Abstract
Metabolism, the network of biochemical reactions that powers life, arose under conditions radically different from those on Earth today. Investigating its origins reveals how initially simple chemical processes gradually integrated nucleic acid and then protein catalysts, becoming progressively more complex and regulated until they evolved into the enzyme-rich systems observed in modern organisms. Here, we integrate multiple perspectives on the origin of metabolism, focusing primarily on an evolutionary trajectory from an RNA-based world, where ribozymes, metal ions, coenzymes, small peptides, and other small organic molecules worked in concert, to enzyme-driven metabolic networks. We also address the longstanding debates on whether these early metabolic pathways were largely autotrophic or heterotrophic, and consider so-called “pre-metabolisms” (non-enzymatic networks) as an alternative conceptual framework. We discuss key examples such as the Wood–Ljungdahl (W–L) pathway and the reverse tricarboxylic acid (TCA) cycle, both posited to function under early Earth conditions. Finally, we examine how the environment (e.g., minerals, clays, hydrothermal vents) shaped early metabolism, describe unresolved questions about the Last Common Ancestor’s catalytic repertoire and propose future directions that link geochemical insights with molecular biology and synthetic approaches.
1. Introduction
The emergence of metabolism is a defining evolutionary innovation in life’s early history. Rather than depending solely on spontaneous abiotic reactions, the first organisms gradually assembled intricate biochemical pathways, enabling them to harness energy and synthesize complex molecules. Understanding the transition from elementary chemical processes to multifaceted enzyme-based networks provides fundamental insights into the origin and early evolution of life.
From a thermodynamic perspective, the evolution of metabolic routes was likely constrained by the need to reduce free energy (ΔG) while maximizing kinetic efficiency. Universal core pathways such as glycolysis and the Wood–Ljungdahl (W–L) pathway may represent optimal solutions to these limitations, effectively coupling exergonic and endergonic reactions under prebiotic conditions [1]. By dissipating energy gradients in this way, early life could have progressively refined these basic routes into the robust, enzyme-based systems observed today. This thermodynamic imperative, together with geochemical gradients, sets the stage for ongoing debates over whether the first metabolic pathways were primarily autotrophic (deriving energy and carbon from inorganic sources like CO2) or heterotrophic (relying on pre-formed organic molecules).
These debates reflect the broader question of how life could have emerged under Earth’s primordial conditions and adapted over billions of years. Autotrophic models emphasize the role of geochemical energy (e.g., from hydrothermal vents) driving the fixation of carbon, whereas heterotrophic models posit that organic building blocks accumulated abiotically and were then exploited by emerging metabolic networks. Exploring these perspectives not only clarifies the chemical diversity observed in modern organisms but also sheds light on the evolutionary pressures that shaped today’s sophisticated biochemical architectures.
This knowledge has broad implications. For instance, insights into early metabolic pathways inform the design of synthetic biological systems, guide the search for astrobiological biosignatures, and have even contributed to advance biomedical research. Understanding how simple prebiotic chemistries were organized into increasingly complex networks helps researchers pinpoint the key steps that may have occurred repeatedly on Earth and potentially elsewhere in the universe.
Here, we support the idea that an RNA-based evolutionary stage, featuring catalytic RNAs (ribozymes), small peptides, metal ions, and other small organic molecules, was key to establishing the first primitive metabolic foundations. We acknowledge, however, that other researchers advocate different points of view, including strictly autotrophic models (with minimal or no RNA involvement at first) and proposals of non-enzymatic “pre-metabolisms” on mineral surfaces [2].
Below, we outline early prebiotic chemistry, discuss the possible roles of clays, coenzymes, and semi-enzymatic processes, and then address the autotrophy versus heterotrophy debate in the context of known primordial pathways. We also emphasize how key pathways, such as the Wood–Ljungdahl pathway and the reverse tricarboxylic acid or reverse Krebs cycle (rTCA), exemplify plausible ancestral chemistries. Finally, we address unanswered questions ranging from horizontal gene transfer to the reconstruction of ancestral enzymes and suggest future directions to clarify the deep evolutionary underpinnings of metabolism.
2. From Prebiotic Chemistry to Early Catalysts
2.1. Geochemical Foundations and Non-Enzymatic Possibilities
The Archean Earth was characterized by diverse environments, ranging from volcanic landmasses and tidal pools to hydrothermal vents rich in redox gradients (Figure 1A,B). Building on the work of Oparin [3,4] and Haldane [5,6], many have argued that organic molecules could accumulate via atmospheric chemistry (as shown by the Miller–Urey experiments [7,8]), producing amino acids, nucleobases, and other precursors (Figure 1C). Alongside these organic molecules, minerals and metal ions (e.g., Fe2+, Ni2+) could have generated localized catalytic niches, a notion consistent with the iron-sulfur world hypothesis [9]. Some authors have referred to these early networks “non-enzymatic metabolisms” or “pre-metabolisms”, highlighting their reliance on spontaneous or mineral-facilitated reactions rather than on genetically encoded proteins [10].
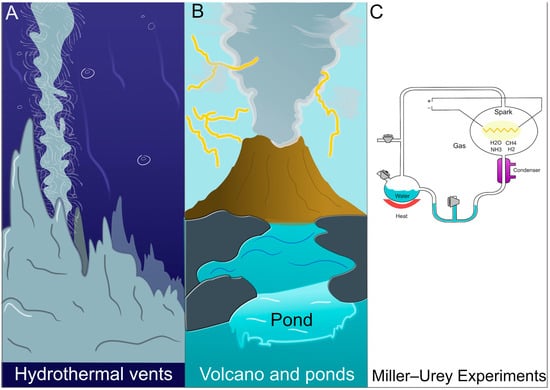
Figure 1.
Schematic representation of potential prebiotic environments on Archean Earth. (A) Hydrothermal vent systems, rich in redox gradients, where gases such as H2, CO2, and H2S interact with metal sulfides (e.g., FeS, NiS) to catalyze the formation of simple organic molecules, including formate and acetate. (B) Volcanic land masses and tidal pools, exposed to atmospheric gases such as CO, CH4, and NH3, could have facilitated prebiotic chemistry through interactions with mineral surfaces and UV radiation, ultimately leading to the accumulation of amino acids and nucleobases. (C) A laboratory simulation of prebiotic chemistry, as demonstrated by the Miller–Urey experiment, in which a mixture of CH4, NH3, H2, and H2O was subjected to electrical discharges, generating amino acids, aldehydes, and simple hydrocarbons under early Earth-like conditions.
Notably, partial segments of glycolysis or the reverse TCA cycle can proceed abiotically under specific pH, temperature, and redox conditions [11,12,13,14]. These findings reveal that key intermediates (e.g., formate or acetate) can form in the absence of sophisticated enzymes, especially when facilitated by reactive metal sulfides or mineral surfaces [15]. However, forming a complete, cyclic process capable of robust energy transduction and biosynthesis likely required additional molecular components (e.g., nucleic acid catalysts, peptides, and coenzymes) to increase specificity and efficiency. This need for more elaborate catalysis sets the stage for the subsequent emergence of proto-biological macromolecules.
