Polysaccharides from Brown Seaweeds (Padina boergesenii and Sargassum euryphyllum) as Promising Inhibitors of SARS-CoV-2: Characterization, Mechanisms, and Therapeutic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Seaweed Samples
2.2. Extraction of the Crude Seaweed Polysaccharides
2.3. Characterization and Identification of Seaweed Polysaccharides
2.3.1. Alginate Extraction
2.3.2. Physiochemical Properties of Alginate (Viscosity, pH, EC, and TDS)
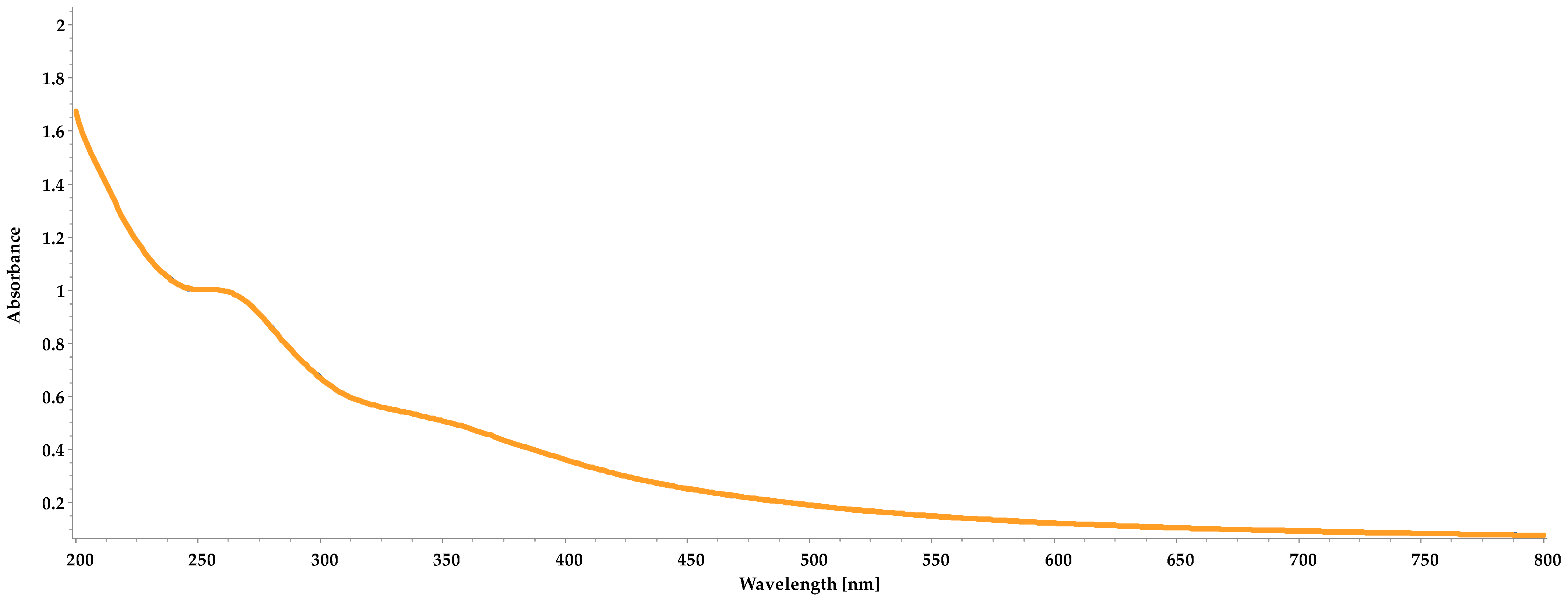
2.3.3. UV/VIS Spectrophotometric Profiles of Alginate Solutions
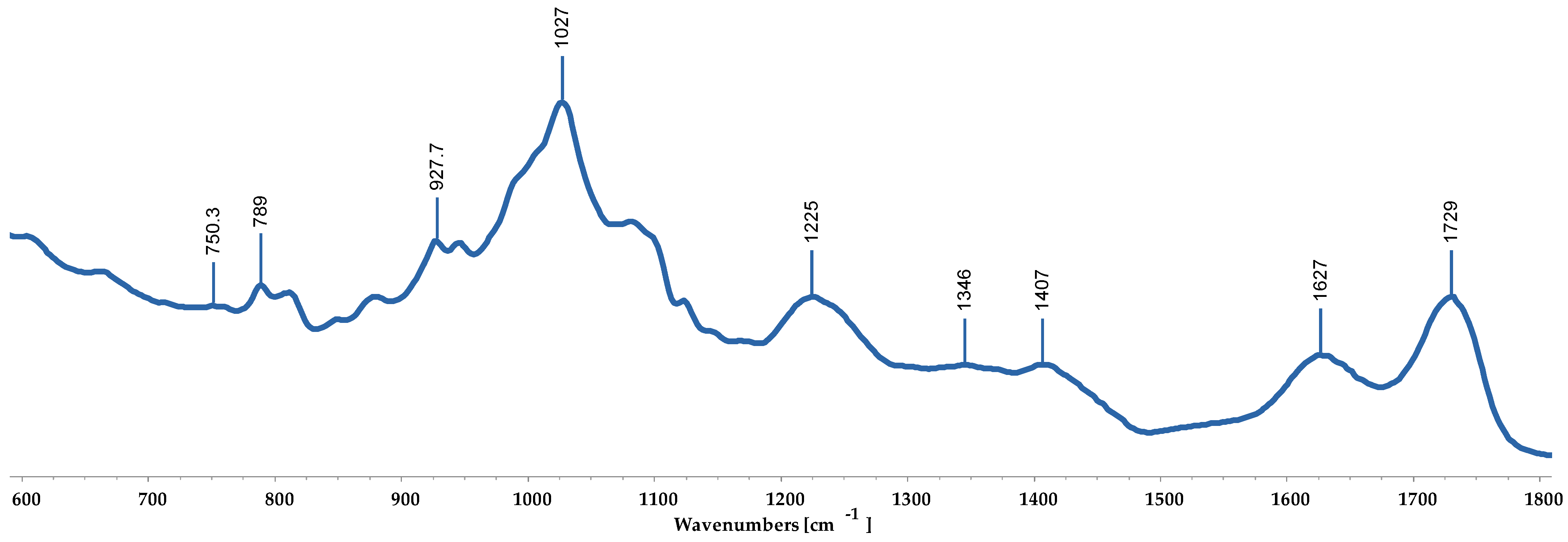
2.3.4. FTIR-ATR Analysis
2.4. SARS-CoV-2 Virus Isolation and Propagation
2.4.1. Cell Line
2.4.2. Detection of Antiviral Activity and Cytotoxicity of Seaweed Polysaccharide Virus Titration by TCID50
2.4.3. Determination of Inhibitory Concentration 50% (IC50)
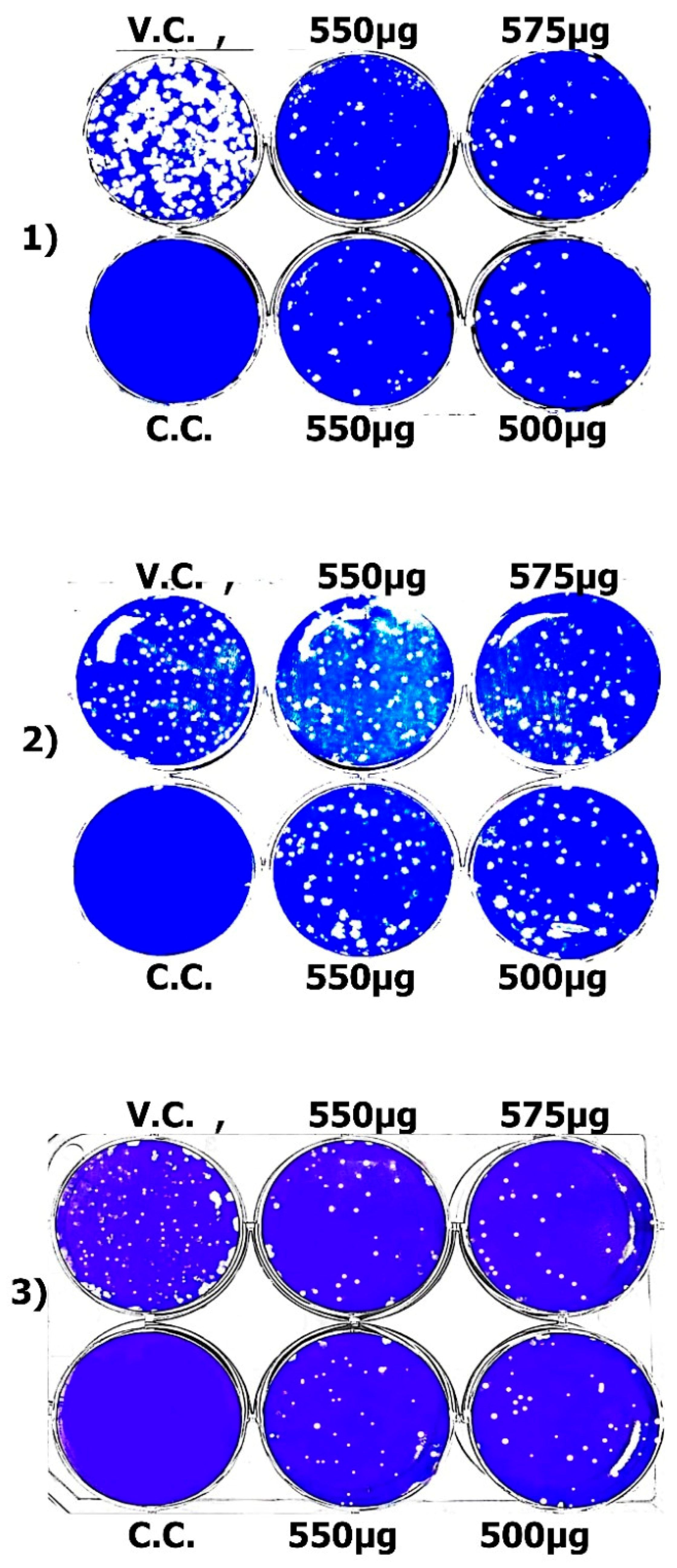
2.4.4. Viral Plaque Titration Assay
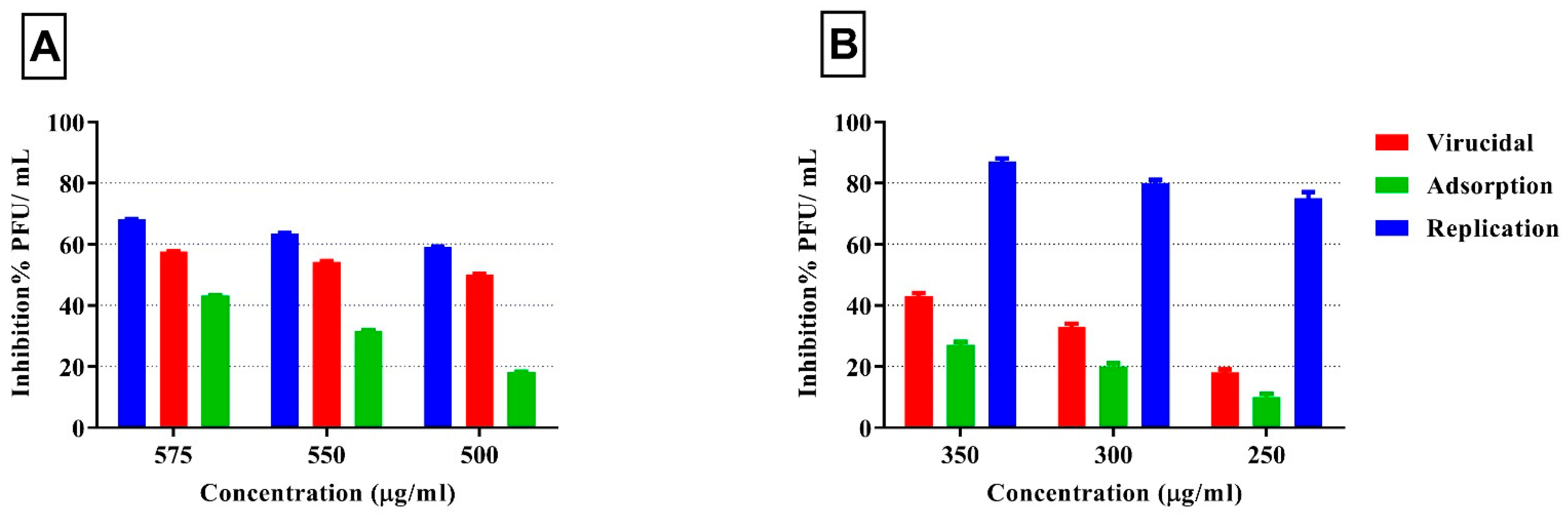
2.4.5. Mode of Action of Virus Inhibition
Viral Adsorption
Viral Replication
Virucidal Mechanism
2.5. Statistical Analyses
3. Results
3.1. Physicochemical Characterization of Seaweed Polysaccharides (Alginate)
3.2. UV/VIS Spectra
3.3. FTIR-ATR
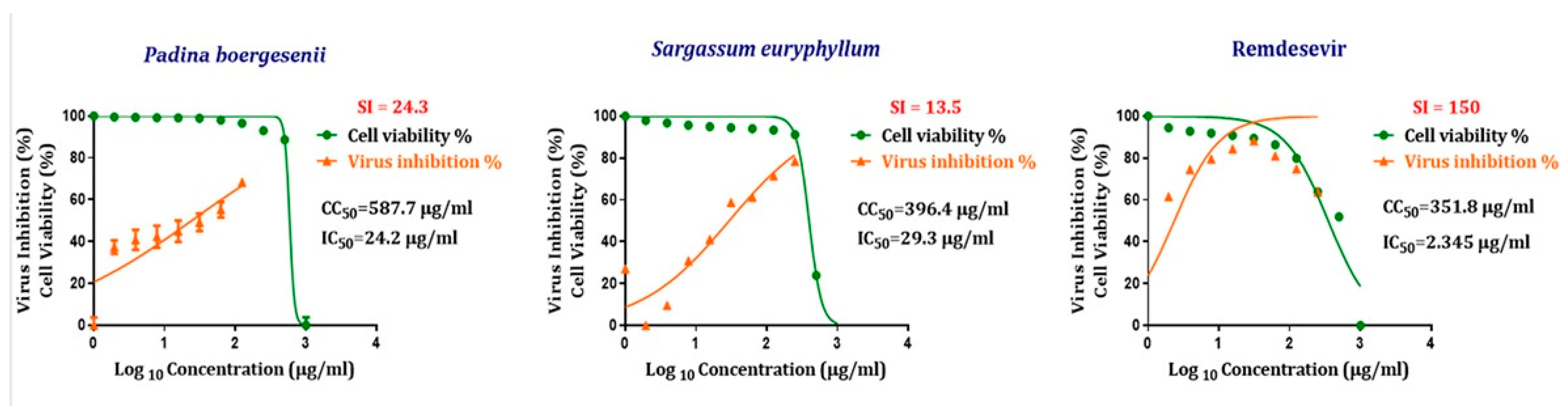
3.4. Antiviral Activity of Seaweed Polysaccharides Against SARS-CoV-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19—16 March 2023; World Health Organization: Geneva, Switzerland, 2023.
