physiology 2018, 33, 16–25. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899. [Google Scholar] [CrossRef]
- Melly, L.; Banfi, A. Fibrin-based factor delivery for therapeutic angiogenesis: Friend or foe? Cell Tissue Res. 2022, 387, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Zamani, M.; Huang, N.F. Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering. J. Cardiovasc. Dev. Dis. 2021, 8, 137. [Google Scholar] [CrossRef]
- Ramirez, F. Pathophysiology of the microfibril/elastic fiber system: Introduction. Matrix Biol. 2000, 19, 455–456. [Google Scholar] [CrossRef]
- Keeley, F.W.; Bellingham, C.M.; Woodhouse, K.A. Elastin as a self–organizing biomaterial: Use of recombinantly expressed human elastin polypeptides as a model for investigations of structure and self-assembly of elastin. Philos. Trans. R. Soc. Lond. B 2002, 357, 185–189. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.-W.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef]
- Jung, J.P.; Hu, D.; Domian, I.J.; Ogle, B.M. An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3D extracellular matrices. Sci. Rep. 2015, 5, 18705. [Google Scholar] [CrossRef]
- Hirata, M.; Yamaoka, T. Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of induced pluripotent stem (iPS) cells at different stages. Acta Biomater. 2018, 65, 44–52. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, S.; Liu, Y.; Brazile, B.; Cooley, J.; Butler, J.R.; McMahan, S.R.; Perez, K.L.; Xu, J.; Eastep, T.; et al. Spatial distribution and network morphology of epicardial, endocardial, interstitial, and Purkinje cell-associated elastin fibers in porcine left ventricle. Bioact. Mater. 2023, 19, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Gilbert, T.; Sellaro, T.; Badylak, S. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Lee, H.; Cho, D.-W. 3D Printing of Organs-on-Chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Tsui, Y.-K.; Cheung, K.M.C.; Chan, D.; Chan, B.P. Decellularization of Chondrocyte-Encapsulated Collagen Microspheres: A Three-Dimensional Model to Study the Effects of Acellular Matrix on Stem Cell Fate. Tissue Eng. Part C Methods 2009, 15, 697–706. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kirita, Y.; Kami, D.; Kitani, T.; Ozaki, C.; Itakura, Y.; Toyoda, M.; Gojo, S. Novel detergent for whole organ tissue engineering: Organ Tissue Engineering for Various Organs. J. Biomed. Mater. Res. 2015, 103, 3364–3373. [Google Scholar] [CrossRef]
- Choi, K.-H.; Choi, B.H.; Park, S.R.; Kim, B.J.; Min, B.-H. The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials 2010, 31, 5355–5365. [Google Scholar] [CrossRef]
- Wolchok, J.C.; Tresco, P.A. The isolation of cell derived extracellular matrix constructs using sacrificial open-cell foams. Biomaterials 2010, 31, 9595–9603. [Google Scholar] [CrossRef] [PubMed]
- Kc, P.; Hong, Y.; Zhang, G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Bai, A.; Jiang, W.; Li, X.; Wang, X.; Mao, Y.; Lu, C.; Qian, R.; Guo, F.; et al. Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction. Biomaterials 2016, 105, 52–65. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, S.; Wang, C. Multifunctional Polymer Nanocomposites Reinforced by Aligned Carbon Nanomaterials. Polymers 2018, 10, 542. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Maia, F.R.; Fonseca, K.B.; Rodrigues, G.; Granja, P.L.; Barrias, C.C. Matrix-driven formation of mesenchymal stem cell–extracellular matrix microtissues on soft alginate hydrogels. Acta Biomater. 2014, 10, 3197–3208. [Google Scholar] [CrossRef]
- Sun, T.; Shi, Q.; Huang, Q.; Wang, H.; Xiong, X.; Hu, C.; Fukuda, T. Magnetic alginate microfibers as scaffolding elements for the fabrication of microvascular-like structures. Acta Biomater. 2018, 66, 272–281. [Google Scholar] [CrossRef]
- Vanwinkle, W.B.; Snuggs, M.B.; Buja, L.M. Cardiogel: A biosynthetic extracellular matrix for cardiomyocyte culture. In Vitro Cell. Dev. Biol. Anim. 1996, 32, 478–485. [Google Scholar] [CrossRef]
- Candiello, J.; Grandhi, T.S.P.; Goh, S.K.; Vaidya, V.; Lemmon-Kishi, M.; Eliato, K.R.; Ros, R.; Kumta, P.N.; Rege, K.; Banerjee, I. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials 2018, 177, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Singelyn, J.M.; Sundaramurthy, P.; Johnson, T.D.; Schup-Magoffin, P.J.; Hu, D.P.; Faulk, D.M.; Wang, J.; Mayle, K.M.; Bartels, K.; Salvatore, M.; et al. Catheter-Deliverable Hydrogel Derived from Decellularized Ventricular Extracellular Matrix Increases Endogenous Cardiomyocytes and Preserves Cardiac Function Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 751–763. [Google Scholar] [CrossRef]