Indeed, while purely mineral-driven “pre-metabolisms” illustrate how simple chemical routes might have arisen, their overall efficiency and stability under Archean conditions remain subjects of debate. Questions persist about whether environmental fluctuations (e.g., shifts in temperature, pH, or availability of metals) would allow sustained growth of these reaction networks. Thus, the transition from sporadic abiotic reactions to increasingly coordinated biochemical pathways likely involved additional stabilizing and catalyzing factors. Below, we examine how small peptides, clays, and coenzymes may have contributed to bridging the gap between geochemical processes and the earliest metabolic frameworks.
2.2. Small Peptides, Coenzymes, and Clays
In addition to minerals acting as early catalytic surfaces, short, non-coded peptides likely played a significant role in connecting geochemical reactions to the first biological processes. Even short polypeptides can supply rudimentary binding pockets for metal ions, stabilizing transition states and enhancing reaction specificity. Unlike modern proteins, these proto-peptides may have emerged sporadically via random polymerization events, potentially aided by mineral surfaces that concentrate and align amino acids. Despite their simplicity, such peptides could have measurably improved the efficiency of prebiotic transformations, thus offering a functional bridge between purely abiotic chemistry and early biological catalysis.
Clays and other minerals (e.g., montmorillonite, kaolinite, hydroxyapatite) are recognized as potential catalytic hubs, aiding in the polymerization of nucleotides and amino acids under relatively mild conditions [16,17]. By adsorbing and localizing reactants, clay minerals can shield fragile intermediates from hydrolytic degradation and provide reactive ionic microenvironments. Experimental studies show that these surfaces support the formation of short RNA strands and assist in incorporating amino acids into nascent peptides [18,19]. Through such processes, clays may have acted as microreactors where proto-peptides and early nucleic acids coexisted, catalyzing each other’s synthesis and gradually building up the molecular scaffolding for primordial metabolic cycles.
In parallel with these processes, cofactors emerged as a crucial link between geochemical conditions and increasingly sophisticated biochemistry. Widely recognized as essential organic cofactors in modern enzymes, many coenzymes retain ribonucleotide components, prompting the hypothesis that they are vestiges of an RNA world [20]. In such a scenario, metal ions and small peptides may have combined with these emerging organic cofactors to enable more intricate reactions than could be achieved by RNA or peptides alone. Over time, as the genetic code and translation machinery evolved, coenzymes were integrated into the active sites of newly evolving protein enzymes, catalyzing redox, group-transfer, and other specialized reactions. Their ubiquity in contemporary metabolism highlights a continuity stretching from prebiotic chemical networks to fully evolved metabolic pathways. By shuttling electrons, stabilizing reactive intermediates, or transferring functional groups, coenzymes provide an evolutionary echo of a period when catalysis still relied heavily on small peptides, mineral surfaces, and ribozymes long before large, genetically encoded proteins originated.
2.3. Evolution of Biological Catalysis: From Ribozymes to Enzymes
One influential framework for the origin of life is the RNA world hypothesis, which posits that, following a period of prebiotic synthesis and accumulation of organic compounds, ribonucleic acid molecules assumed pivotal roles in both genetic replication and catalysis [2]. According to this model, RNA initially served as both genome and catalyst, laying the groundwork for subsequent molecular innovations that would drive the emergence of modern metabolism. Among the supporting evidence is the observation that numerous coenzymes carry ribonucleotide moieties, prompting proposals that these cofactors are molecular fossils of an RNA-centric era [20,21]. Experimental findings indicate that some coenzymes can independently catalyze reactions akin to those they now facilitate in protein-based systems, reinforcing the notion that such cofactors once played direct catalytic roles before the widespread advent of protein enzymes.
Although ribozymes may have provided sufficient catalytic rate enhancement and specificity to support early life, they likely faced challenges related to stability and substrate range. Short peptides may have helped address these limitations by offering additional catalytic groups and structural reinforcement (Figure 2B) [22,23,24]. These peptide-RNA interactions likely gave rise to early ribonucleoprotein (RNP) complexes, which boosted reaction efficiency well before the evolution of the genetic code and the formation of longer polypeptides [25]. The advent of a proto-ribosome (Figure 2C), a rudimentary catalytic RNP assembly, was a pivotal breakthrough, enabling more reliable peptide synthesis and driving the emergence of increasingly specialized polypeptides.
Throughout this early period, metal ions (e.g., Fe2+, Ni2+) and coenzymes remained integral, bridging geochemically driven processes with the expanding biochemical repertoire of early cells [26]. Initially operating alongside ribozymes as small RNA-like molecules, many coenzymes gradually became ubiquitous mediators of oxidation-reduction, group transfers, and other essential reactions in protein-based systems [20]. As the translational apparatus evolved, these cofactors were systematically adopted into the nascent enzymes, ultimately paving the way for sophisticated catalysts in modern metabolism [27].
This progression from ribozymes to protein enzymes (Figure 2A–D) encapsulates not only the gradual shift from RNA-centric to protein-centric catalysis but also the interplay among environmental factors, prebiotic chemistry, and an evolving genetic code [28,29]. In this way, metal ions, small peptides, and nucleic-acid-based cofactors likely contributed to the earliest functional biochemical networks [30], shedding light on how rudimentary chemical systems may have transitioned into the enzyme-driven pathways that sustain contemporary life.
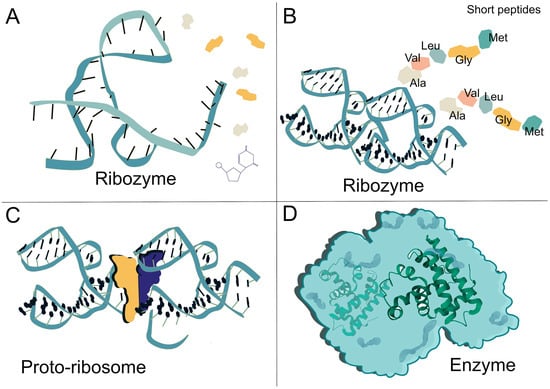
Figure 2.
Evolutionary transition from ribozyme-catalyzed reactions to protein-based enzymatic systems. (A) A catalytic ribozyme, representative of early RNA-based biochemistry, independently facilitates chemical reactions. The colored shapes represent amino acids. (B) A ribozyme bound to short peptides, where amino acid side chains contribute additional catalytic groups and enhance structural stability, marking an intermediate stage toward ribonucleoprotein enzymes. (C) A proto-ribosome, an early ribonucleoprotein complex capable of more reliable peptide synthesis, represents a pivotal step in the evolution of translation. The colored shapes depict a representation of the semi 2-fold symmetry. The illustration is a conceptual depiction inspired by Figure 1E from [31]. (D) A fully formed protein enzyme, illustrating the shift from RNA-based to protein-based catalysis as the dominant mode of enzymatic activity. Throughout this transition, metal ions (e.g., Fe2+, Ni2+) and RNA-derived coenzymes played fundamental roles in catalyzing redox reactions, group transfers, and early metabolic transformations. These cofactors helped bridge prebiotic chemistry with the emergence of modern enzymatic networks, enabling more efficient and specialized catalytic systems.
