Chemical, Textural and Antioxidant Properties of Oat-Fermented Beverages with Different Starter Lactic Acid Bacteria and Pectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Oat Material, Starter Cultures and Samples
2.2. Preparing of Starters and Fermentation of Oat Base
2.3. Chemical Assays
2.4. Preparing Protein-Free Extract (PFE) and Total Phenolic Compound (TPC) Assays
2.4.1. Preparing of Protein-Free Extract (PFE)
2.4.2. Total Phenolic Compound (TPC) Assays
2.5. Isolation and Yield of Extractable Polysaccharides (EPSs)
2.6. Apparent Viscosity and Thixotropic Property Assays
2.7. Syneresis Analysis
2.8. Water-Holding Capacity Analysis
2.9. Texture Profile Analysis (TPA)
2.10. Sensory Analysis
2.11. Antioxidant Assays
2.11.1. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.11.2. Evaluation of Radical-Scavenging Ability (RSA) by 2,2-Di-Phenyl-1-Picrylhydrazyl (DPPH) Assay
2.11.3. OH Free-Radical-Scavenging Assay
2.12. Statistical Analysis
3. Results
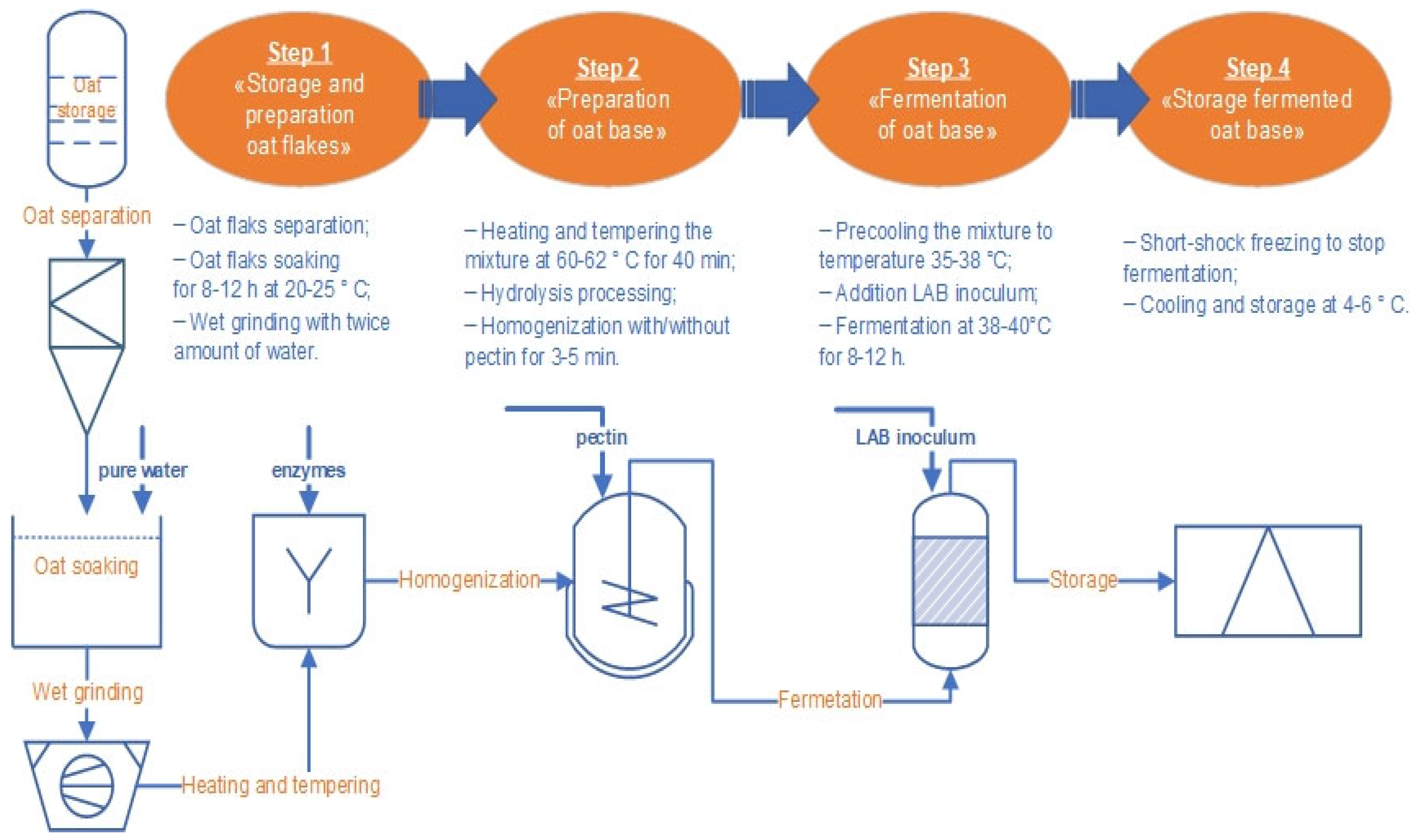
3.1. Reasons for Choosing Raw Materials, Preparation Oat Flakes and Base
3.2. The Oat Base Fermentation
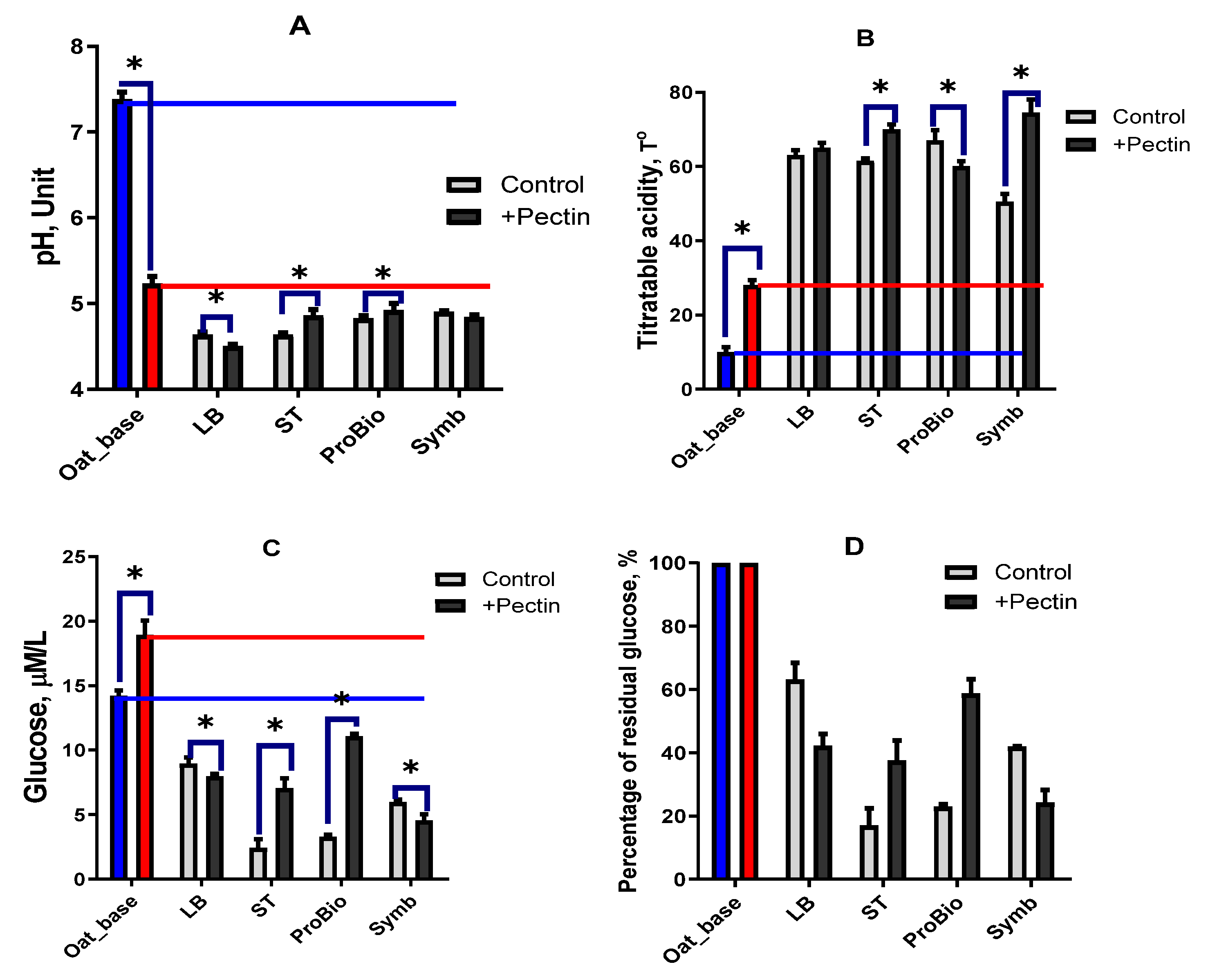
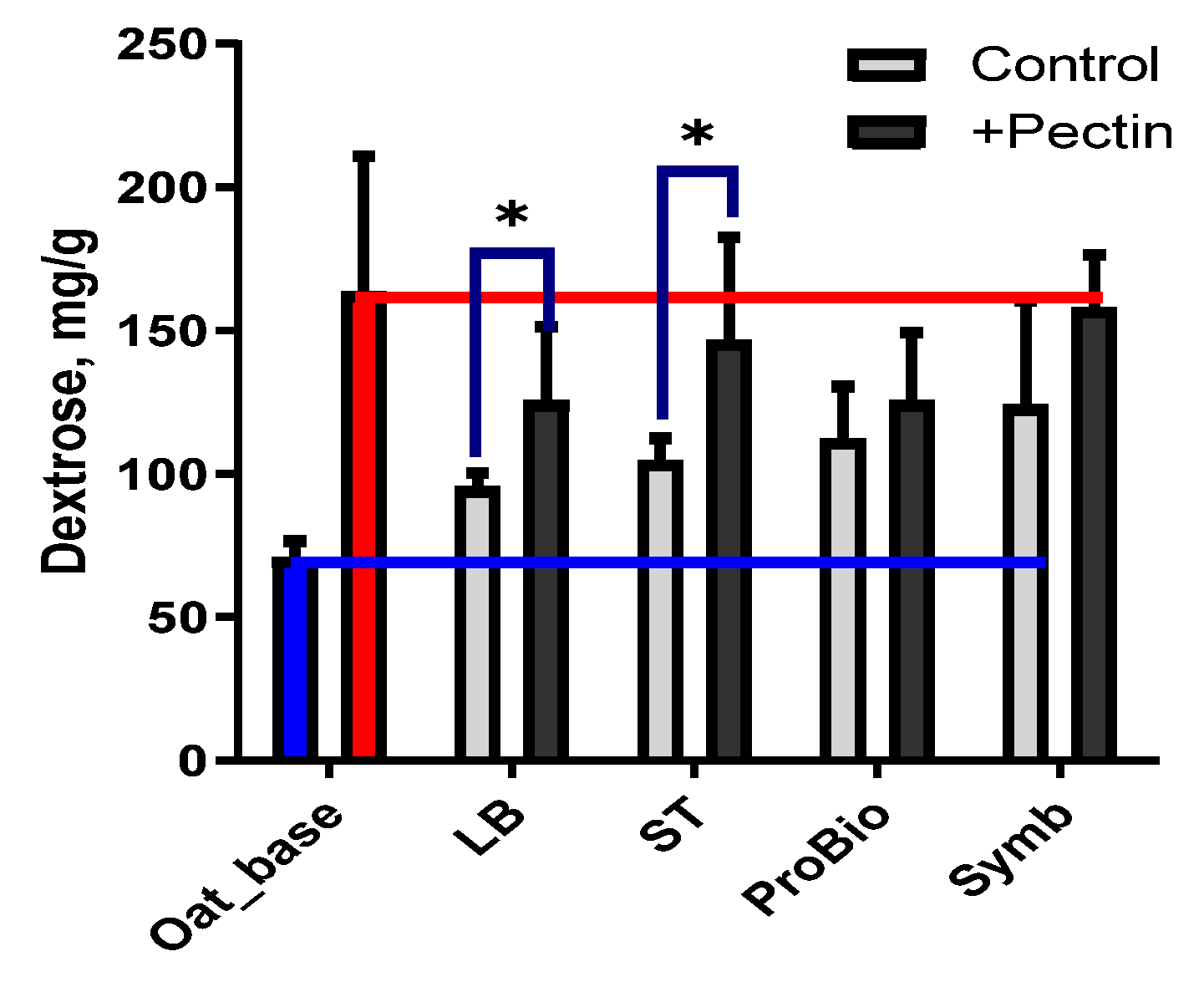
3.2.1. Chemical Composition
3.2.2. Sensory Estimation
3.2.3. Structural and Textural Properties
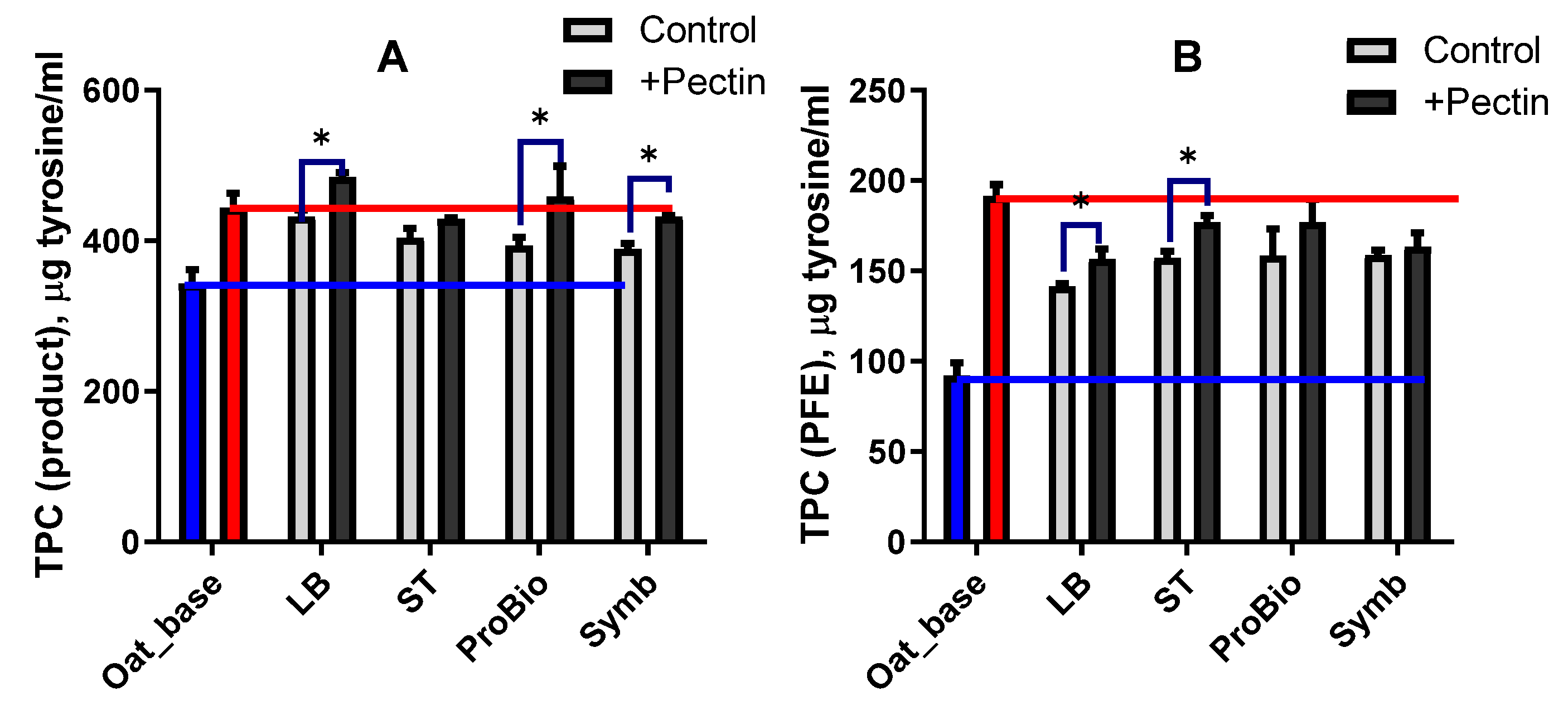
3.2.4. Antioxidant Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Tuccillo, F.; Lampi, A.M.; Knaapila, A.; Pulkkinen, M.; Kariluoto, S.; Coda, R.; Edelmann, M.; Jouppila, K.; Sandell, M.; et al. Flavor challenges in extruded plant-based meat alternatives: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2898–2929. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, N.U.; Rao, P.S. Effect of processing on storage stability of millet flour: A review. Trends Food Sci. Technol. 2021, 112, 58–74. [Google Scholar] [CrossRef]
- Kamal, N.; Tsardakas Renhuldt, N.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mohd Basri, M.S.; Mohd Jais, N.; Sulaiman, A.; Mohd Nor, M.Z.; Abdul Karim Shah, N.N.; Ariffin, S.H. Optimizing the Processing Factor and Formulation of Oat-Based Cookie Dough for Enhancement in Stickiness and Moisture Content Using Response Surface Methodology and Superimposition. Processes 2020, 8, 797. [Google Scholar] [CrossRef]
- Brückner-Gühmann, M.; Benthin, A.; Drusch, S. Enrichment of yoghurt with oat protein fractions: Structure formation, textural properties and sensory evaluation. Food Hydrocoll. 2019, 86, 146–153. [Google Scholar] [CrossRef]
- Guo, X.N.; Gao, F.; Zhu, K.X. Effect of fresh egg white addition on the quality characteristics and protein aggregation of oat noodles. Food Chem. 2020, 330, 127319. [Google Scholar] [CrossRef]
- McGorrin, R.J. Key aroma compounds in oats and oat cereals. J. Agric. Food Chem. 2019, 67, 13778–13789. [Google Scholar] [CrossRef]
