Development of Biodegradable Cups from Corn and Fruit Processing Waste and Its Characterization: A Sustainable Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Raw Material Preparation
2.2.2. Experimental Design
2.2.3. Development of Cups
2.2.4. Characterization
Texture Analysis
Colour Analysis
Water-Holding Capacity
Physical and Dimensional Analysis
Fourier Transform Infrared Spectroscopy (FTIR)
X-Ray Diffraction (XRD)
Scanning Electron Microscopy
Biodegradability Testing by Soil Burial Test
2.2.5. Statistical Analysis
3. Results and Discussion
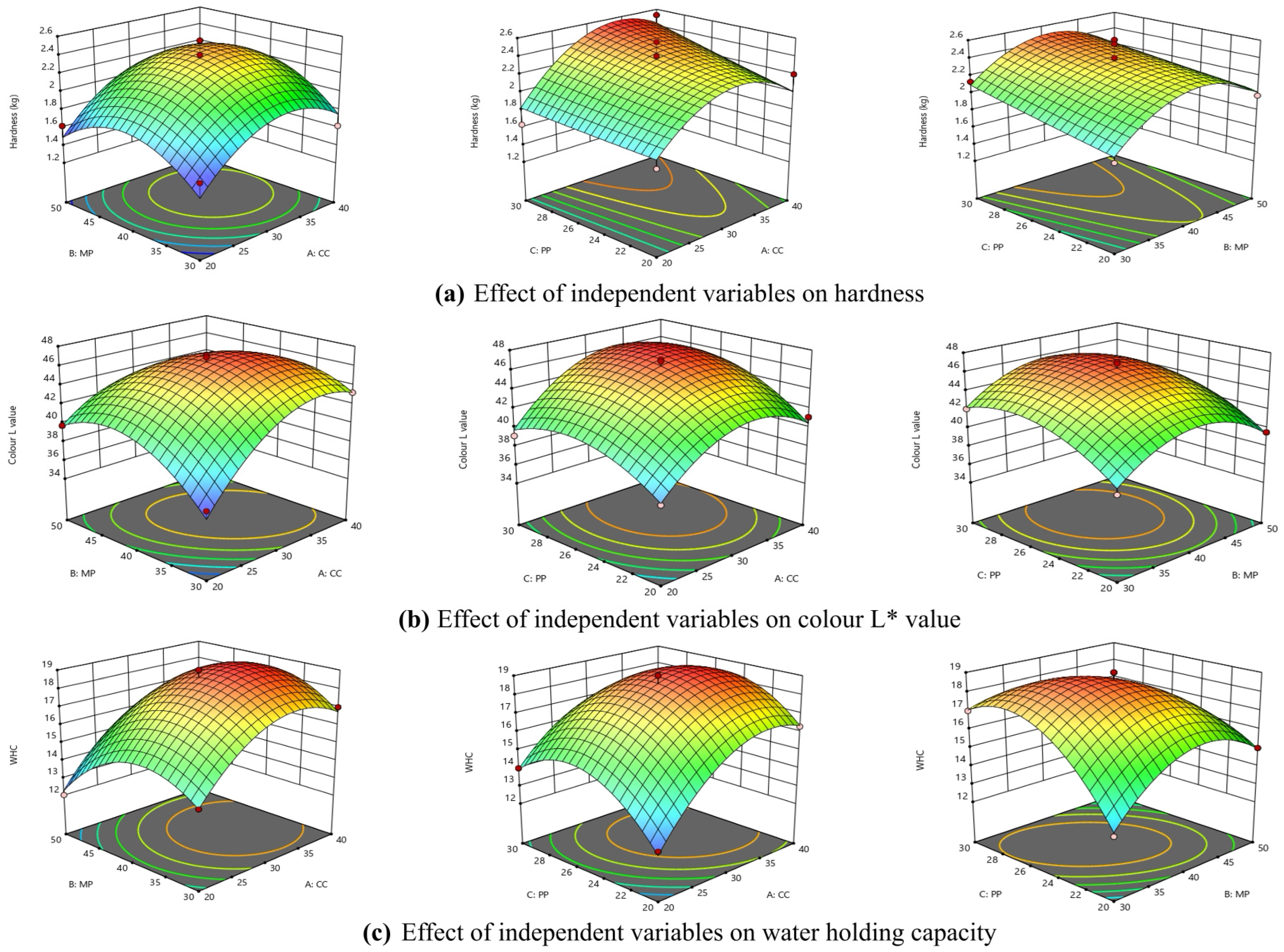
3.1. Effect of Various Independent Variables on Responses and Predictive Model
3.1.1. Effect of Independent Variables on Hardness of Cups
3.1.2. Effect of Independent Variables on Colour (L*) Value of Cups
3.1.3. Effect of Independent Variables on Water-Holding Capacity of Cups
3.2. Prediction and Validation
3.3. Characterization
3.3.1. Dimensional Analysis
3.3.2. Colour, Hardness, Fracturability, and Water-Holding Capacity
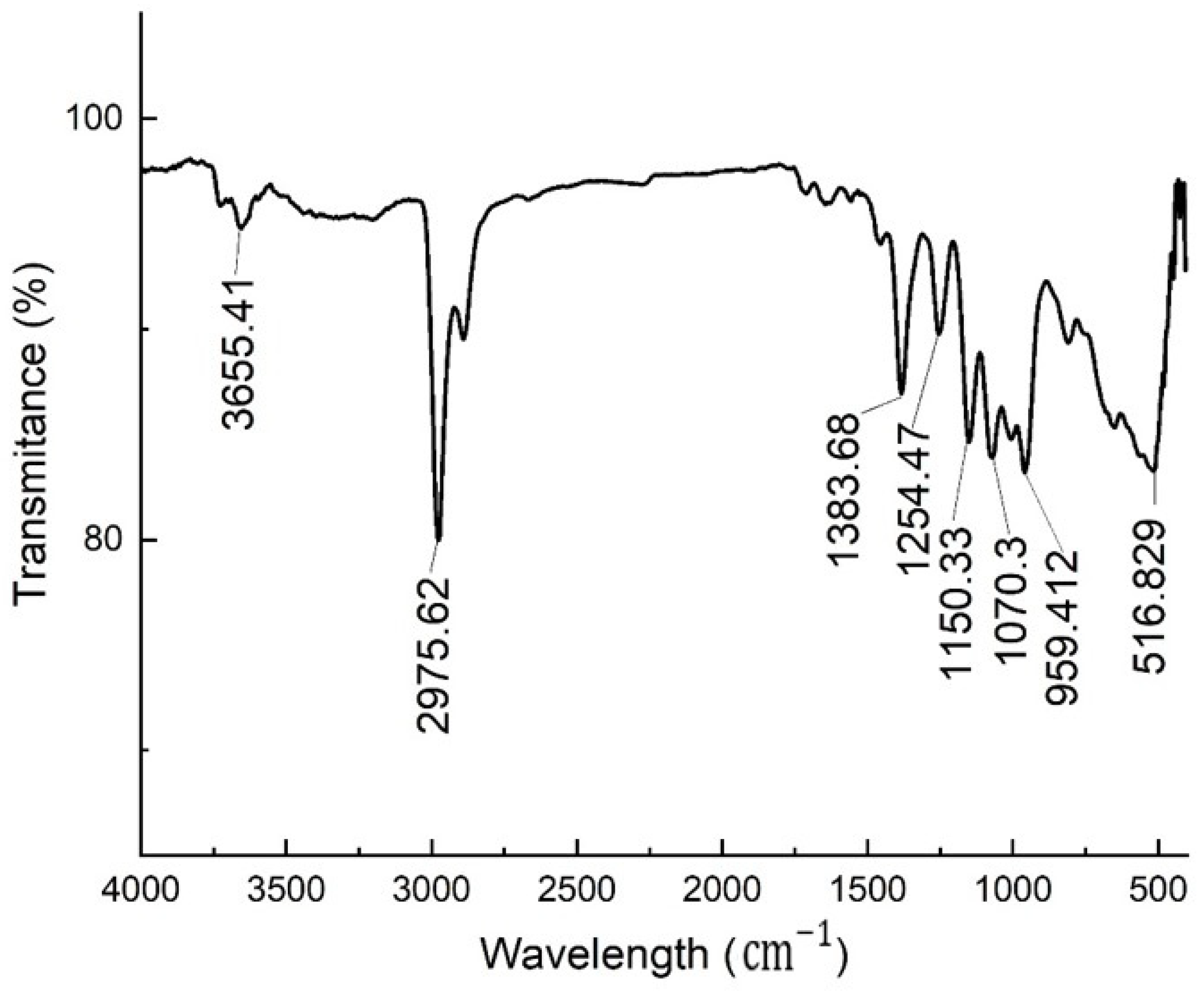
3.3.3. FTIR Analysis
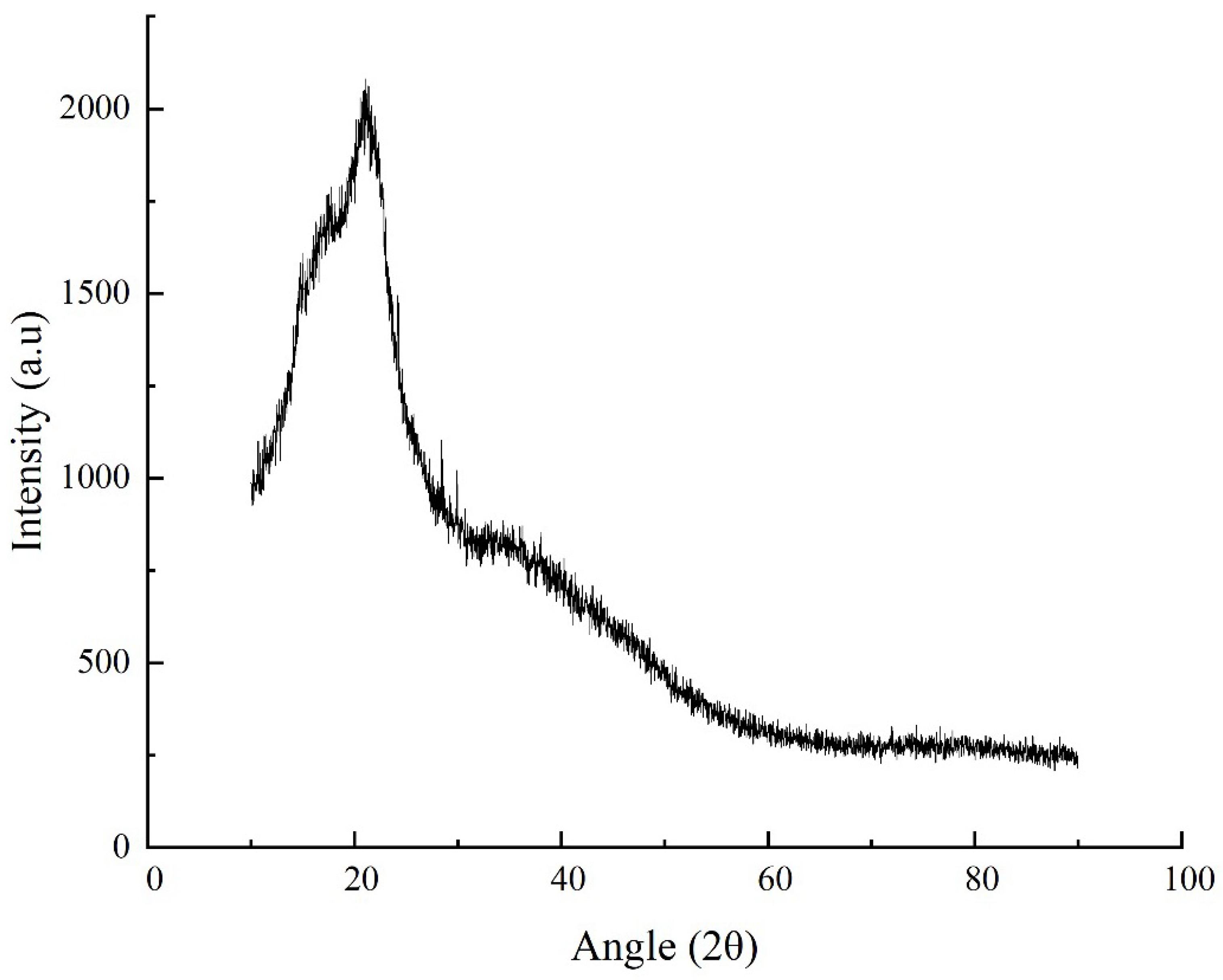
3.3.4. X-Ray Diffraction (XRD)
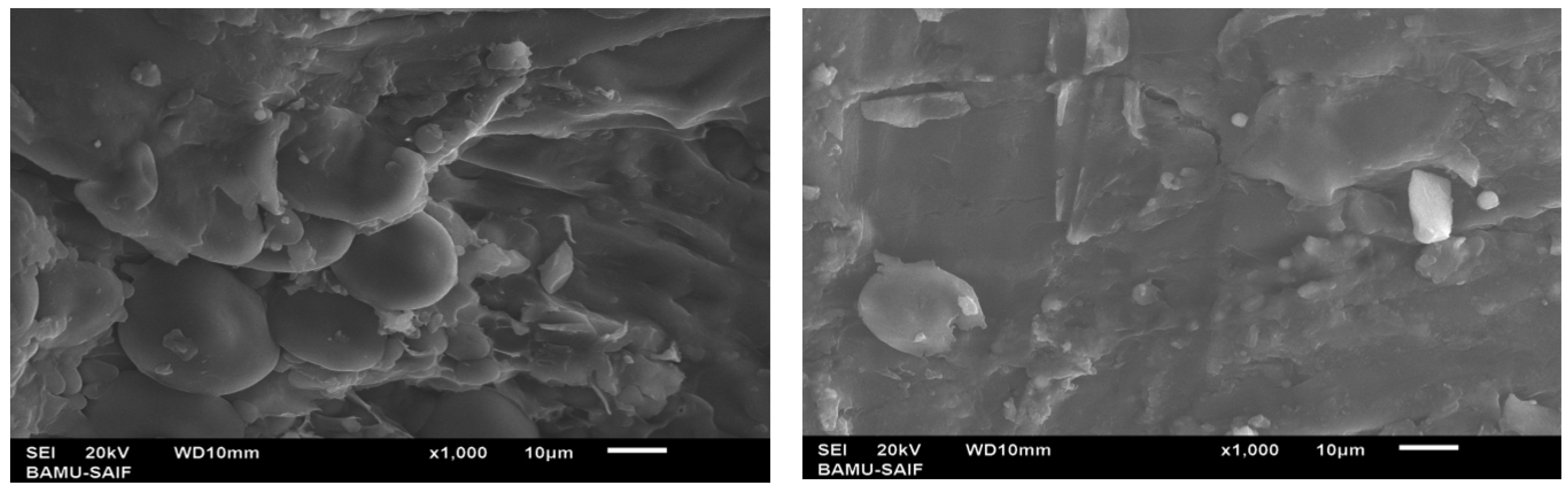
3.3.5. Scanning Electron Microscopy
3.3.6. Biodegradability by Soil Burial Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RSM | Response surface methodology |
| BBD | Box–Behnken design |
| WHC | Water-holding capacity |
| ANOVA | Analysis of variance |
| CC | Corn cob |
| MP | Mango peel |
| PP | Pineapple peel |
| FTIR | Fourier transfer infrared spectroscopy |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Pilapitiya, P.G.C.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Chen, X.; Chen, F.; Jiang, H.; Wang, J.; Li, Y.X.; Wang, G. Replacing plastic with bamboo: Eco-friendly disposable tableware based on the separation of bamboo fibers and the reconstruction of their network structure. ACS Sustain. Chem. Eng. 2023, 1119, 7407–7418. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of biodegradable materials in food packaging: A review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Arijeniwa, V.F.; Akinsemolu, A.A.; Chukwugozie, D.C.; Onawo, U.G.; Ochulor, C.E.; Nwauzoma, U.M.; Kawino, D.A.; Onyeaka, H. Closing the loop: A framework for tackling single-use plastic waste in the food and beverage industry through circular economy—A review. J. Environ. Manag. 2024, 359, 120816. [Google Scholar] [CrossRef]
- Mulawekar, S.; Talaulikar, A. To study the effect of single-use plastic ban on fast food & take-away outlets in Pimpri–Chinchwad region. Strad Res. 2020, 7, 607–614. [Google Scholar] [CrossRef]
- Hananeh, W.M.; Al Rukibat, R.; Jaradat, S.; Borhan Al-Zghoul, M. Exposure assessment of bisphenol A by drinking coffee from plastic cups. Rocz. Panstw. Zakl. Hig. 2021, 72, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.L.; Lorencatto, F.; Miodownik, M.; Michie, S. Influences on single-use and reusable cup use: A multidisciplinary mixed-methods approach to designing interventions reducing plastic waste. UCL Open Environ. 2021, 3, e025. [Google Scholar] [CrossRef] [PubMed]
- Sheri, R.; Hanji, A.; Khasbag, A.; Nalaband, S.; Sidnal, V. Design and development of bio cups from agricultural waste. Int. J. Adv. Res. Sci. Commun. Technol. 2025, 5, 663–670. [Google Scholar]
- Purghorbani, F.; Pirsa, S.; Qarachoboogh, A.F. Production of disposable biodegradable cups based on watermelon peel waste modified with zinc oxide nanoparticles and red cabbage extract. Appl. Food Res. 2025, 5, 100975. [Google Scholar] [CrossRef]
- Rahul, S.; Nikshitha, K.; Keerthana, S.; Bhavani, M.; Samreen; Swamy, R. Development of biodegradable cups from pomegranate peel powder. J. Curr. Res. Food Sci. 2023, 4, 39–44. [Google Scholar]
- Varghese, S.A.; Pulikkalparambil, H.; Promhuad, K.; Srisa, A.; Laorenza, Y.; Jarupan, L.; Nampitch, T.; Chonhenchob, V.; Harnkarnsujarit, N. Renovation of Agro-Waste for Sustainable Food Packaging: A Review. Polymers 2023, 15, 648. [Google Scholar] [CrossRef]