3. The Heterotrophic Origins of Metabolism
A long-standing debate concerns whether early metabolic pathways were primarily autotrophic (involving CO2 fixation) or heterotrophic (dependent on preformed organic molecules). Although evidence exists for both views, a heterotrophic origin, where the first organisms consumed abiotically generated organic compounds, remains compelling [3,4,7,8,31,32,33]. Notably, heterotrophy does not require a sophisticated CO2-fixation mechanism at the outset; it merely presupposes a sufficient reservoir of organic molecules accumulating on the prebiotic Earth. This aligns with Oparin’s early proposals, situating Darwinian and biochemical considerations at the forefront while avoiding the need for specialized autotrophic enzymes during life’s initial stages [3,4].
3.1. From Simple Heterotrophy to Early Non-Enzymatic Networks
Under this heterotrophic model, the first cells likely harvested existing organic compounds, amino acids, simple sugars, or other reactive intermediates from a primordial soup. Unrestricted by the requirement for CO2 fixation, these early life forms could harness non-enzymatic reactions present in their environment, thereby deriving significant metabolic advantages. Such reactions, often promoted by metal ions, mineral surfaces, or chemical gradients, could have been readily co-opted as proto-metabolic routes [11,12,13]. Over time, the integration of these partial chemical cycles with rudimentary biological regulation may have laid the groundwork for more robust metabolic pathways [10].
This model accommodates phenomena traditionally linked to “autotrophic” or “surface-metabolism” approaches without discarding the importance of external organic substrates. For instance, non-enzymatic segments of a TCA-like cycle or metal-facilitated acetyl–CoA precursor formation can fit into a heterotrophic framework if early cells opportunistically harnessed these steps [9]. In this sense, features of the iron–sulfur world or partial rTCA cycles might be incorporated into a broad heterotrophic narrative, especially once catalytic networks were co-opted and refined.
3.2. Rapid Innovation Within a Heterotrophic Context
The notion that the earliest heterotrophs rapidly incorporated beneficial, non-enzymatic reactions helps explain how more advanced metabolic processes arose so rapidly. If primordial cells were not hindered by the need to generate all of their carbon internally, they could focus on stabilizing and channeling any prebiotic reactions that delivered energy or building blocks. Over evolutionary time, genetic systems would begin encoding newly advantageous catalytic steps, eventually converting them into fully enzyme-driven pathways.
3.3. Reconciling Autotrophic-like Features and Pre-Metabolism
Even with a heterotrophic foundation, autotrophic-like processes or “pre-metabolism” cycles could still have emerged in lineages adapting to environments favoring CO2-fixation (e.g., metal-rich hydrothermal vents). The patchy distribution of metabolic pathways in modern organisms supports the idea that proto-autotrophic modules arose in specific contexts, while other lineages continued to rely on heterotrophy.
Thus, a heterotrophic origin, where exogenous organics are abundant [32], melds naturally with observations of non-enzymatic or semi-enzymatic reaction networks [33]. These networks, initially chemical prototypes, were gradually integrated or superseded by broad-specificity enzymes, gene duplications, and horizontal gene transfers [34]. Over billions of years, environmental pressures, fortuitous chemical discoveries, and natural selection combined to yield the extensive metabolic architectures observed today. The heterotrophic theory, in which simple, preformed organic molecules served as the first fuel, remains a compelling explanation for the origin of metabolism [35]. Proposing that early life began by consuming existing organics, then co-opted and refined non-enzymatic processes, provides a unifying framework for reconciling partial rTCA, Wood–Ljungdahl-like pathways, and other chemical cycles [36]. It also supports the evolutionary logic that simpler modes of carbon and energy acquisition would precede more complex autotrophic mechanisms [26,37]. In this way, “autotrophy” and “pre-metabolism” become valuable extensions of a heterotrophic starting point rather than mutually exclusive alternatives.
4. Primordial Metabolism: Semi-Enzymatic Pathways
Semi-Enzymatic or Partial Enzymatic Models
A notable concept for explaining how early metabolic pathways evolved is the “semi-enzymatic” hypothesis proposed by Lazcano and Miller [38]. This model integrates elements of previous ideas, such as the retrograde evolution hypothesis [39], Granick’s forward-evolution concept [40], and the patchwork assembly approach [41,42], but places a strong emphasis on prebiotic or partial catalytic processes. Specifically, it posits that:
- The shift from non-enzymatic networks probably unfolded in three key phases: (i) the formation of feedback-driven reaction loops, such as the formose reaction, which create self-amplifying feedback; (ii) compartmentalization within mineral pores or protocells that favors certain reaction subsets; and (iii) the genetic coding of functional peptides and ribozymes that stabilize these networks, as demonstrated by the in vitro evolution of RNA ligases [17].
- Initial metabolic-like routes emerged from spontaneous or metal-assisted reactions (Figure 3A), which gradually became entwined with ribozyme-mediated steps.
- Semi-enzymatic processes provided key building blocks, e.g., certain amino acids or bases, without fully specialized enzymes (Figure 3B). These systems were intrinsically unstable until the advent of compartmentalization and genetic memory, a bottleneck that the semi-enzymatic model clarifies through gradual evolutionary enhancements.
- Gene duplication and non-specific starter enzymes eventually replaced or augmented these partial catalysts (Figure 3B), enabling improved reaction rates and specificity [43,44].
- These transitions occurred close to the origin of life, before the Last Common Ancestor, thus bridging the gap between purely geochemical “pre-metabolisms” and modern enzymatic pathways [45,46].
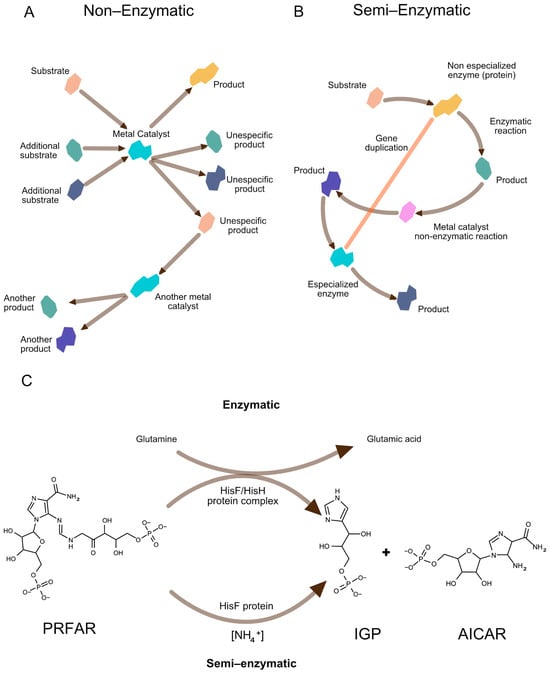
Figure 3.
