Evaluation of Chemical and Functional Properties of Pectin-like Polymers Extracted from Tomato Using Conventional Acid Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tomato Polysaccharide (TP) Extraction
2.3. Chemical Properties
2.4. Structural Analysis
2.5. Functional Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
3.2. Chemical Characterisation
3.3. FTIR Spectroscopy of Tomato Polysaccharides (TP)
3.4. Antioxidant Activity (DPPH Assay)
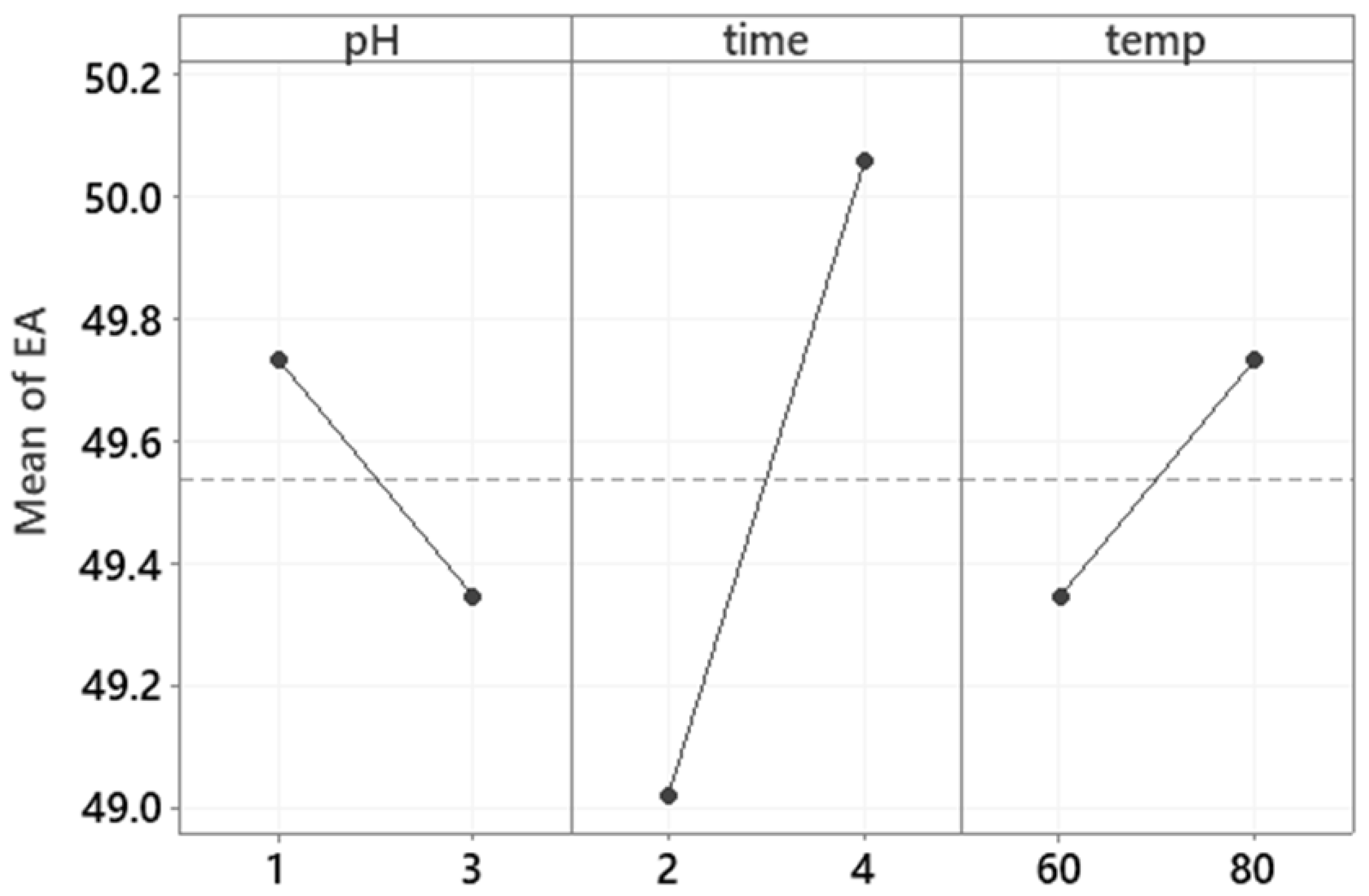
3.5. Emulsifying Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyomugasho, C.; Willemsen, K.L.D.D.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. Pectin-Interactions and in Vitro Bioaccessibility of Calcium and Iron in Particulated Tomato-Based Suspensions. Food Hydrocoll. 2015, 49, 164–175. [Google Scholar] [CrossRef]
- Patova, O.A.; Golovchenko, V.V.; Ovodov, Y.S. Pectic Polysaccharides: Structure and Properties. Russ. Chem. Bull. 2014, 63, 1901–1924. [Google Scholar] [CrossRef]
- Tombs, M.P.; Harding, S.E. An Introduction to Polysaccharide Biotechnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging Concepts in the Nutraceutical and Functional Properties of Pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zouambia, Y.; Youcef Ettoumi, K.; Krea, M.; Moulai-Mostefa, N. A New Approach for Pectin Extraction: Electromagnetic Induction Heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 5th ed; CRC Press: Boca Raton, FL, USA, 2017; pp. 91–117. [Google Scholar]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Alba, K.; Laws, A.P.; Kontogiorgos, V. Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll. 2015, 43, 726–735. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Smith, A.M.; Morris, G.A.; Kontogiorgos, V. Structure and Physicochemical Properties of Ghanaian Grewia Gum. Int. J. Biol. Macromol. 2018, 122, 866–872. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Structure-Function Relationships in Pectin Emulsification. Food Biophys. 2018, 13, 71–79. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.-C.; Thibault, J.-F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.-C. A copolymer analysis approach to estimate the neutral sugar distribution of sugar beet pectin using size exclusion chromatography. Carbohydr. Polym. 