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Grubaugh, N.D.; Hodcroft, E.B.; Fauver, J.R.; Phelan, A.L.; Cevik, M. Public health actions to control new SARS-CoV-2 variants. Cell 2021, 184, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Kupferschmidt, K. A very, very bad look’for remdesivir. Science 2020, 370, 642–643. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Bhatt, A.; Arora, P.; Prajapati, S.K. Can Algal Derived Bioactive Metabolites Serve as Potential Therapeutics for the Treatment of SARS-CoV-2 Like Viral Infection? Front. Microbiol. 2020, 11, 596374. [Google Scholar] [CrossRef]
- Abotaleb, S.; Gheda, S.; Alam, N.; ELMehalawy, A.; Saeed, A. In vitro Assessment of Antimicrobial, Antioxidant and Anticancer Activities of Some Marine Macroalgae. Egypt. J. Bot. 2019, 60, 81–96. [Google Scholar] [CrossRef]
- Ismail, G.A.; Gheda, S.F.; Abo-Shady, A.M.; Abdel-Karim, O.H. In vitro potential activity of some seaweeds as antioxidants and inhibitors of diabetic enzymes. Food Sci. Technol. 2020, 40, 681–691. [Google Scholar] [CrossRef]
- Gheda, S.; Hamouda, R.A.; Naby, M.A.; Mohamed, T.M.; Al-Shaikh, T.M.; Khamis, A. Potent Effect of Phlorotannins Derived from Sargassum linifolium as Antioxidant and Antidiabetic in a Streptozotocin-Induced Diabetic Rats Model. Appl. Sci. 2023, 13, 4711. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2019, 18, 2. [Google Scholar] [CrossRef]