- Kumar, L.; Sehrawat, R.; Kong, Y. Oat proteins: A perspective on functional properties. Lebensm. Wiss. Technol. 2021, 152, 112307. [Google Scholar] [CrossRef]
- Varma, P.; Bhankharia, H.; Bhatia, S. Oats: A multi-functional grain. J. Clin. Prev. Cardiol. 2016, 5, 9. [Google Scholar] [CrossRef]
- Rollan, G.C.; Gerez, C.L.; Leblanc, J.G. Lactic fermentation as a strategy to improve the nutritional and functional values of pseudocereals. Front. Nutr. 2019, 6, 98. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Wronkowska, M.; Rostek, D.; Lenkiewicz, M.; Kurantowicz, E.; Yaneva, T.G.; Starowicz, M. Oat flour fermented by Lactobacillus strains—Kinetics of volatile compound formation and antioxidant capacity. J. Cereal Sci. 2022, 103, 103392. [Google Scholar] [CrossRef]
- Dallagnol, A.M.; Pescuma, M.; De Valdez, G.F.; Rollán, G. Fermentation of quinoa and wheat slurries by Lactobacillus plantarum CRL 778: Proteolytic activity. Appl. Microbiol. Biotechnol. 2013, 97, 3129–3140. [Google Scholar] [CrossRef]
- Wang, X.; Lu, H.; Gu, X.; Li, C.; Tian, H.; Wang, M.; Kang, H. Technology of oat milk fermentation by lactobacillus casei and product quality analysis. J. Chin. Inst. Food Sci. Technol. 2021, 21, 126–134. [Google Scholar]
- Chen, H.Y.; Hsieh, C.W.; Chen, P.C.; Lin, S.P.; Lin, Y.F.; Cheng, K.C. Development and Optimization of Djulis Sourdough Bread Fermented by Lactic Acid Bacteria for Antioxidant Capacity. Molecules 2021, 26, 5658. [Google Scholar] [CrossRef]
- Lee, S.M.; Oh, J.; Hurh, B.-S.; Jeong, G.-H.; Shin, Y.-K.; Kim, Y.-S. Volatile Compounds Produced by Lactobacillus paracasei During Oat Fermentation. J. Food Sci. 2016, 81, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Vasile, A.; Bahrim, G.E.; Orpianesi, C.; Cresci, A. Effect of buckwheat flour and oat bran on growth and cell viability of the probiotic strains Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and their combination SYNBIO®, in synbiotic fermented milk. Int. J. Food Microbiol. 2013, 167, 261–268. [Google Scholar] [CrossRef]
- Khrundin, D.; Ponomarev, V.; Yunusov, E. Fermented oat milk as a base for lactose-free sauce. Foods Raw Mater. 2022, 10, 155–162. [Google Scholar] [CrossRef]
- Masiá, C.; Geppel, A.; Jensen, P.E.; Buldo, P. Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods 2021, 10, 573. [Google Scholar] [CrossRef]
- Chen, L.; Wu, D.; Schlundt, J.; Conway, P.L. Development of a Dairy-Free Fermented Oat-Based Beverage with Enhanced Probiotic and Bioactive Properties. Front. Microbiol. 2020, 11, 609734. [Google Scholar] [CrossRef]
- Tao, L.; Wang, J.; Zhu, Q.; Zhang, J.; Li, Y.; Song, S.; Yu, L. Effect of fermentation with Lactobacillus fermentum FL-0616 on probiotic-rich bean powders. J. Food Sci. Technol. 2023, 60, 1144–1152. [Google Scholar] [CrossRef]