2012, 87, 1139–1143. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F.; Harding, S.E. Physical Characterisation of the Rhamnogalacturonan and Homogalacturonan Fractions of Sugar Beet (Beta vulgaris) Pectin. Carbohydr. Polym. 2010, 82, 1161–1167. [Google Scholar] [CrossRef]
- Chaliha, M.; Williams, D.; Smyth, H.; Sultanbawa, Y. Extraction and Characterization of a Novel Terminalia Pectin. Food Sci. Biotechnol. 2018, 27, 65–71. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound Assisted Extraction and Characterization of Pectin from Tomato Waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Ronkart, S.N.; Robert, C.; Wathelet, B.; Paquot, M. Characterisation of Pectins Extracted from Banana Peels (Musa AAA) under Different Conditions Using an Experimental Design. Food Chem. 2008, 108, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Obodo-Ovie, O.; Alyassin, M.; Smith, A.M.; Morris, G.A. The Effect of Different Extraction Conditions on the Physicochemical Properties of Novel High Methoxyl Pectin-like Polysaccharides from Green Bell Pepper (GBP). Macromol 2024, 4, 420–436. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Kandri, Y.R.; Chahdi, F.O.; Touzani, H.; Errachidi, F. Yield, Esterification Degree and Molecular Weight Evaluation of Pectins Isolated from Orange and Grapefruit Peels under Different Conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef]
- McComb, E.A.; McCready, R.M. Determination of Acetyl in Pectin and in Acetylated Carbohydrate Polymers. Anal. Chem. 1957, 29, 819–821. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-Scavenging Activities of Chickpea Protein Hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of Microwave Assisted Extraction of Pectin from Orange Peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohydr. Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of Extraction Conditions on the Yield, Purity and Surface Properties of Sugar Beet Pulp Pectin Extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Nguyễn, H.V.; Savage, G.P. The effects of temperature and pH on the extraction of oxalate and pectin from green kiwifruit (Actinidia deliciosa L.), golden kiwifruit (Actinidia chinensis L.), kiwiberry (Actinidia arguta) and persimmon (Diospyros kaki). Int. J. Food Sci. Technol. 