- Gheda, S.F.; El-Adawi, H.I.; El-Deeb, N.M. Antiviral Profile of Brown and Red Seaweed Polysaccharides Against Hepatitis C Virus. Iran. J. Pharm. Res. 2016, 15, 483–491. [Google Scholar]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and Characterization of Griffithsin, a Novel HIV-inactivating Protein, from the Red Alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic Activities of Sulfated Polysaccharides from Green Algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Tark, D.; Song, B.-M.; Lee, G.-H.; Yang, J.-H.; Han, H.-J.; Yim, S.K. Evaluation of Antiviral Effect against SARS-CoV-2 Propagation by Crude Polysaccharides from Seaweed and Abalone Viscera In Vitro. Mar. Drugs 2022, 20, 296. [Google Scholar] [CrossRef]
- Ziyaei, K.; Ataie, Z.; Mokhtari, M.; Adrah, K.; Daneshmehr, M.A. An insight to the therapeutic potential of algae-derived sulfated polysaccharides and polyunsaturated fatty acids: Focusing on the COVID-19. Int. J. Biol. Macromol. 2022, 209, 244–257. [Google Scholar] [CrossRef]
- Binsuwaidan, R.; El-Masry, T.A.; El-Sheekh, M.; Seadawy, M.G.; Makhlof, M.E.M.; Aboukhatwa, S.M.; El-Shitany, N.A.; Elmorshedy, K.E.; El-Nagar, M.M.F.; El-Bouseary, M.M. Prospective Antiviral Effect of Ulva lactuca Aqueous Extract against COVID-19 Infection. Mar. Drugs 2024, 22, 30. [Google Scholar] [CrossRef]
- You, L.; Gong, Y.; Li, L.; Hu, X.; Brennan, C.; Kulikouskaya, V. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. Int. J. Food Sci. Technol. 2020, 55, 1199–1206. [Google Scholar] [CrossRef]
- Nova, P.; Pimenta-Martins, A.; Laranjeira Silva, J.; Silva, A.M.; Gomes, A.M.; Freitas, A.C. Health benefits and bioavailability of marine resources components that contribute to health—what’s new? Crit. Rev. Food Sci. Nutr. 2020, 60, 3680–3692. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.d.C.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef]