- Seif-Naraghi, S.B.; Singelyn, J.M.; Salvatore, M.A.; Osborn, K.G.; Wang, J.J.; Sampat, U.; Kwan, O.L.; Strachan, G.M.; Wong, J.; Schup-Magoffin, P.J.; et al. Safety and Efficacy of an Injectable Extracellular Matrix Hydrogel for Treating Myocardial Infarction. Sci. Transl. Med. 2013, 5, 173ra25. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Liu, X.; Hou, X.; Chen, J.; Zhang, H.; Song, S.; Han, X.; Shi, C. Specific angiogenic peptide binding with injectable cardiac ECM collagen gel promotes the recovery of myocardial infarction in rat. J. Biomed. Mater. Res. 2020, 108, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Xie, W.; Zhao, J.; Wang, Y.; Yu, W. Therapeutic angiogenesis based on injectable hydrogel for protein delivery in ischemic heart disease. iScience 2023, 26, 106577. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Brigham, M.D.; Bick, A.; Lo, E.; Bendali, A.; Burdick, J.A.; Khademhosseini, A. Mechanically Robust and Bioadhesive Collagen and Photocrosslinkable Hyaluronic Acid Semi-Interpenetrating Networks. Tissue Eng. Part A 2009, 15, 1645–1653. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Zhou, X.; Zhang, J.; Zhou, H.; Hao, H.; Ding, L.; Li, H.; Gu, Y.; Ma, J.; et al. An Overview of Extracellular Matrix-Based Bioinks for 3D Bioprinting. Front. Bioeng. Biotechnol. 2022, 10, 905438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Fu, C.-P.; Li, X.-Y.; Lu, X.-C.; Hu, L.-G.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Three-Dimensional Bioprinting of Decellularized Extracellular Matrix-Based Bioinks for Tissue Engineering. Molecules 2022, 27, 3442. [Google Scholar] [CrossRef]
- Kafili, G.; Kabir, H.; Jalali Kandeloos, A.; Golafshan, E.; Ghasemi, S.; Mashayekhan, S.; Taebnia, N. Recent advances in soluble decellularized extracellular matrix for heart tissue engineering and organ modeling. J. Biomater. Appl. 2023, 38, 577–604. [Google Scholar] [CrossRef]
- Das, S.; Nam, H.; Jang, J. 3D bioprinting of stem cell-laden cardiac patch: A promising alternative for myocardial repair. APL Bioeng. 2021, 5, 031508. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for heart tissue repair. Adv. Drug Deliv. Rev. 2008, 60, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Di Pasquale, E. Toward Cardiac Regeneration: Combination of Pluripotent Stem Cell-Based Therapies and Bioengineering Strategies. Front. Bioeng. Biotechnol. 2020, 8, 455. [Google Scholar] [CrossRef]
- Vunjak Novakovic, G.; Eschenhagen, T.; Mummery, C. Myocardial Tissue Engineering: In Vitro Models. Cold Spring Harb. Perspect. Med. 2014, 4, a014076. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Huang, N.F.; Lee, R.J.; Li, S. Chemical and Physical Regulation of Stem Cells and Progenitor Cells: Potential for Cardiovascular Tissue Engineering. Tissue Eng. 2007, 13, 1809–1823. [Google Scholar] [CrossRef]
- Kane, R. Patterning proteins and cells using soft lithography. Biomaterials 1999, 20, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Politi, S.; Carotenuto, F.; Rinaldi, A.; Di Nardo, P.; Manzari, V.; Albertini, M.C.; Araneo, R.; Ramakrishna, S.; Teodori, L. Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials 2020, 10, 1781. [Google Scholar] [CrossRef]
- Su, Y.; Toftdal, M.S.; Le Friec, A.; Dong, M.; Han, X.; Chen, M. 3D Electrospun Synthetic Extracellular Matrix for Tissue Regeneration. Small Sci. 2021, 1, 2100003. [Google Scholar] [CrossRef]
- Broadwin, M.; Imarhia, F.; Oh, A.; Stone, C.R.; Sellke, F.W.; Bhowmick, S.; Abid, M.R. Exploring Electrospun Scaffold Innovations in Cardiovascular Therapy: A Review of Electrospinning in Cardiovascular Disease. Bioengineering 2024, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, D.; Ding, L.; Li, X.-L.; Liu, X.-T.; Li, W.-J.; Wei, T.; Yan, S.; Xie, J.-H.; Wei, L.; et al. Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling. BMC Cell Biol. 2015, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Broadwin, M.; St. Angelo, K.; Petersen, M.; Teixeira, R.B.; Harris, D.D.; Stone, C.R.; Xu, C.; Kanuparthy, M.; Sellke, F.W.; Morgan, J.; et al. Lab-grown, 3D extracellular matrix particles improve cardiac function and morphology in myocardial is chemia. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H221–H234. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamze, J.; Broadwin, M.; Stone, C.; Muir, K.C.; Sellke, F.W.; Abid, M.R. Developments in Extracellular Matrix-Based Angiogenesis Therapy for Ischemic Heart Disease: A Review of Current Strategies, Methodologies and Future Directions. BioTech 2025, 14, 23. https://doi.org/10.3390/biotech14010023
Hamze J, Broadwin M, Stone C, Muir KC, Sellke FW, Abid MR. Developments in Extracellular Matrix-Based Angiogenesis Therapy for Ischemic Heart Disease: A Review of Current Strategies, Methodologies and Future Directions. BioTech. 2025; 14(1):23. https://doi.org/10.3390/biotech14010023
Chicago/Turabian StyleHamze, Jad, Mark Broadwin, Christopher Stone, Kelsey C. Muir, Frank W. Sellke, and M. Ruhul Abid. 2025. "Developments in Extracellular Matrix-Based Angiogenesis Therapy for Ischemic Heart Disease: A Review of Current Strategies, Methodologies and Future Directions" BioTech 14, no. 1: 23. https://doi.org/10.3390/biotech14010023
APA StyleHamze, J., Broadwin, M., Stone, C., Muir, K. C., Sellke, F. W., & Abid, M. R. (2025). Developments in Extracellular Matrix-Based Angiogenesis Therapy for Ischemic Heart Disease: A Review of Current Strategies, Methodologies and Future Directions. BioTech, 14(1), 23. https://doi.org/10.3390/biotech14010023