Gradual emergence of metabolic pathways from geochemical to enzymatic processes. (A) Non-enzymatic metabolic-like routes, where specific substrates and additional non-specific reactants interact with catalytic metals (e.g., Fe2+, Ni2+), generating specific and non-specific products. Some of these products serve as substrates for further reactions catalyzed by other metal ions, forming rudimentary biochemical networks. (B) Semi-enzymatic processes, where a (primitive) non-specialized enzyme catalyzes a reaction, producing metabolites that enter additional metal-assisted transformations. Gene duplication leads to the emergence of a specialized enzyme, improving reaction efficiency and specificity. (C) A contemporary example of semi-enzymatic flexibility is found in histidine biosynthesis, where the imidazole glycerol phosphate (IGP) synthase complex typically transforms N′-(5′-phosphoribosyl)formimino-5-aminoimidazole ribonucleotide (PRFAR) into IGP plus 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). This reaction requires both the HisH (glutamine amidotransferase) and HisF (cyclase) subunits for full enzymatic function. However, in Klebsiella pneumoniae, elevated NH4+ concentrations allow the system to bypass HisH (semi-enzymatic), illustrating how primitive metabolic processes may have operated prior to the evolution of fully specialized enzymes. Image inspired by [38,43,47].
A distinguishing aspect of the semi-enzymatic proposal is its emphasis on partial catalytic processes that bridge prebiotic chemistry and the eventual emergence of fully enzyme-mediated routes [46,48,49,50]. While it posits that non-enzymatic or semi-enzymatic steps preceded contemporary metabolic networks, it also acknowledges that genetic and ribosomal components had to arise to refine and inherit these early routes. Lazcano and Miller [38] emphasized that even in modern biochemistry, some reactions can proceed spontaneously or under alternative conditions without enzymatic catalysis. One illustrative example involves the histidine biosynthesis pathway in the γ-proteobacterium Klebsiella pneumoniae, where imidazole glycerol phosphate (IGP) formation persists despite inactivation of the hisH gene, encoding a glutamine amidotransferase. In this scenario, high concentrations of NH4+ circumvent the need for enzyme-mediated amine transfer [51,52,53,54], suggesting that environmental fluctuations could have supported metabolic processes prior to full genetic regulation.
Recent computational work using network expansion [48,49] and experimental demonstrations of partial rTCA cycles under non-enzymatic conditions [11,12] lend strong support to the plausibility of “semi-enzymatic” networks. Together, these findings suggest that a small set of catalytic precursors, working alongside simple cofactors and peptides, may have progressively generated more coherent metabolic pathways as life transitioned from geochemical to biological evolution [45,50]. Through a sequence of feedback-driven reaction loops, compartmentalization, and eventual genetic control, these early chemical processes could have been refined and propagated, gradually transforming chemical randomness into biological order.
The semi-enzymatic model offers a vital bridge between prebiotic chemistries and the emergence of life’s first metabolic frameworks. It also converges with the patchwork logic, in which broad-specificity enzymes initially catalyzed multiple steps before diverging into specialized forms [42,51]. Consequently, modern metabolic networks may reflect successive layers of refinement stacked atop an initially flexible yet basic chemical foundation.
5. The Wood–Ljungdahl Pathway
Among the various routes proposed to have fueled early autotrophy, the Wood–Ljungdahl pathway stands out for its broad distribution across Bacteria and Archaea [52,53]. This pathway is central to acetogenesis and methanogenesis and, in some lineages, enables CO2 fixation under strictly anaerobic conditions (Figure 4A). Phylogenetic reconstructions suggest that, of the known carbon fixation strategies, only W–L appears to bridge both domains [52]. Consequently, it has been posited that a geochemically driven “proto–W–L” might have existed before the consolidation of a fully enzyme-mediated version [55,56].
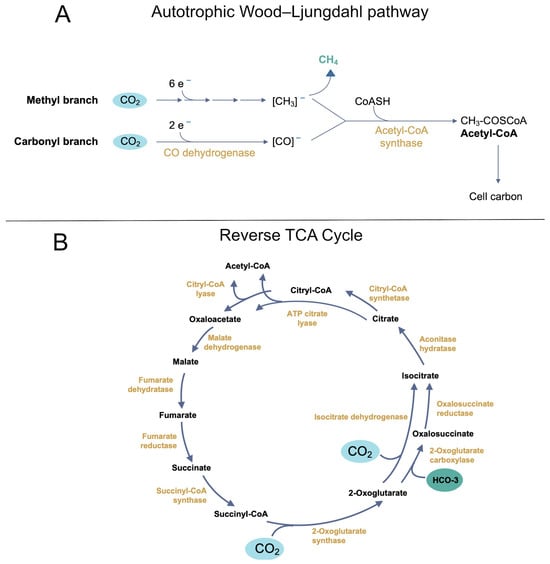
Figure 4.
Two major carbon fixation pathways are proposed to have played key roles in early autotrophic metabolism. (A) The Wood–Ljungdahl pathway is a linear carbon fixation route found in both Bacteria and Archaea. This pathway enables the conversion of CO2 into acetyl-CoA, fueling acetogenesis and methanogenesis. Key intermediates include formate, carbon monoxide, and acetate, with methanogenesis producing methane as a final product. Transition metals (e.g., Fe2+, Ni2+, Co2+) and mineral surfaces may have facilitated prebiotic analogs of this pathway, suggesting a geochemically driven proto–W–L cycle. (B) The reductive tricarboxylic acid (rTCA) cycle is a cyclic carbon fixation route that operates in reverse compared to the oxidative Krebs cycle. This pathway generates key biosynthetic precursors, including succinate, 2-oxoglutarate, and citrate, through ATP-dependent reactions. Enzyme complexes such as ATP-citrate lyase or alternative two-enzyme systems enable citrate cleavage. Abiotic analogs of rTCA reactions, mediated by metal ions and mineral catalysts, support the idea that portions of this cycle may have functioned in prebiotic environments before the evolution of enzymatic catalysis.
Studies of methanogenic clades underscore the antiquity of certain W–L components, as geological evidence places methane production at 3.4 Ga or earlier [57]. However, debates persist over whether hydrogenotrophic methanogenesis, which shares part of the W–L pathway, truly dates to the Last Common Ancestor (LCA). Recent genomic analyses reveal that certain coenzymes and key enzymes, such as methyl-coenzyme M reductase (MCR), exhibit signs of extensive horizontal gene transfer and lineage-specific adaptation [58]. Furthermore, many archaeal groups with partial methanogenic machinery appear to have acquired genes from distantly related taxa [59]. Thus, while W–L–type carbon fixation may be ancient, it is not necessarily universal in the deepest archaeal branches.