- Goncerzewicz, A.; Misiewicz, A.; Owczarek, L.; Jasińska, U.; Skąpska, S. The Effect of a Newly Developed Oat-Banana Fermented Beverage with a Beta-glucan Additive on ldhL Gene Expression in Streptococcus thermophilus TKM3 KKP 2030p. Curr. Microbiol 2016, 73, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Qian, J.; Guo, Y.; Qian, H.; Yao, W.; Cheng, Y. Applicable Strains, Processing Techniques and Health Benefits of Fermented Oat Beverages: A Review. Foods 2023, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Angelov, A.; Yaneva, T.; Gotcheva, V. Oats as a matrix of choice for developing fermented functional beverages. J. Food Sci. Technol. 2018, 55, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Tomita, S.; Mercenier, A.; Kleerebezem, M. Cell surface-associated compounds of probiotic lactobacilli sustain the strain-specificity dogma. Curr. Opin. Microbiol. 2013, 16, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Cicenia, A.; Scirocco, A.; Carabotti, M.; Pallotta, L.; Marignani, M.; Severi, C. Postbiotic activities of Lactobacilli-derived factors. J. Clin. Gastroenterol. 2014, 48, 18–22. [Google Scholar] [CrossRef]
- Kim, K.W.; Kang, S.S.; Woo, S.J.; Park, O.J.; Ahn, K.B.; Song, K.D.; Lee, H.K.; Yun, C.H.; Han, S.H. Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates poly I: C-induced IL-8 production in porcine intestinal epithelial cells. Front. Microbiol. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy. Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 5–14. [Google Scholar] [CrossRef]
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 24, 20399. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid. Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Niu, Y.; Li, M.; Liu, Y.; Wang, K.; Gao, Z.; Wang, Z.; Yue, T.; Yuan, Y. Emerging trends in pectin functional processing and its fortification for synbiotics: A review. Trends Food Sci. Technol. 2023, 134, 80–97. [Google Scholar] [CrossRef]
- Hua, Y.; Wei, Z.; Xue, C.; Si, J. Stability and programmed sequential release of Lactobacillus plantarum and curcumin encapsulated in bilayer-stabilized W1/O/W2 double emulsion: Effect of pectin as protective shell. Int. J. Biol. Macromol. 2024, 265 Pt 1, 130805. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Qiu, B.; Liu, Z.; Gao, X.; Zhang, N.; Liu, X.; Qi, S.; Li, L.; Liu, W. Encapsulation of Lactobacillus plantarum in W1/O/W2 double emulsions stabilized with the high-intensity ultrasound-treated pea protein and pectin. Ultrason. Sonochem. 2024, 107, 106936. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Jia, W.; Du, A.; Shi, L. Complex pectin metabolism by Lactobacillus and Streptococcus suggests an effective control approach for Maillard harmful products in brown fermented milk. Fundam. Res. 2022, in press. [Google Scholar] [CrossRef]