2013, 48, 794–800. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion Stabilizing Properties of Pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical Profile, Functional and Antioxidant Properties of Tomato Peel Fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Li, N.; Feng, Z.; Niu, Y.; Yu, L. Structural, Rheological and Functional Properties of Modified Soluble Dietary Fiber from Tomato Peels. Food Hydrocoll. 2018, 77, 557–565. [Google Scholar] [CrossRef]
- Garna, H.; Mabon, N.; Robert, C.; Cornet, C.; Nott, K.; Legros, H.; Wathelet, B.; Paquot, M. Effect of Extraction Conditions on the Yield and Purity of Apple Pomace Pectin Precipitated but Not Washed by Alcohol. J. Food Sci. 2007, 72, C001–C009. [Google Scholar] [CrossRef]
- Morales-Contreras, B.E.; Rosas-Flores, W.; Contreras-Esquivel, J.C.; Wicker, L.; Morales-Castro, J. Pectin from Husk Tomato (Physalis ixocarpa Brot.): Rheological Behavior at Different Extraction Conditions. Carbohydr. Polym. 2018, 179, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M. Pectin quantity, composition and physicochemical behaviour as influenced by the purification process. Food Res. Int. 2009, 42, 1197–1202. [Google Scholar] [CrossRef]
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Hydrophobic interaction in the gelation of high methoxyl pectins. Food Sci. 1994, 49, 1093–1098. [Google Scholar] [CrossRef]
- Guillotin, S.E.; Bakx, E.J.; Boulenguer, P.; Schols, H.A.; Voragen, A.G.J. Determination of the Degree of Substitution, Degree of Amidation and Degree of Blockiness of Commercial Pectins by Using Capillary Electrophoresis. Food Hydrocoll. 2007, 21, 444–451. [Google Scholar] [CrossRef]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventós, R.; Buxaderas, S.; Codina, C. An Industrial Approach in the Search of Natural Antioxidants from Vegetable and Fruit Wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin Isolation and Characterization from Six Okra Genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Nep, E.I.; Carnachan, S.M.; Ngwuluka, N.C.; Kontogiorgos, V.; Morris, G.A.; Sims, I.M.; Smith, A.M. Structural Characterisation and Rheological Properties of a Polysaccharide from Sesame Leaves (Sesamum Radiatum Schumach. & Thonn.). Carbohydr. Polym. 2016, 152, 541–547. [Google Scholar] [CrossRef]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef]