Nonetheless, experimental work shows that formate, acetate, and other W–L intermediates can form abiotically in the presence of specific minerals and transition metals, reinforcing the idea that early Earth settings, like hydrothermal vents, could support a proto–W–L cycle [60]. Although its ultimate status as the “first metabolism” remains unproven, this pathway stands among the strongest candidates for anchoring autotrophic CO2 fixation before the diversification of modern lineages (see Table 1).

Table 1.
Comparison of what are believed to be the primordial metabolic pathways.
6. The Reverse Krebs Cycle (rTCA)
Another primordial carbon fixation pathway is the reductive or reverse TCA cycle (rTCA), sometimes called the Arnon cycle (Figure 4B) [61]. Initially characterized in Chlorobium limicola [62] and later identified in other strictly anaerobic or microaerophilic bacteria, the rTCA was once considered widespread. More detailed surveys indicate a patchy distribution, mainly in certain Proteobacteria, Chlorobi, and Aquificales [63]. It remains absent from most archaeal clades, suggesting that although rTCA is evolutionarily important, it is not universal among the earliest lineages [52].
Functionally, the rTCA requires specific enzymes (or enzyme complexes) different from those of the oxidative Krebs cycle. For example, ATP-citrate lyase, or a split system of citryl-CoA synthetase and citryl-CoA lyase, facilitates the cleavage of citrate into oxaloacetate and acetyl-CoA, reversing the oxidative pathway. Likewise, converting 2-oxoglutarate to isocitrate can involve a single isocitrate dehydrogenase (operating in reverse) or a two-enzyme system of 2-oxoglutarate carboxylase and oxalosuccinate reductase [64].
Advocates of rTCA as an early metabolism also point to abiotic analogs: some metal-ion- or mineral-facilitated reactions effectively mimic portions of the cycle without proteins [11,12]. A partial “horseshoe” rTCA combined with Wood–Ljungdahl–type reactions has been proposed to account for the initial carbon skeletons of primitive cells [14]. Whether such reconstructions truly replicate an ancestral “pre-genetic” metabolism remains subject to debate, as modern rTCA enzymes clearly depend on genetically encoded proteins. Nevertheless, the existence of non-enzymatic rTCA-like segments implies that simple catalysis could precede and guide the eventual emergence of dedicated enzyme complexes [12].
7. Evolutionary Implications
7.1. Metabolic Diversification and Early Lineages
As rudimentary metabolic pathways gained efficiency, cells acquired the capacity to exploit diverse energy and carbon sources. This broadening of metabolism represented a crucial step in early life’s evolution, enabling organisms to adapt to multiple ecological niches. Gene duplication, horizontal gene transfer (HGT), and enzyme specialization shaped this process, dispersing metabolic traits among various lineages [65]. The mosaic distribution of metabolic features in modern organisms implies that early metabolic innovations circulated rapidly among nascent life forms, possibly before the LCA had consolidated a single canonical repertoire [66].
Gene duplication furnished the raw material for metabolic innovation, allowing one gene copy to retain its original function while the other assumed new roles [67]. This mechanism may have promoted the development of specialized enzymes capable of novel reactions, enriching the metabolic mosaic of early cells. Horizontal gene transfer could have further expedited diversification by enabling genetic material exchange between distantly related organisms, spreading new and sometimes advantageous traits [68].
The concept of an LCA with a partially consolidated metabolic repertoire challenges the traditional view of a fully formed LCA possessing a fixed set of pathways. Instead, it underscores that early life was metabolically flexible, with lineages independently acquiring and refining various pathways [69]. The fact that metabolic pathways are patchily distributed across the Tree of Life suggests multiple origins and losses throughout evolution [70].
7.2. Environmental Constraints and Selective Pressures
The evolution of modern metabolic pathways can be traced back to the environmental constraints and selective pressures that shaped life on early Earth. Hydrothermal vents and shallow ponds offered distinct physicochemical conditions that drove the emergence and improvement of metabolic strategies. Hydrothermal vents offer stable redox gradients, sustained by the continuous flow of reduced chemical compounds (i.e., H2, H2S, CH4) from beneath the Earth’s surface interacting with oxidized seawater [71]. These conditions may have favored metabolic pathways capable of exploiting chemiosmotic coupling and redox reactions, such as the Wood–Ljungdahl pathway, which remains central, with some different steps, in some metabolisms (acetogenesis and methanogenesis) in modern organisms [58,72].
However, the evolutionary history of methanogenesis, reliant on W–L components, suggests that while it may be ancient, it need not be primordial. Methanogenesis probably arose after the divergence of Archaea and Bacteria, partly because it needs complex enzymatic machinery, including methyl-coenzyme M reductase (MCR), which is not universally conserved [73]. Similarly, the carbon monoxide dehydrogenase/acetyl-CoA synthase (CODH/ACS) complex is an elaborate multi-subunit protein (Figure 5), making it unlikely to have existed at life’s origin. Phylogenomic data indicate that some subunits are archaeal-specific, others bacterial-specific, pointing to domain-specific modifications that arose after divergence [58,73]. Such structural and functional intricacy contrasts with the simpler catalytic systems expected in early networks.
Conversely, shallow ponds, with oscillating temperatures and intermittent UV exposure, may have favored metabolisms adaptable to variable energy inputs while also managing UV-related damage [74]. These and other diverse habitats served as natural laboratories, testing and refining metabolic traits that eventually became foundational to life (see Table 2).

Table 2.
Environmental conditions and their influence on early metabolic pathways. Timescales are based on observed geological/experimental cycles [71,75], and spatial scale ranges reflect microenvironments where prebiotic reactions likely occurred [56,76].
Each niche exerted distinct selective pressures on early metabolisms, favoring pathways such as W–L or partial rTCA cycles that were best suited to harness local energy or carbon. Modern extremophiles, which thrive in conditions analogous to early Earth, illustrate how varied or even extreme environments can maintain specialized ancient pathways.
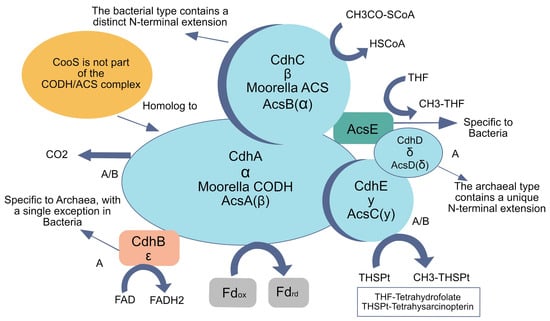
Figure 5.