- Yadav, M.; Sehrawat, N.; Sharma, A.K.; Kumar, S.; Singh, R.; Kumar, A.; Kumar, A. Synbiotics as potent functional food: Recent updates on therapeutic potential and mechanistic insight. J. Food Sci. Technol. 2024, 61, 1–15. [Google Scholar] [CrossRef]
- Nikitina, E.; Petrova, T.; Vafina, A.; Ezhkova, A.; Nait Yahia, M.; Kayumov, A. Textural and Functional Properties of Skimmed and Whole Milk Fermented by Novel Lactiplantibacillus plantarum AG10 Strain Isolated from Silage. Fermentation 2022, 8, 290. [Google Scholar] [CrossRef]
- Nikitina, E.; Petrova, T.; Sungatullina, A.; Bondar, O.; Kharina, M.; Mikshina, P.; Gavrilova, E.; Kayumov, A. The Profile of Exopolysaccharides Produced by Various Lactobacillus Species from Silage during Not-Fat Milk Fermentation. Fermentation 2023, 9, 197. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Analyt. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sungatullina, A.; Petrova, T.; Nikitina, E. Investigation on Fermented Milk Quality after the Addition of Flaxseed Mucilage and the Use of Lactobacillus delbrueckii subsp. bulgaricus and Lactiplantibacillus plantarum AG9. Front. Biosci.-Elite 2024, 16, 11. [Google Scholar] [CrossRef]
- Sungatullina, A.; Petrova, T.; Kharina, M.; Mikshina, P.; Nikitina, E. Effect of Flaxseed Mucilage on the Probiotic, Antioxidant, and Structural-Mechanical Properties of the Different Lactobacillus Cells. Fermentation 2023, 9, 486. [Google Scholar] [CrossRef]
- Smith, N.W.; Dave, A.C.; Hill, J.P.; McNabb, W.C. Nutritional assessment of plant-based beverages in comparison to bovine milk. Front. Nutr. 2022, 9, 957486. [Google Scholar] [CrossRef] [PubMed]
- Chalupa-Krebzdak, S.; Long, C.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Cui, L.; Jia, Q.; Zhao, J.; Hou, D.; Zhou, S. A comprehensive review on oat milk: From oat nutrients and phytochemicals to its processing technologies, product features, and potential applications. Food Funct. 2023, 14, 5858–5869. [Google Scholar] [CrossRef]
- Sargautis, D.; Kince, T.; Gramatina, I. Characterisation of the Enzymatically Extracted Oat Protein Concentrate after Defatting and Its Applicability for Wet Extrusion. Foods 2023, 12, 2333. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.Z.; Zhang, X.; Wu, Z.F.; Liu, L.Y.; Liu, L.L. Whole grain benefit: Synergistic effect of oat phenolic compounds and beta-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Mårtensson, O.; Dueñas-Chasco, M.; Irastorza, A.; Öste, R.; Holst, O. Comparison of growth characteristics and exopolysaccharide formation of two lactic acid bacteria strains, Pediococcus damnosus 2.6 and Lactobacillus brevis G-77, in an oat-based, nondairy medium. LWT—Food Sci. Technol. 2003, 36, 353–357. [Google Scholar] [CrossRef]