- Hariharan, V.; Arulraj, R. Design and analysis of innovative machine to make bio cups and plates from agricultural waste. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 1597–1608. [Google Scholar] [CrossRef]
- Aghaei, S.; Alavijeh, M.K.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass Bioenergy 2022, 161, 106447. [Google Scholar] [CrossRef]
- Castorina, G.; Cappa, C.; Negrini, N.; Criscuoli, F.; Casiraghi, M.C.; Marti, A.; Rollini, M.; Consonni, G.; Erba, D. Characterization and nutritional valorization of agricultural waste corncobs from Italian maize landraces through the growth of medicinal mushrooms. Sci. Rep. 2023, 13, 21148. [Google Scholar] [CrossRef]
- Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Bhatia, S.K.; Kumar, A.S.; Rajkumar, M.; Kumar, G.; Pugazhendhi, A.; Chi, N.T.L.; Banu, J.R. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar] [CrossRef]
- Sinaga, M.Z.E.; Gea, S.; Panindia, N.; Sihombing, Y.A. The Preparation of All-Cellulose Nanocomposite Film from Isolated Cellulose of Corncobs as Food Packaging. Orient. J. Chem. 2018, 34, 562–567. [Google Scholar] [CrossRef]
- Sha, S.P.; Modak, D.; Sarkar, S.; Roy, S.K.; Sah, S.P.; Ghatani, K.; Bhattacharjee, S. Fruit waste: A current perspective for the sustainable production of pharmacological, nutraceutical, and bioactive resources. Front. Microbiol. 2023, 14, 1260071. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Baddi, J.; Vijayalakshmi, D.; Durgannavar, N.A.; Chandru, R. Mango peel: A potential source of natural bioactive phyto-nutrients in functional food. Asian J. Dairy Food Res. 2015, 34, 75–77. [Google Scholar] [CrossRef]
- Meena, L.; Sengar, A.S.; Neog, R.; Sunil, C.K. Pineapple processing waste (PPW): Bioactive compounds, their extraction, and utilisation: A review. J. Food Sci. Technol. 2022, 59, 4152–4164. [Google Scholar] [CrossRef]