- Liu, J.; Wen, X.; Zhang, X.; Pu, H.; Kan, J.; Jin, C. Extraction, Characterization and in Vitro Antioxidant Activity of Polysaccharides from Black Soybean. Int. J. Biol. Macromol. 2015, 72, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.E.; Hosseini, S.S. Optimization of Aqueous Pectin Extraction from Citrus Medica Peel. Carbohydr. Polym. 2017, 178, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Alancay, M.M.; Lobo, M.O.; Quinzio, C.M.; Iturriaga, L.B. Extraction and Physicochemical Characterization of Pectin from Tomato Processing Waste. J. Food Meas. Charact. 2017, 11, 2119–2130. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A. Extraction and rheological properties of pectin from fresh peach pomace. J. Food Eng. 1999, 39, 193–201. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Denman, L.J.; Binhamad, H.A.S.; Morris, G.A. The Effect of Different Extraction Conditions on the Physical Properties, Conformation and Branching of Pectins Extracted from Cucumis Melo Inodorus. Polysaccharides 2020, 1, 3–20. [Google Scholar] [CrossRef]
- Sharma, R.; Kamboj, S.; Khurana, R.; Singh, G.; Rana, V. Physicochemical and Functional Performance of Pectin Extracted by QbD Approach from Tamarindus indica L. Pulp. Carbohydr. Polym. 2015, 134, 364–374. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Xiang, C.; Teng, H.; Sheng, Z.; Zhao, C.; Deng, J.; Zhao, C.; He, B.; Chen, L.; Ai, C. Structural Characterization and Antioxidant Activity Mechanism of the Ferulic Acid-Rich Subfraction from Sugar Beet Pectin. Carbohydr. Polym. 2025, 347, 122691. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Yu, Y.; Wang, Y.; Xue, D.; Zhou, Y.; Li, X. A Ginseng-Derived Rhamnogalacturonan I (RG-I) Pectin Promotes Longevity via TOR Signalling in Caenorhabditis Elegans. Carbohydr. Polym. 2023, 312, 120818. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; de la Rosa, L.A.; Alvarez-Parrilla, E. Effect of Pectin on the Interactions among Phenolic Compounds Determined by Antioxidant Capacity. J. Mol. Struct. 2020, 1199, 126967. [Google Scholar] [CrossRef]