- Aatif, M.; Muteeb, G.; Alsultan, A.; Alshoaibi, A.; Khelif, B.Y. Dieckol and Its Derivatives as Potential Inhibitors of SARS-CoV-2 Spike Protein (UK Strain: VUI 202012/01): A Computational Study. Mar. Drugs 2021, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Important Roles of Oligo- and Polysaccharides against SARS-CoV-2: Recent Advances. Appl. Sci. 2021, 11, 3512. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.-I.; Kim, C.W.; Lee, H.-R. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Lombardo, M.E.; Dogliotti, A.; Flynn, L.P.; Giugliano, R.; Simonelli, G.; Valentini, R.; Ramos, A.; Romano, P.; Marcote, M.; et al. Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease. Int. J. Gen. Med. 2021, 14, 6277–6286. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, W.; Mitra, D.; McCandless, M.G.; Sharma, P.; Tandon, R.; Zhang, F.; Linhardt, R.J. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020, 163, 1649–1658. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef]
- Garcia-Ruiz, D.; Villalobos-Sánchez, E.; Alam-Escamilla, D.; Elizondo-Quiroga, D. In vitro inhibition of SARS-CoV-2 Infection by dry algae powders. Sci. Rep. 2022, 12, 17101. [Google Scholar] [CrossRef]
- Aleem, A.A. The Marine Algae of Alexandria; Faculty of Science, University of Alexandria: Alexandria, Egypt, 1993. [Google Scholar]