- Ghadge, S.; Jadhav, H.B.; Shewale, S.R.; Annapure, U. Production of probiotic sweet lime juice powder: Process optimization, quality assessment, and storage stability. J. Food Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Muralidharan, V.; Jebathomas, C.R.T.; Sundaramoorthy, S.; Madhan, B.; Palanivel, S. Preparation and evaluation of novel biodegradable Kombucha cellulose-based multi-layered composite tableware. Ind. Crops Prod. 2024, 215, 118689. [Google Scholar] [CrossRef]
- Rana, A.; Dogiparthi, O.; Sakhare, S.D.; Sathyendra Rao, B.V.; Inamdar, A.A. Study on the utilization of by-products of wheat milling industry for the development of biodegradable plates. J. Food Sci. Technol. 2023, 60, 2042–2049. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, G.; Ghosh, P.; Arora, K.; Sharma, S. Development of biodegradable tableware from novel combination of paddy straw and pine needles: A potential alternative against plastic cutlery. J. Environ. Chem. Eng. 2023, 11, 111310. [Google Scholar] [CrossRef]
- Nur Azura, Z.; Radhiah, S.; Wan Zunairah, W.I.; Nurul Shazini, R.; Nur Hanani, Z.A.; Ismail-Fitry, M.R. Physicochemical, cooking quality, and sensory characterization of yellow alkaline noodle: Impact of mango peel powder level. Food Res. 2020, 4, 70–76. [Google Scholar]
- Ganjizadeh Zavareh, S.; Javanmard Dakheli, M.; Tajeddin, B. Optimization of biodegradable paper cup packaging coated with whey protein isolate and rice bran wax as potential popcorn package. Food Sci. Nutr. 2021, 9, 6762–6775. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.W.; Majewski, Ł.; Puszka, A. Modern Biodegradable Plastics-Processing and Properties Part II. Materials 2021, 14, 2523. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, S.; Su, J.; Zhu, M.; Zhou, M.; Chen, T.; Han, Y. Recent advances in carrageenan-based films for food packaging applications. Front. Nutr. 2022, 9, 1004588. [Google Scholar] [CrossRef]