- Ürüncüoğlu, Ş.; Alba, K.; Morris, G.A.; Kontogiorgos, V. Influence of Cations, PH and Dispersed Phases on Pectin Emulsification Properties. Curr. Res. Food Sci. 2021, 4, 398–404. [Google Scholar] [CrossRef]
- Niu, H.; Chen, X.; Luo, T.; Chen, H.; Fu, X. Relationships between the Behavior of Three Different Sources of Pectin at the Oil-Water Interface and the Stability of the Emulsion. Food Hydrocoll. 2022, 128, 107566. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Koch, L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Effect of Molecular Weight Reduction, Acetylation and Esterification on the Emulsification Properties of Citrus Pectin. Food Biophys. 2015, 10, 217–227. [Google Scholar] [CrossRef]
- Narasimman, P.; Sethuraman, P. An Overview on the Fundamentals of Pectin. Int. J. Adv. Res. 2016, 4, 1855–1860. [Google Scholar] [CrossRef]
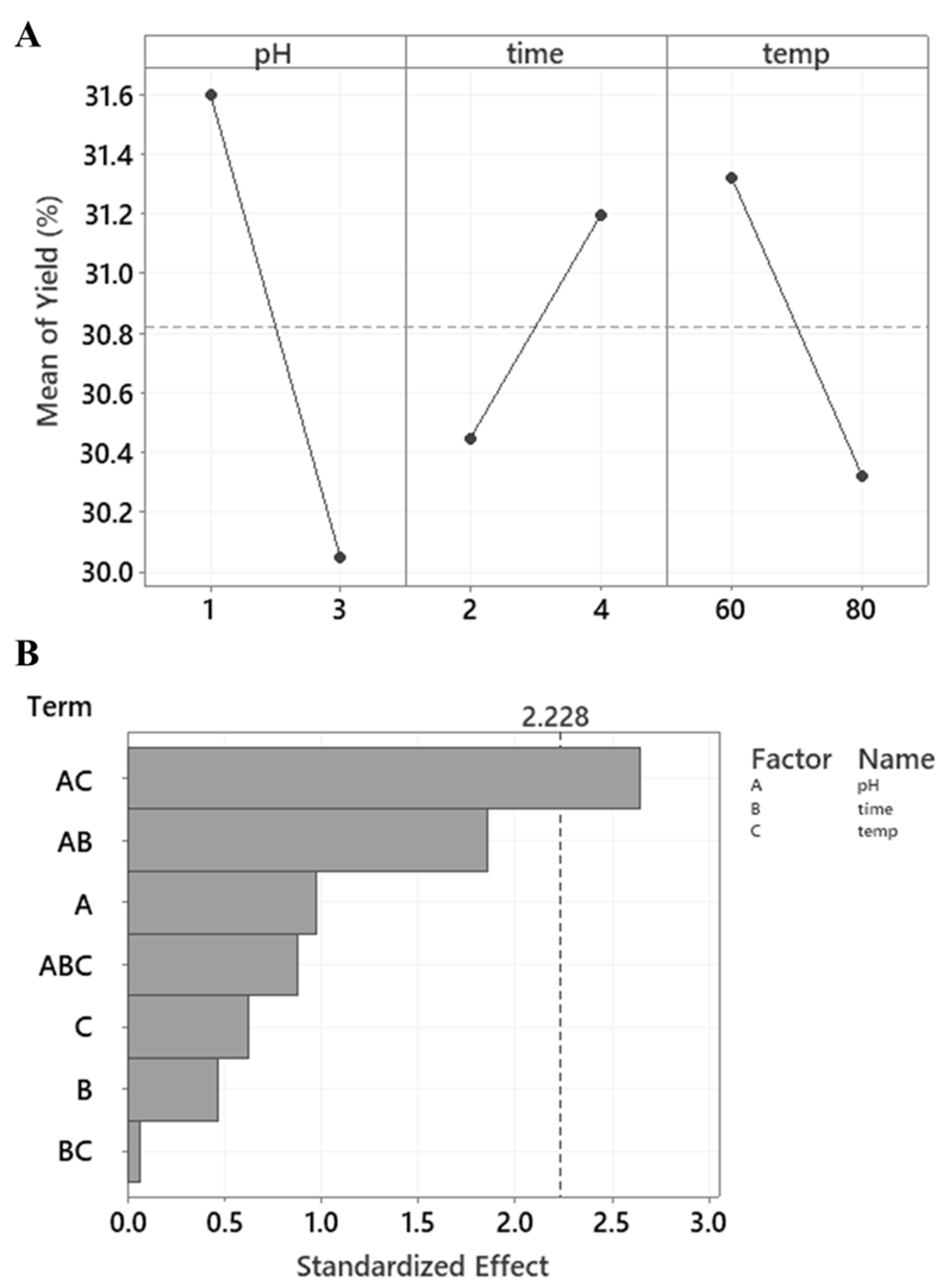


| Sample | pH | Time (Hours) | Temperature (°C) | Yield (%AIR) | Protein (%) | GalA (wt. %) | DE (%) | DA (%) | Glc (wt. %) | Phenolics (mg GAE/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 60 | 28.60 ± 4.81 a | 12.90 ± 2.05 a | 20.5 ± 0.7 a | 80.55 ± 4.26 a | 26.1 ± 1.1 a b | 14.9 ± 2.3 a b | 25.5 ± 0.2 a |
| 2 | 3 | 2 | 60 | 32.80 ± 3.94 a | 11.52 ± 3.01 a | 18.1 ± 5.0 a | 77.85 ± 1.53 a | 36.4 ± 1.8 a | 13.8 ± 1.0 b c | 26.4 ± 0.2 a |
| 3 | 1 | 4 | 60 | 30.80 ± 1.70 a | 13.75 ± 3.63 a | 22.6 ± 9.1 a | 79.05 ± 2.11 a | 24.8 ± 2.6 b | 17.8 ± 0.4 a | 23.9 ± 0.1 a |
| 4 | 3 | 4 | 60 | 31.90 ± 4.70 a | 12.60 ± 0.40 a | 22.5 ± 2.1 a | 83.45 ± 2.60 a | 25.5 ± 2.8 a b | 16.5 ± 0.4 a b c | 26.4 ± 0.2 a |
| 5 | 1 | 2 | 80 | 30.30 ± 3.36 a | 15.50 ± 4.21 a | 23.4 ± 3.7 a | 82.85 ± 4.00 a | 26.2 ± 5.0 a b | 16.2 ± 0.4 a b c | 26.0 ± 0.1 a |
| 6 | 3 | 2 | 80 | 28.90 ± 0.10 a | 11.95 ± 0.84 a | 20.4 ± 3.0 a | 80.90 ± 8.30 a | 25.3 ± 4.5 a b | 15.8 ± 1.0 a b c | 28.0 ± 0.2 a |
| 7 | 1 | 4 | 80 | 35.50 ± 3.52 a | 12.25 ± 0.91 a | 21.9 ± 6.4 a | 74.45 ± 0.82 a | 25.7 ± 5.0 a b | 14.6 ± 0.4 a b c | 25.3 ± 0.1 a |