Structural organization and evolutionary divergence of the carbon monoxide dehydrogenase/acetyl-CoA synthase (CODH/ACS) complex. This intricate multi-subunit enzyme is central to the Wood–Ljungdahl pathway, playing a key role in both methanogens and homoacetogens by catalyzing the reduction of CO2 and the synthesis of acetyl-CoA. Its complexity makes it unlikely to have been present at the origin of life. Phylogenomic analyses reveal domain-specific modifications, with certain subunits exclusive to Bacteria (B), others unique to Archaea (A), and a subset shared by both domains (A/B). This distribution suggests that while CODH/ACS is fundamental to autotrophic metabolism, its current form emerged after the divergence of Bacteria and Archaea. Modified from [77], with information from [58,73].
8. Future Directions and Open Questions
Despite these advances, many questions remain:
Ancestral Enzyme Reconstruction: Recreating proteins that likely existed billions of years ago can illuminate how early enzymes coordinated with metal ions or ribonucleotides. This approach may clarify whether certain catalytic motifs stemmed from an RNA-based era. Future work should emphasize promiscuous generalist enzymes capable of multiple reactions in proto-metabolic contexts. Examining how mineral surfaces, such as iron-sulfur clusters, templated initial active sites is similarly crucial
Synthetic Biology of Primitive Systems: Engineering minimal cells or vesicles incorporating partial autotrophic or heterotrophic pathways could reveal how metal ions, small peptides, and coenzymes bridged non-enzymatic catalysis and robust enzyme networks. Major obstacles include designing self-replicating vesicles that couple metabolic reactions with the encapsulation of genetic material, and simulating prebiotic thermal gradients under realistic early Earth conditions.
Horizontal Gene Transfer Mapping: The extensive lateral gene transfer among early prokaryotes likely accelerated the spread of novel metabolic features. Detailed phylogenomic work might unveil which core traits originated near the LCA and which emerged later. Advanced machine-learning approaches could detect “metabolic fossils” in the genomes of present-day extremophiles and reconstruct the evolutionary history of coenzyme-binding domains throughout the tree of life.
Astrobiological Context: If these early metabolic pathways operated under plausible prebiotic conditions, they could be applicable on Mars or icy moons. Detecting isotopic or organic signatures reflecting early carbon fixation may guide the search for extraterrestrial life or prebiotic chemistries. Building microfluidic devices to simulate extraterrestrial environments (e.g., Europa’s ocean), while enlarging databases of abiotic and biotic isotopic fractionation signatures, is an essential next step.
Biomedical Applications: Insights into ancestral coenzymes and minimal metabolic networks could spur innovations in metabolic bioengineering. For instance, archaeal-inspired cofactors (modified derivatives of F420) might be tested as redox mediators in enzyme therapies. Likewise, reconstructing ancestral ribozyme-peptide complexes might yield biocatalysts compatible with harsh conditions (low pH, high temperature). These designs hold promise for heat-stable enzymes in industrial biotech and pioneering therapeutics based on primordial redox chemistries.
Eco-Evolutionary Modeling: In silico simulations incorporating geochemical data (pH, temperature, redox gradients) can predict which catalytic regimes (RNA-based, metal-based, semi-enzymatic) likely thrived on early Earth, uniting geochemistry and molecular biology. Future avenues include employing quantum-chemical simulations for prebiotic networks and agent-based models to examine protocell populations competing for limited resources in Archaean conditions.
Less Studied Prebiotic Environments: While hydrothermal vents and tidal pools are important in origin-of-life discussions, other locales may yield fresh insights. Subglacial settings could shed light on metabolic pathways under high pressure and low temperature, approximating icy moons, whereas volcanic aerosol droplets might clarify the role of atmospheric chemistry, through dehydration-UV cycles, in metabolic innovation.
Metabolic “Time Travel” Experiments: Novel methods could unravel the timeline of metabolic evolution. For instance, evolving contemporary microbes under simulated Archaean conditions might reveal “early” adaptations. Additionally, designing “synthetic panchronic” organisms (engineered life forms embedded in mineral matrices to mimic long-term geological persistence) could shed light on how primitive biochemical systems achieve stability and fossilization potential over geological timescales.
9. Conclusions
Across the hypotheses presented in this study, thermodynamic optimization stands out as a unifying principle. By reducing activation energies (ΔG‡) and taking advantage of redox gradients, proto-metabolic networks could generate products or intermediates that, in turn, helped to sustain or amplify the same reactions, a process sometimes referred to as self-reinforcement. Under these conditions, the Wood–Ljungdahl pathway’s dependence on low-potential electron donors (H2/CO2) and metal catalysis aligns well with Archaean hydrothermal environments, indicating that both energy availability and environmental factors guided the emergence of early metabolism.
Investigating how carbon fixation arose, whether via the reverse citric acid cycle or the W–L pathway, offers valuable clues for identifying potential universal biosignatures. For example, isotopic fractionation of carbon (e.g., 12C/13C ratios) or sulfur-based compounds in exoplanet atmospheres could mirror analogous processes, thereby informing the search for life on hydrogen-rich worlds like icy moons or sub-Neptunian exoplanets.
Ultimately, the complexity of metabolic origins seems to involve multiple threads: geochemically driven synthesis of essential organic molecules, an RNA world centered on ribozymes and small peptides, and semi-enzymatic pathways that incrementally integrated genetic and translational systems. While certain approaches emphasize purely autotrophic beginnings or non-enzymatic routes, a holistic perspective suggests that diverse mechanisms may have merged in the Last Common Ancestor’s metabolism.
Key pathways, such as the Wood–Ljungdahl and reverse Krebs (rTCA) cycle, provide examples of ancient carbon fixation under limited catalytic conditions, while ribonucleotide-bearing coenzymes highlight a profound relationship with RNA-based catalysis. By combining comparative genomics, ancestral enzyme reconstructions, and synthetic biology, future work can delve deeper into this pivotal era, ultimately bridging geochemical processes and biological innovation, and revealing how modern metabolism took shape.
Author Contributions
I.M.-V. and A.V.-S. contributed equally to the conceptualization, investigation, writing—original draft, and writing—review and editing. All authors participated in the research and writing process. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were generated or analyzed in this study. All data supporting the reported results are derived from the published literature cited in the manuscript.