- Choeybundit, W.; Shiekh, K.A.; Rachtanapun, P.; Tongdeesoontorn, W. Fabrication of edible and biodegradable cutlery from morning glory (Ipomoea aquatic) stem fiber-reinforced onto soy protein isolate. Heliyon 2022, 8, e09529. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Lal, A.B.; Thakur, D.; Singh, K.; Vashisht, P. Development and evaluation of biodegradable cutlery from sweet lime peel waste: A sustainable and efficient alternative to recycled plastics. Pharma Innov. J. 2024, 13, 262–268. [Google Scholar]
- Ranganath, K.G.; Shivashankara, K.S.; Roy, T.K.; Dinesh, M.R.; Geetha, G.A.; Pavithra, K.C.; Ravishankar, K.V. Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J. Food Sci. Technol. 2018, 55, 4566–4577. [Google Scholar] [CrossRef]
- Calvo-Brenes, P.; Fanning, K.; O’Hare, T. Does kernel position on the cob affect zeaxanthin, lutein and total carotenoid contents or quality parameters, in zeaxanthin-biofortified sweet-corn? Food Chem. 2019, 277, 490–495. [Google Scholar] [CrossRef]
- Kondo, T. The assignment of IR absorption bands due to free hydroxyl groups in cellulose. Cellulose 1997, 4, 281–292. [Google Scholar] [CrossRef]
- Baker, M.T.; Oguntoye, O.S. Physical and spectroscopic characterization of the microcrystalline cellulose derivatives from corn cob and Daniella oliveri wastes. J. Turk. Chem. Soc. Sect. A Chem. 2023, 10, 31–38. [Google Scholar] [CrossRef]
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R.; et al. Fruit Characteristics, Peel Nutritional Compositions, and Their Relationships with Mango Peel Pectin Quality. Plants 2021, 10, 1148. [Google Scholar] [CrossRef]
- Lubaina, A.S.; Renjith, P.R.; Dinesh Babu, K.V. Phytochemical analysis and FT-IR fingerprinting of pineapple peel—A natural resource of bioactive compounds. Int. J. Pharm. Biol. Sci. 2019, 9, 1229–1237. [Google Scholar]