- Wang, Y.; Mehmood, S.; Maina, N.H.; Katina, K.; Coda, R. Synthesis in situ of heteropolysaccharide by Levilactobacillus brevis AM7 during fermentation of oat and hemp and its effect on the techno-functional properties of oat yogurt type model. Food Hydrocoll. 2024, 147 Pt B, 109416. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Li, Z.; Xi, J.; Chen, H.; Chen, W.; Chen, W.; Zhong, Q.; Zhang, M. Effect of glycosylation with apple pectin, citrus pectin, mango pectin and sugar beet pectin on the physicochemical, interfacial and emulsifying properties of coconut protein isolate. Food Res. Int. 2022, 156, 111363. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Kanareikina, S.G.; Kanareikin, V.I.; Ganieva, E.S.; Burakovskaya, N.V.; Shadrin, M.A.; Khalepo, O.; Babaeva, M.V.; Voskanyan, O.S. The structure development of yogurt with vegetable ingredients. Int. J. Recent. Technol. Eng. 2019, 8, 1587–1592. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Witek, M.; Żmudziński, D.; Ptaszek, A. Changes in the viscosity, textural properties, and water status in yogurt gel upon supplementation with green and Pu-erh teas. J. Dairy. Sci. 2020, 103, 11039–11049. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A.J. Human Oral Processing and Texture Profile Analysis Parameters—Bridging the gap between the sensory evaluation and the instrumental measurements. J. Texture Stud. 2019, 50, 12404. [Google Scholar] [CrossRef]
- McMullen, R.L.; Gorcea, M.; Chen, S.S. Emulsions and their Characterization by Texture Profile Analysis. In Apply Topically: A Practical Guide to Formulating Topical Application; Dayan, N., Ed.; Allured Publishing: Carol Stream, IL, USA, 2016; Chapter 6; pp. 131–154. [Google Scholar]
- Shendurse, A.M.; Khedkar, C.D. Glucose: Properties and Analysis. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; Volume 3, pp. 239–247. [Google Scholar]
- Collard, K.M.; McCormick, D.P. A nutritional comparison of cow’s milk and alternative milk products. Acad. Pediatr. 2021, 21, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, Y.; Zhang, Y.; Liu, L.; Wu, Z.; Weng, P. Insights into oat polyphenols constituent against advanced glycation end products mechanism by spectroscopy and molecular interaction. Food Biosci. 2021, 43, 101313. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Dong, L.; Li, Y.; Liu, Y.; Liu, Y.; Liu, L.; Liu, L. Fermentation of Lactobacillus fermentum NB02 with feruloyl esterase production increases the phenolic compounds content and antioxidant properties of oat bran. Food Chem. 2024, 437 Pt 1, 137834. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.Y.; Li, W.; Sun, Z. Structure characterization, antioxidant capacity, rheological characteristics and expression of biosynthetic genes of exopolysaccharides produced by Lactococcus lactis subsp. lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Feng, J.; Cai, Z.; Chen, Y.; Zhu, H.; Chang, X.; Wang, X.; Liu, Z.; Zhang, J.; Nie, G. Effects of an exopolysaccharide from Lactococcus lactis Z-2 on innate immune response, antioxidant activity, and disease resistance against Aeromonas hydrophila in Cyprinus carpio L. Fish. Shellfish. Immunol. 2020, 98, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Ngamsamer, C.; Muangnoi, C.; Tongkhao, K.; Sae-Tan, S.; Treesuwan, K.; Sirivarasai, J. Potential Health Benefits of Fermented Vegetables with Additions of Lacticaseibacillus rhamnosus GG and Polyphenol Vitexin Based on Their Antioxidant Properties and Prohealth Profiles. Foods 2024, 13, 982. [Google Scholar] [CrossRef] [PubMed]
- de Assis, B.B.T.; Pimentel, T.C.; Dantas, A.M.; Dos Santos Lima, M.; da Silva, C.B.G.; Magnani, M. Biotransformation of the Brazilian Caatinga fruit-derived phenolics by Lactobacillus acidophilus La-5 and Lacticaseibacillus casei 01 impacts bioaccessibility and antioxidant activity. Food Res. Int. 2021, 146, 110435. [Google Scholar] [CrossRef] [PubMed]
- Dzandu, B.; Chotiko, A.; Sathivel, S. Antioxidant activity and viability of Lacticaseibacillus rhamnosus, Lacticaseibacillus casei, and Co-culture in fermented tomato juice during refrigerated storage. Food Biosci. 2022, 50, 102085. [Google Scholar] [CrossRef]
- Zhong, H.; Li, B.; Fan, Z.; Fang, Z.; Li, Y.; Lu, S.; Shen, Y.; Wang, W.; Zhang, R. Isolation, purification, characterization, and analysis of the antioxidant activity of antioxidant peptides from walnut milk fermented with Lactobacillus paracasei SMN-LBK. Food Biosci. 2024, 61, 104547. [Google Scholar] [CrossRef]
- Xu, J.G.; Tian, C.R.; Hu, Q.P.; Luo, J.Y.; Wang, X.D.; Tian, X.D. Dynamic changes in phenolic compounds and antioxidant activity in Oats (Avena nuda L.) during steeping and germination. J. Agric. Food Chem. 2009, 57, 10392–10398. [Google Scholar] [CrossRef]
- Chen, D.; Shi, J.; Hu, X.; Du, S. Alpha-amylase treatment increases extractable phenolics and antioxidant capacity of oat (Avena nuda L.) flour. J. Cereal Sci. 2015, 65, 60–66. [Google Scholar] [CrossRef]
- del Amo-Mateos, E.; Pérez, R.; Merino, A.; Lucas, S.; García-Cubero, M.T.; Coca, M. Rhamnogalacturonan–I pectin and derived oligosaccharides obtained from sugar beet pulp and discarded red beetroot: Characterization and comparative study of their antioxidant and prebiotic properties. Food Hydrocoll. 2024, 152, 109955. [Google Scholar] [CrossRef]