Acknowledgments
We thank our mentors, Antonio Lazcano, Arturo Becerra, and Juli Peretó, for their inspiration, intellectual guidance, and unwavering support, which motivated us to develop and refine the ideas presented here. We are deeply indebted to them for fostering a passion for understanding life’s earliest stages. The authors would like to thank Thalía Garcés-Jurado for her contribution to the images.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TCA | Tricarboxylic acid cycle |
| RNA | Ribonucleic acid |
| RNP | Ribonucleoprotein |
| W–L | Wood–Ljungdahl (pathway) |
| rTCA | Reverse tricarboxylic acid cycle |
| LCA | Last Common Ancestor |
| MCR | Methyl-coenzyme M reductase |
| Fe2+ | Ferrous ion (iron) |
| Ni2+ | Nickel ion |
| CO2 | Carbon dioxide |
| NH4+ | Ammonium ion |
| Ga | Giga years |
| UV | Ultraviolet |
| HGT | Horizontal gene transfer |
| H2 | Hydrogen gas |
| H2S | Hydrogen sulfide |
| CH4 | Methane gas |
| CODH/ACS | Carbon monoxide dehydrogenase/acetyl-CoA synthase |
References
- Morowitz, H.; Smith, E. Energy flow and the organization of life. Complexity 2007, 13, 51–59. [Google Scholar] [CrossRef]
- Muñoz-Velasco, I.; Cruz-González, A.; Hernández-Morales, R.; Campillo-Balderas, J.A.; Cottom-Salas, W.; Jácome, R.; Vázquez-Salazar, A. Pioneering role of RNA in the early evolution of life. Genet. Mol. Biol. 2024, 47, e20240028. [Google Scholar] [CrossRef]
- Oparin, A.I. The Origin of Life; MacMillan: New York, NY, USA, 1938. [Google Scholar]
- Oparin, A.I. Proiskhozhdenie Zhizny; Synge, A., Translator; Izd. Moskovskii Rabochii: Moscow, Russia, 1924. [Google Scholar]
- Haldane, J.B.S. Origin of Life. In The Rationalist Annual; Rationalist Press Association: London, UK, 1929. [Google Scholar]
- Tirard, S.J.B.S. Haldane and the origin of life. J. Genet. 2017, 96, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Lazcano, A.; Bada, J.L. The 1953 Stanley L. Miller Experiment: Fifty Years of Prebiotic Organic Chemistry. Discov. Life 2003, 33, 235–242. [Google Scholar] [CrossRef]
- Tessera, M. Is pre-Darwinian evolution plausible? Biol. Direct 2018, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Dherbassy, Q.; Mayer, R.J.; Moran, J. Coenzymes in a pre-enzymatic metabolism. Sci. Adv. 2024, 10, eadr5357. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Chevallot-Beroux, E.; Moran, J. Recreating ancient metabolic pathways before enzymes. Bioorganic Med. Chem. 2019, 27, 2292–2297. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 2019, 569, 104–107. [Google Scholar] [CrossRef]
- Preiner, M.; Xavier, J.C.; Vieira, A.D.N.; Kleinermanns, K.; Allen, J.F.; Martin, W.F. Catalysts, autocatalysis and the origin of metabolism. Interface Focus 2019, 9, 20190072. [Google Scholar] [CrossRef]
- Li, Y.; Kitadai, N.; Nakamura, R. Chemical Diversity of Metal Sulfide Minerals and Its Implications for the Origin of Life. Life 2018, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E.; Lohrmann, R. Prebiotic chemistry and nucleic acid replication. Accounts Chem. Res. 1974, 7, 368–377. [Google Scholar] [CrossRef]
- Kawamura, K.; Takeya, H.; Kushibe, T.; Koizumi, Y. Mineral-Enhanced Hydrothermal Oligopeptide Formation at the Second Time Scale. Astrobiology 2011, 11, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Horning, D.P.; Joyce, G.F. Amplification of RNA by an RNA polymerase ribozyme. Proc. Natl. Acad. Sci. USA 2016, 113, 9786–9791. [Google Scholar] [CrossRef] [PubMed]
- Kloprogge, J.T.; Hartman, H. Clays and the Origin of Life: The Experiments. Life 2022, 12, 259. [Google Scholar] [CrossRef]
- White, H.B., 3rd. Coenzymes as fossils of an earlier metabolic state. J. Mol. Evol. 1976, 7, 101–104. [Google Scholar] [CrossRef]
- White, H.B. Evolution of Coenzymes and the Origin of Pyridine Nucleotides. In The Pyridine Nucleotide Coenzymes [Internet]; Elsevier: Amsterdam, The Netherlands, 1982; pp. 1–17. [Google Scholar] [CrossRef]
- Yarus, M. Getting Past the RNA World: The Initial Darwinian Ancestor. Cold Spring Harb. Perspect. Biol. 2011, 3, a003590. [Google Scholar] [CrossRef]
- Vázquez-Salazar, A.; Lazcano, A. Early Life: Embracing the RNA World. Curr. Biol. 2018, 28, R220–R222. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Cech, T.R. The RNA Worlds in Context. Cold Spring Harb. Perspect. Biol. 2012, 4, a006742. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.; Martin, W.F. The Origin of Membrane Bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef]
- Szathmáry, E. The origin of replicators and reproducers. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F. The antiquity of RNA-based evolution. Nature 2002, 418, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Prebiotic Chemistry and the Origin of the RNA World. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [CrossRef]
- Higgs, P.G.; Lehman, N. The RNA World: Molecular cooperation at the origins of life. Nat. Rev. Genet. 2015, 16, 7–17. [Google Scholar] [CrossRef]
- Bose, T.; Fridkin, G.; Davidovich, C.; Krupkin, M.; Dinger, N.; Falkovich, A.H.; Peleg, Y.; Agmon, I.; Bashan, A.; Yonath, A. Origin of life: Protoribosome forms peptide bonds and links RNA and protein dominated worlds. Nucleic Acids Res. 2022, 50, 1815–1828. [Google Scholar] [CrossRef]
- Smith, E.; Morowitz, H.J. Universality in intermediary metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 13168–13173. [Google Scholar] [CrossRef]
- Kauffman, S.A. The Origins of Order: Self-Organization and Selection in Evolution; Oxford University Press (OUP): Oxford, UK, 1993. [Google Scholar] [CrossRef]
- Woese, C.R. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747. [Google Scholar] [CrossRef]
- Lazcano, A. On the origins of organisms The Origin of Life Aleksandr I. Oparin Moscovky Rabotchii, 1924. Science 2024, 386, 1098–1099. [Google Scholar] [CrossRef]
- Morowitz, H.J.; Kostelnik, J.D.; Yang, J.; Cody, G.D. The origin of intermediary metabolism. Proc. Natl. Acad. Sci. USA 2000, 97, 7704–7708. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. Evolution of the first metabolic cycles. Proc. Natl. Acad. Sci. USA 1990, 87, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, A.; Miller, S.L. On the origin of metabolic pathways. J. Mol. Evol. 1999, 49, 424–431. [Google Scholar] [CrossRef]