- Anirudh, M.K.; Lal, A.M.N.; Harikrishnan, M.P.; Jose, J.; Thasim, J.; Warrier, A.S.; Venkatesh, R.; Vaddevolu, U.B.P.; Kothakota, A. Sustainable seedling pots: Development and characterisation of banana waste and natural fibre-reinforced composites for horticultural applications. Int. J. Biol. Macromol. 2024, 270, 132070. [Google Scholar] [CrossRef] [PubMed]
- Watt, E.; Abdelwahab, M.A.; Mohanty, A.K.; Misra, M. Biocomposites from biobased polyamide 4,10 and waste corn cob based biocarbon. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106340. [Google Scholar] [CrossRef]
- Almeida, A.; Araújo, M.; Novoa-Carballal, R.; Andrade, F.; Gonçalves, H.; Reis, R.L.; Lúcio, M.; Schwartz, S., Jr.; Sarmento, B. Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Mater. Sci. Eng. C 2020, 112, 110920. [Google Scholar] [CrossRef] [PubMed]
- Warrier, A.S.; Krishnapriya, R.; Harikrishnan, M.P.; Lal, A.M.N.; Anirudh, M.K.; Kothakota, A. Developing sustainable packaging alternatives for plastic carry bags: Utilizing reinforced lotus fiber with casein bio-coating for enhanced performance. Sustain. Chem. Pharm. 2024, 39, 101564. [Google Scholar] [CrossRef]
- Karim, R.; Nahar, K.; Zohora, F.T.; Islam, M.M.; Bhuiyan, R.H.; Jahan, M.S.; Shaikh, M.A.A. Pectin from lemon and mango peel: Extraction, characterisation and application in biodegradable film. Carbohydr. Polym. Technol. Appl. 2022, 4, 100258. [Google Scholar] [CrossRef]
- Basil, M.; Anirudh, M.K.; Nandhu Lal, A.M.; Harikrishnan, M.P.; Kundu, P.; Kothakota, A. Development and characterization of microfiber incorporated with industrial biopolymer composite based biodegradable cutlery: An alternative to single use plastic. Ind. Crops Prod. 2023, 205, 117526. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Raghunathan, R.; Warrier, A.S.; Basil, M.; Sahoo, S.K.; Pandiselvam, R.; Venkatesh, T.; Pillai, S.; Kundu, P.; Kothakota, A. Reinforced water hyacinth based biodegradable cutlery: Green alternative to single-use plastics. Food Packag. Shelf Life 2023, 40, 101211. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Thampi, A.; Lal, A.M.N.; Warrier, A.S.; Basil, M.; Kothakota, A. Effect of chitosan-based bio coating on mechanical, structural and physical characteristics of microfiber-based paper packaging: An alternative to wood pulp/plastic packaging. Int. J. Biol. Macromol. 2023, 253, 126888. [Google Scholar] [CrossRef]