| 8 | 3 | 4 | 80 | 25.40 ± 0.61 a | 11.50 ± 0.00 a | 23.5 ± 2.2 a | 74.50 ± 9.83 a | 28.1 ± 0.5 a b | 13.0 ± 4.8 c | 25.2 ± 0.1 a |
| Sample | Free Radicals’ Scavenging Effect of DPPH (%) |
|---|---|
| 1 | 82.81 ± 5.32 |
| 2 | 73.35 ± 7.41 |
| 3 | 78.56 ± 5.36 |
| 4 | 85.03 ± 3.60 |
| 5 | 85.08 ± 3.72 |
| 6 | 71.40 ± 6.70 |
| 7 | 82.56 ± 1.70 |
| 8 | 82.76 ± 1.61 |
| Sample | EA (%) | ES at 22 °C After 1 Day (%) | ES at 22 °C After 30 Days (%) | ES at 4 °C After 1 Day (%) | ES at 4 °C After 30 Days (%) |
|---|---|---|---|---|---|
| 1 | 48.7 ± 1.8 | 99.0 ± 1.4 | 98.0 ± 2.8 | 98.0 ± 3.1 | 98.0 ± 3.5 |
| 2 | 48.5 ± 1.5 | 97.0 ± 1.3 | 100.0 ± 0.0 | 91.0 ± 9.4 | 100.0 ± 0.0 |
| 3 | 50.0 ± 0.0 | 97.0 ± 1.4 | 100.0 ± 0.0 | 100.0 ± 0.0 | 93.0 ± 4.2 |
| 4 | 50.0 ± 0.0 | 98.0 ± 0.1 | 100.0 ± 0.0 | 97.0 ± 4.2 | 96.0 ± 5.7 |
| 5 | 50.0 ± 0.0 | 98.0 ± 2.8 | 100.0 ± 0.0 | 96.0 ± 5.7 | 98.0 ± 2.8 |
| 6 | 48.7 ± 1.8 | 97.0 ± 4.2 | 99.0 ± 1.4 | 95.0 ± 1.4 | 98.0 ± 3.5 |
| 7 | 50.0 ± 0.0 | 97.0± 4.2 | 99.0 ± 1.4 | 100.0 ± 0.0 | 94.0 ± 5.7 |
| 8 | 50.0 ± 0.0 | 99.0 ± 1.6 | 99.0 ± 1.4 | 100.0 ± 0.0 | 97.0 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obodo-Ovie, O.; Alyassin, M.; Smith, A.M.; Morris, G.A. Evaluation of Chemical and Functional Properties of Pectin-like Polymers Extracted from Tomato Using Conventional Acid Extraction. Macromol 2025, 5, 46. https://doi.org/10.3390/macromol5040046
Obodo-Ovie O, Alyassin M, Smith AM, Morris GA. Evaluation of Chemical and Functional Properties of Pectin-like Polymers Extracted from Tomato Using Conventional Acid Extraction. Macromol. 2025; 5(4):46. https://doi.org/10.3390/macromol5040046
Chicago/Turabian StyleObodo-Ovie, Onome, Mohammad Alyassin, Alan M. Smith, and Gordon A. Morris. 2025. "Evaluation of Chemical and Functional Properties of Pectin-like Polymers Extracted from Tomato Using Conventional Acid Extraction" Macromol 5, no. 4: 46. https://doi.org/10.3390/macromol5040046
APA StyleObodo-Ovie, O., Alyassin, M., Smith, A. M., & Morris, G. A. (2025). Evaluation of Chemical and Functional Properties of Pectin-like Polymers Extracted from Tomato Using Conventional Acid Extraction. Macromol, 5(4), 46. https://doi.org/10.3390/macromol5040046