- Horowitz, N.H. On the Evolution of Biochemical Syntheses. Proc. Natl. Acad. Sci. USA 1945, 31, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Granick, S. Speculations on the Origins and Evolution of Photosynthesis. Ann. New York Acad. Sci. 1957, 69, 292–308. [Google Scholar] [CrossRef]
- Yčas, M. On earlier states of the biochemical system. J. Theor. Biol. 1974, 44, 145–160. [Google Scholar] [CrossRef]
- Jensen, R.A. Enzyme Recruitment in Evolution of New Function. Annu. Rev. Microbiol. 1976, 30, 409–425. [Google Scholar] [CrossRef]
- Fani, R.; Fondi, M. Origin and evolution of metabolic pathways. Phys. Life Rev. 2009, 6, 23–52. [Google Scholar] [CrossRef]
- Noda-Garcia, L.; Liebermeister, W.; Tawfik, D.S. Metabolite–Enzyme Coevolution: From Single Enzymes to Metabolic Pathways and Networks. Annu. Rev. Biochem. 2018, 87, 187–216. [Google Scholar] [CrossRef]
- Delaye, L.; Lazcano, A. Prebiological evolution and the physics of the origin of life. Phys. Life Rev. 2005, 2, 47–64. [Google Scholar] [CrossRef]
- Becerra, A.; Delaye, L.; Islas, S.; Lazcano, A. The Very Early Stages of Biological Evolution and the Nature of the Last Common Ancestor of the Three Major Cell Domains. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 361–379. [Google Scholar] [CrossRef]
- Becerra, A. The Semi-Enzymatic Origin of Metabolic Pathways: Inferring a Very Early Stage of the Evolution of Life. J. Mol. Evol. 2021, 89, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an Ancient Metabolism without Phosphate. Cell 2017, 168, 1126–1134.e9. [Google Scholar] [CrossRef] [PubMed]
- Goldford, J.E.; Hartman, H.; Marsland, R.; Segrè, D. Environmental boundary conditions for the origin of life converge to an organo-sulfur metabolism. Nat. Ecol. Evol. 2019, 3, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- AL Anet, F. The place of metabolism in the origin of life. Curr. Opin. Chem. Biol. 2004, 8, 654–659. [Google Scholar] [CrossRef]
- Fani, R. The Origin and Evolution of Metabolic Pathways: Why and How did Primordial Cells Construct Metabolic Routes? Evol. Educ. Outreach 2012, 5, 367–381. [Google Scholar] [CrossRef]
- Fuchs, G. Alternative Pathways of Carbon Dioxide Fixation: Insights into the Early Evolution of Life? Annu. Rev. Microbiol. 2011, 65, 631–658. [Google Scholar] [CrossRef]
- Berg, I.A.; Kockelkorn, D.; Ramos-Vera, W.H.; Say, R.F.; Zarzycki, J.; Hügler, M.; Alber, B.E.; Fuchs, G. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol. 2010, 8, 447–460. [Google Scholar] [CrossRef]
- Vázquez-Salazar, A.; Becerra, A.; Lazcano, A. Evolutionary convergence in the biosyntheses of the imidazole moieties of histidine and purines. PLoS ONE 2018, 13, e0196349. [Google Scholar] [CrossRef]
- Martin, W.; Russell, M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 59–85. [Google Scholar] [CrossRef]
- Preiner, M.; Igarashi, K.; Muchowska, K.B.; Yu, M.; Varma, S.J.; Kleinermanns, K.; Nobu, M.K.; Kamagata, Y.; Tüysüz, H.; Moran, J.; et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 2020, 4, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 2006, 440, 516–519. [Google Scholar] [CrossRef]
- Adam, P.S.; Borrel, G.; Gribaldo, S. An archaeal origin of the Wood–Ljungdahl H4MPT branch and the emergence of bacterial methylotrophy. Nat. Microbiol. 2019, 4, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.N.; Boyd, J.A.; Leu, A.O.; Woodcroft, B.J.; Parks, D.H.; Hugenholtz, P.; Tyson, G.W. An evolving view of methane metabolism in the Archaea. Nat. Rev. Microbiol. 2019, 17, 219–232. [Google Scholar] [CrossRef]
- Ferry, J.G.; House, C.H. The Stepwise Evolution of Early Life Driven by Energy Conservation. Mol. Biol. Evol. 2006, 23, 1286–1292. [Google Scholar] [CrossRef]
- Arnon, D.I. Role of ferredoxin in photosynthesis. Sci. Nat. 1969, 56, 295–305. [Google Scholar] [CrossRef]
- Evans, M.C.; Buchanan, B.B.; Arnon, D.I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc. Natl. Acad. Sci. USA 1966, 55, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Hügler, M.; Sievert, S.M. Beyond the Calvin Cycle: Autotrophic Carbon Fixation in the Ocean. Annu. Rev. Mar. Sci. 2011, 3, 261–289. [Google Scholar] [CrossRef]
- Aoshima, M. Novel enzyme reactions related to the tricarboxylic acid cycle: Phylogenetic/functional implications and biotechnological applications. Appl. Microbiol. Biotechnol. 2007, 75, 249–255. [Google Scholar] [CrossRef]
- Smith, E.; Morowitz, H.J. The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere, 1st ed.; Cambridge University Press (CUP): Cambridge, UK, 2016. [Google Scholar] [CrossRef]
- Sousa, F.L.; Thiergart, T.; Landan, G.; Nelson-Sathi, S.; Pereira, I.A.C.; Allen, J.F.; Lane, N.; Martin, W.F. Early bioenergetic evolution. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130088. [Google Scholar] [CrossRef]
- Zhang, J. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Koonin, E.V.; Wolf, Y.I. Genomics of bacteria and archaea: The emerging dynamic view of the prokaryotic world. Nucleic Acids Res. 2008, 36, 6688–6719. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Sathi, S.; Sousa, F.L.; Roettger, M.; Lozada-Chávez, N.; Thiergart, T.; Janssen, A.; Bryant, D.; Landan, G.; Schönheit, P.; Siebers, B.; et al. Origins of major archaeal clades correspond to gene acquisitions from bacteria. Nature 2015, 517, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Jiao, J.-Y.; Fu, L.; Hua, Z.-S.; Liu, L.; Salam, N.; Liu, P.-F.; Lv, A.-P.; Wu, G.; Xian, W.-D.; Zhu, Q.; et al. Insight into the function and evolution of the Wood–Ljungdahl pathway in Actinobacteria. ISME J. 2021, 15, 3005–3018. [Google Scholar] [CrossRef]
- Muñoz-Velasco, I.; García-Ferris, C.; Hernandez-Morales, R.; Lazcano, A.; Peretó, J.; Becerra, A. Methanogenesis on Early Stages of Life: Ancient but Not Primordial. Discov. Life 2018, 48, 407–420. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef]
- Deamer, D.; Damer, B.; Kompanichenko, V. Hydrothermal Chemistry and the Origin of Cellular Life. Astrobiology 2019, 19, 1523–1537. [Google Scholar] [CrossRef]
- Baaske, P.; Weinert, F.M.; Duhr, S.; Lemke, K.H.; Russell, M.J.; Braun, D. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl. Acad. Sci. USA 2007, 104, 9346–9351. [Google Scholar] [CrossRef]
- Lessner, D.J. Methanogenesis Biochemistry. In Encyclopedia of Life Sciences, 1st ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).