| Variables | Range of Levels (g) | |||||
|---|---|---|---|---|---|---|
| Actual | Coded | Actual | Coded | Actual | Coded | |
| A (Corn cob) | 20 | −1 | 30 | 0 | 40 | 1 |
| B (Mango peel) | 30 | −1 | 40 | 0 | 50 | 1 |
| C (Pineapple peel | 20 | −1 | 25 | 0 | 30 | 1 |
| Run Order | CC (A) | MP (B) | PP (C) | Hardness (Kg) | Colour (L* Value) | WHC (min) |
|---|---|---|---|---|---|---|
| 1 | 40 (1) | 40 (0) | 30 (1) | 2.531 | 45.00 | 16 |
| 2 | 30 (0) | 40 (0) | 25 (0) | 2.308 | 46.03 | 18 |
| 3 | 20 (−1) | 30 (−1) | 25 (0) | 1.540 | 36.30 | 14 |
| 4 | 30 (0) | 50 (1) | 30 (1) | 2.256 | 43.23 | 13 |
| 5 | 20 (−1) | 40 (0) | 20 (−1) | 1.673 | 37.35 | 12.3 |
| 6 | 30 (0) | 30 (−1) | 20 (−1) | 1.723 | 38.23 | 13 |
| 7 | 30 (0) | 40 (0) | 25 (0) | 2.216 | 46.86 | 19 |
| 8 | 30 (0) | 40 (0) | 25 (0) | 2.401 | 46.13 | 18 |
| 9 | 30 (0) | 40 (0) | 25 (0) | 2.360 | 45.92 | 19 |
| 10 | 40 (1) | 30 (−1) | 25 (0) | 1.623 | 43.25 | 17 |
| 11 | 40 (1) | 50 (1) | 25 (0) | 1.823 | 39.23 | 16 |
| 12 | 20 (−1) | 50 (1) | 25 (0) | 1.621 | 39.76 | 12 |
| 13 | 30 (0) | 30 (−1) | 30 (1) | 2.132 | 42.08 | 17 |
| 14 | 40 (1) | 40 (0) | 20 (−1) | 2.203 | 41.12 | 16.3 |
| 15 | 20 (−1) | 40 (0) | 30 (1) | 1.634 | 39.13 | 14 |
| 16 | 30 (0) | 50 (1) | 20 (−1) | 1.974 | 39.56 | 15 |
| 17 | 30 (0) | 40 (0) | 25 (0) | 2.556 | 47.06 | 19 |
| Source | Hardness (Kg) | Colour (L* Value) | WHC (Min) | |||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| Model | 5.43 | 0.0182 | 39.5 | 0.0001 | 47.66 | 0.0001 |
| (A): CC | 10.36 | 0.0147 | 56.83 | 0.0001 | 98.91 | 0.0001 |
| (B): MP | 1.52 | 0.2572 | 0.8123 | 0.3974 | 14.63 | 0.0065 |
| (C): PP | 3.39 | 0.1079 | 38.28 | 0.0005 | 6.77 | 0.0354 |