- Prandi, B.; Baldassarre, S.; Babbar, N.; Bancalari, E.; Vandezande, P.; Hermans, D.; Bruggeman, G.; Gatti, M.; Elst, K.; Sforza, S. Pectin oligosaccharides from sugar beet pulp: Molecular characterization and potential prebiotic activity. Food Funct. 2018, 9, 1557–1569. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Innovative technologies for manufacturing plant-based non-dairy alternative milk and their impact on nutritional, sensory and safety aspects. Future Foods 2021, 5, 100098. [Google Scholar] [CrossRef]
- Nasseri, A.T.; Thibault, J.-F.; Ralet, M.C. Citrus pectin: Structure and application in acid dairy drinks. Tree Sci. Biotechnol. 2018, 2, 60–70. [Google Scholar]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.; Petkova, N.; Todorova, M.; Dobreva, V.; Vlaseva, R.; Denev, P.; Hadjikinov, D.; Bouvard, V. Influence of citrus and celery pectins on physicochemical and sensory characteristic of fermented dairy products. Sci. Study Res. 2020, 21, 533–545. [Google Scholar]
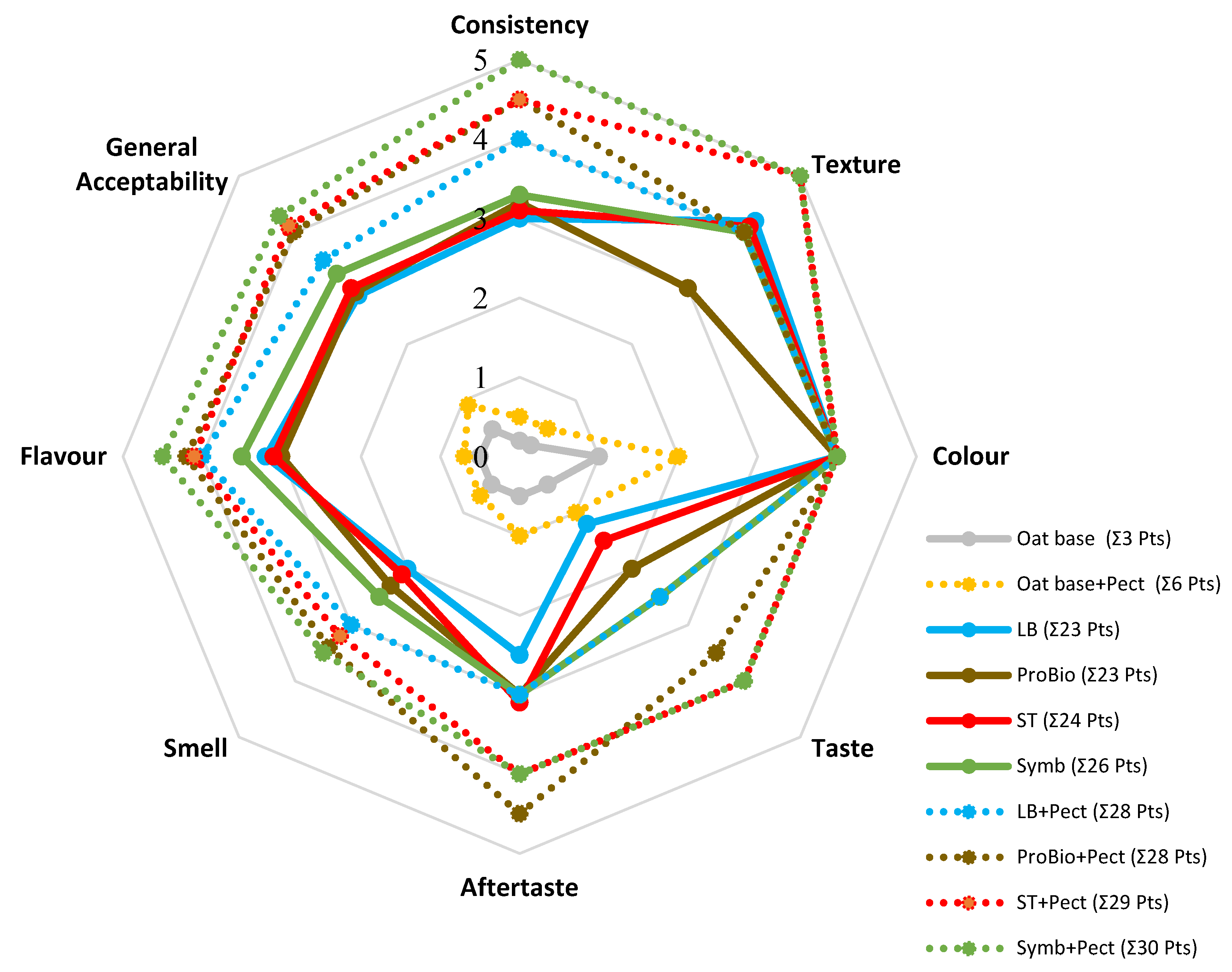
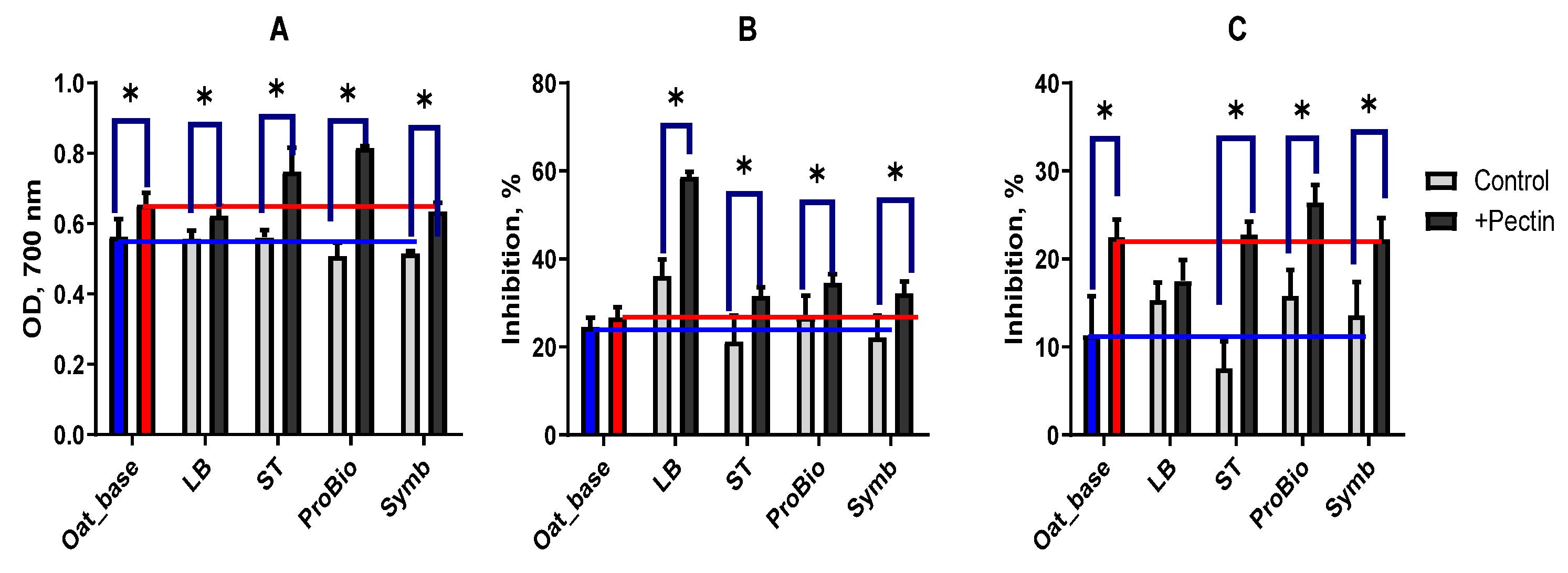
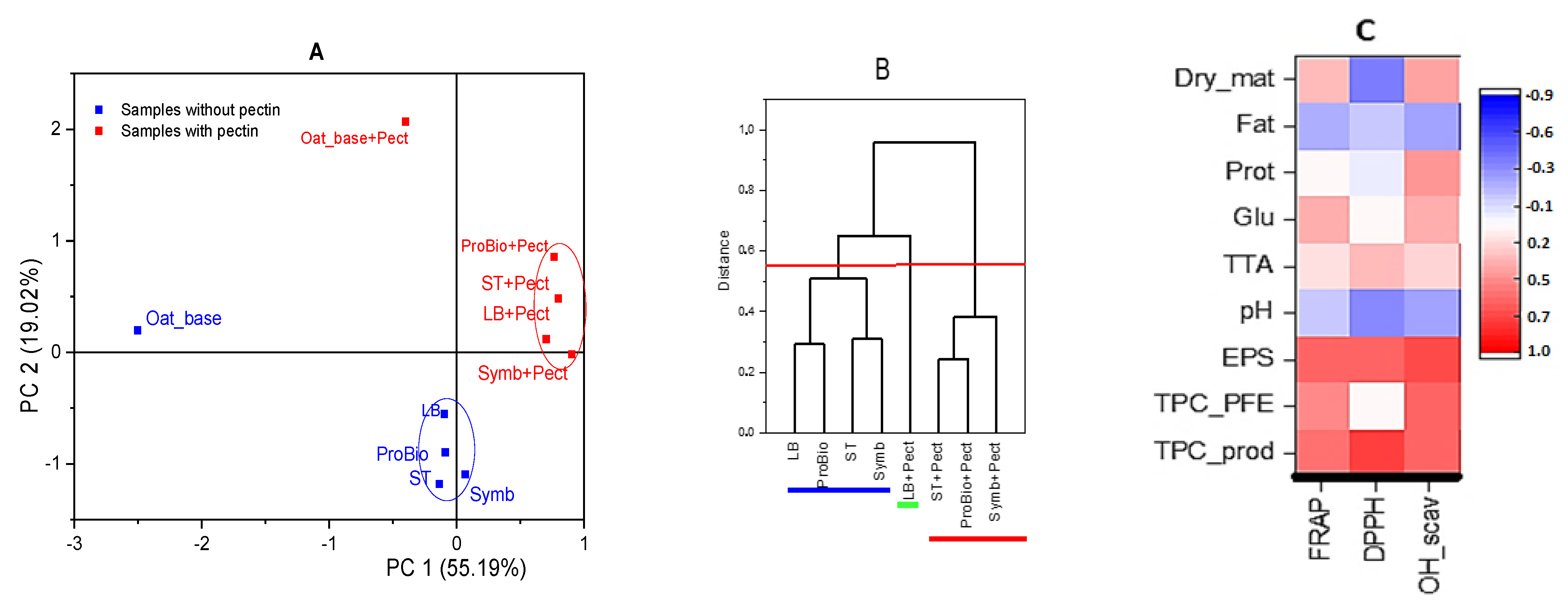
| Sampel | Starter Cultures | Short Description | ||
|---|---|---|---|---|
| Lactobacilli | Strepto/Lactococci | Bifidobacteria | ||
| LB | L. delbrueckii subsp. bulgaricus | - | - | The oat base was treated by amylolytic enzymes and then fermented by appropriate LAB starter cultures. |
| ST | - | S. thermophilus | - | |
| ProBio | L. delbrueckii ssp. bulgaricus L. acidophilus, L. casei, L. rhamnosus, L. paracasei | S. thermophilus | B. lactis, B. infantis | |
| Symb | L. delbrueckii ssp. bulgaricus L. acidophilus | S. thermophilus L. lactis ssp. Cremoris, L. lactis ssp. lactis, L. lactis ssp. lactis var. diacetylactis | B. lactis | |
| Control | Oat_base without pectin was fermented by starter cultures LAB | |||
| +Pectin | Oat_base with pectin (1%) was fermented by starter cultures LAB | |||
| Parameter | Before | After |
|---|---|---|
| pH | 7.54 ± 0.08 * | 7.34 ± 0.07 * |
| Titrable acidity, °T | 8.0 ± 0.05 * | 10.0 ± 0.06 * |
| Protein, % | 2.66 ± 0.08 * | 3.07 ± 0.25 * |
| Fat, % | 1.33 ± 0.07 * | 1.66 ± 0.21 * |
| Dry matter, % | 12.37 ± 0.09 * | 14.80 ± 0.38 * |
| Total sugar, % | 2.73 ± 0.02 * | 6.36 ± 0.05 * |
| Glucose, mmol/L | 0.1 ± 0.01 * | 16.0 ± 0.02 * |
| Sample | Protein, % | Fat, % | Dry Matter, % | |||
|---|---|---|---|---|---|---|
| Control | +Pectin | Control | +Pectin | Control | +Pectin | |
| Oat_base | 3.07 ± 0.25 | 3.29 ± 0.15 b | 1.33 ± 0.03 | 0.86 ± 0.01 b | 11.81 ± 0.32 | 13.56 ± 0.24 b |
| LB | 3.23 ± 0.06 | 3.24 ± 0.06 | 0.91 ± 0.02 a | 0.94 ± 0.02 a | 10.74 ± 0.21 a | 11.10 ± 0.22 a |
| ST | 3.10 ± 0.06 | 3.40 ± 0.07 b | 0.81 ± 0.02 a | 0.94 ± 0.02 ab | 11.39 ± 0.23 a | 11.99 ± 0.24 ab |
| ProBio | 3.47 ± 0.17 a | 3.44 ± 0.12 | 0.97 ± 0.02 a | 0.91 ± 0.02 ab | 11.48 ± 0.23 a | 12.09 ± 0.24 ab |
| Symb | 3.25 ± 0.07 | 3.16 ± 0.06 a | 0.92 ± 0.02 a | 0.89 ± 0.02 | 11.12 ± 0.22 a | 11.58 ± 0.23 ab |
| Sample | Apparent Viscosity, mPa·s−1 | Lη, % | CMR | |||