| AB | 0.1001 | 0.7609 | 24.66 | 0.0016 | 1.17 | 0.3151 |
| AC | 0.9522 | 0.3617 | 1.94 | 0.2059 | 4.68 | 0.0672 |
| BC | 0.114 | 0.7455 | 0.0143 | 0.9082 | 42.14 | 0.0003 |
| A2 | 15.75 | 0.0054 | 88.09 | 0.0001 | 67.47 | 0.0001 |
| B2 | 14.81 | 0.0063 | 81.81 | 0.0001 | 78.86 | 0.0001 |
| C2 | 0.004 | 0.9515 | 39.43 | 0.0004 | 86.94 | 0.0001 |
| LOF | 3.89 NS | 0.1113 | 3.54 NS | 0.127 | 0.3278 NS | 0.8068 |
| R2 | 0.8747 | 0.9807 | 0.9839 | |||
| Adj.R2 | 0.7135 | 0.9559 | 0.9633 | |||
| Pre. R2 | 0.5434 | 0.7674 | 0.9292 | |||
| CV (%) | 9.25 | 1.79 | 2.92 | |||
| Responses | Predicted Values | Experimental Values |
|---|---|---|
| Hardness | 2.45 | 2.41 |
| L* value | 47.05 | 47.03 |
| WHC | 18.7 | 18.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wandhekar, S.S.; Kshirsagar, R.B.; Sadawarte, S.K.; Gosavi, R.A.; Gabor, V.; Shaikh, A.M.; Bela, K. Development of Biodegradable Cups from Corn and Fruit Processing Waste and Its Characterization: A Sustainable Approach. Macromol 2025, 5, 53. https://doi.org/10.3390/macromol5040053
Wandhekar SS, Kshirsagar RB, Sadawarte SK, Gosavi RA, Gabor V, Shaikh AM, Bela K. Development of Biodegradable Cups from Corn and Fruit Processing Waste and Its Characterization: A Sustainable Approach. Macromol. 2025; 5(4):53. https://doi.org/10.3390/macromol5040053
Chicago/Turabian StyleWandhekar, Sangram S., Rajesh B. Kshirsagar, Surendra K. Sadawarte, Rinkesh A. Gosavi, Vaszko Gabor, Ayaz Mukarram Shaikh, and Kovács Bela. 2025. "Development of Biodegradable Cups from Corn and Fruit Processing Waste and Its Characterization: A Sustainable Approach" Macromol 5, no. 4: 53. https://doi.org/10.3390/macromol5040053
APA StyleWandhekar, S. S., Kshirsagar, R. B., Sadawarte, S. K., Gosavi, R. A., Gabor, V., Shaikh, A. M., & Bela, K. (2025). Development of Biodegradable Cups from Corn and Fruit Processing Waste and Its Characterization: A Sustainable Approach. Macromol, 5(4), 53. https://doi.org/10.3390/macromol5040053