|---|---|---|---|---|---|---|
| −Pectin | +Pectin | −Pectin | +Pectin | −Pectin | +Pectin | |
| Oat_base | 22.4 ± 1.1 | 40.3 ± 2.0 ab | 6.5 | 3.5 | 0.87 | 0.96 |
| LB | 26.3 ± 1.3 a | 44.6 ± 2.2 ab | 4.2 | 2.4 | 0.94 | 0.98 |
| ST | 68.8 ± 3.4 a | 146.8 ± 7.3 ab | 4.4 | 2.0 | 0.94 | 0.98 |
| ProBio | 59.5 ± 3.0 a | 101.2 ± 5.1 ab | 5.2 | 2.5 | 0.92 | 0.97 |
| Symb | 64.8 ± 3.2 a | 141.7 ± 7.1 ab | 3.0 | 1.0 | 0.94 | 0.98 |
| Sample | Syn, % | Δ Syn, % to −Pectin | WHC, % | Δ WHC, % to −Pectin | ||
| −Pectin | +Pectin | −Pectin | +Pectin | |||
| Oat_base | 45.0 ± 2.3 ab | 28.0 ± 1.4 ab | −42.6 | 23.0 ± 1.2 ab | 32.0 ± 1.6 ab | +35.4 |
| LB | 36.9 ± 1.8 ab | 14.4 ± 0.7 ab | −61.1 | 27.6 ± 1.4 ab | 41.0 ± 2.1 ab | +48.7 |
| ST | 27.4 ± 1.4 ab | 3.5 ± 0.2 ab | −87.2 | 28.0 ± 1.4 ab | 39.0 ± 2.0 ab | +39.3 |
| ProBio | 31.0 ± 1.6 ab | 3.7 ± 0.2 ab | −81.0 | 29.3 ± 1.5 ab | 32.5 ± 1.6 ab | +11.0 |
| Symb | 27.5 ± 1.4 ab | 5.2 ± 0.3 ab | −88.1 | 29.9 ± 1.5 ab | 41.5 ± 2.1 ab | +38.9 |
| Sample | Hardness, g | Cohesiveness, % | ||
|---|---|---|---|---|
| −Pectin | +Pectin | −Pectin | +Pectin | |
| LB | 11.22 ± 0.56 | 12.11 ± 0.61 | 94.34 ± 4.72 | 97.61 ± 4.88 |
| ST | 12.53 ± 0.63 | 13.21 ± 0.69 | 75.90 ± 3.80 | 81.51 ± 4.08 |
| ProBio | 11.92 ± 0.60 | 13.12 ± 0.66 a | 92.68 ± 4.63 | 96.37 ± 4.92 |
| Symb | 11.93 ± 0.60 | 12.91 ± 0.65 | 92.19 ± 4.61 | 95.84 ± 4.79 |
| Sample | Gumminess, g | Adhesiveness, g·mm | ||
| −Pectin | +Pectin | −Pectin | +Pectin | |
| LB | 10.60 ± 0.53 | 11.81 ± 0.59 a | 45.44 ± 2.27 | 51.53 ± 2.58 a |
| ST | 9.5 ± 0.48 b | 11.16 ± 0.56 a | 52.2 ± 2.61 b | 56.85 ± 2.84 a |
| ProBio | 11.72 ± 0.59 b | 12.12 ± 0.61 a | 49.63 ± 2.48 b | 54.86 ± 2.74 a |
| Symb | 10.97 ± 0.55 | 12.38 ± 0.62 a | 51.28 ± 2.56 b | 60.22 ± 3.01 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrundin, D.V.; Nikitina, E.V. Chemical, Textural and Antioxidant Properties of Oat-Fermented Beverages with Different Starter Lactic Acid Bacteria and Pectin. BioTech 2024, 13, 38. https://doi.org/10.3390/biotech13040038
Khrundin DV, Nikitina EV. Chemical, Textural and Antioxidant Properties of Oat-Fermented Beverages with Different Starter Lactic Acid Bacteria and Pectin. BioTech. 2024; 13(4):38. https://doi.org/10.3390/biotech13040038
Chicago/Turabian StyleKhrundin, Dmitrii V., and Elena V. Nikitina. 2024. "Chemical, Textural and Antioxidant Properties of Oat-Fermented Beverages with Different Starter Lactic Acid Bacteria and Pectin" BioTech 13, no. 4: 38. https://doi.org/10.3390/biotech13040038
APA StyleKhrundin, D. V., & Nikitina, E. V. (2024). Chemical, Textural and Antioxidant Properties of Oat-Fermented Beverages with Different Starter Lactic Acid Bacteria and Pectin. BioTech, 13(4), 38. https://doi.org/10.3390/biotech13040038