- Jha, B.; Reddy, C.R.K.; Thakur, M.C.; Rao, M.U. Seaweeds of India; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication. 2023. Available online: http://www.algaebase.org (accessed on 16 October 2024).
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. Appl. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Cotas, J.; Pacheco, D.; Araujo, G.S.; Valado, A.; Critchley, A.T.; Pereira, L. On the Health Benefits vs. Risks of Seaweeds and Their Constituents: The Curious Case of the Polymer Paradigm. Mar. Drugs 2021, 19, 164. [Google Scholar] [CrossRef]
- Mamede, M.; Cotas, J.; Bahcevandziev, K.; Pereira, L. Seaweed polysaccharides on seed germination of Brassica napus L. Algal Res. 2023, 76, 103288. [Google Scholar] [CrossRef]
- Kandeil, A.; Mostafa, A.; El-Shesheny, R.; Shehata, M.; Roshdy, W.H.; Ahmed, S.S.; Gomaa, M.; El Taweel, A.; Kayed, A.E.; Mahmoud, S.H.; et al. Coding-Complete Genome Sequences of Two SARS-CoV-2 Isolates from Egypt. Microbiol. Resour. Announc. 2020, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef]
- Mostafa, A.; Kandeil, A.; A. M. M. Elshaier, Y.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals 2020, 13, 443. [Google Scholar] [CrossRef]
- Tobita, K. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med. Microbiol. Immunol. 1975, 162, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhan, B.; Yao, X.; Gao, Y.; Shong, J. Antiviral activity of tannin from the pericarp of Punica granatum L. against genital Herpes virus in vitro. Zhongguo Zhong Yao Za Zhi 1995, 20, 556–558, 576. [Google Scholar]
- Kuo, Y.-C.; Lin, L.-C.; Tsai, W.-J.; Chou, C.-J.; Kung, S.-H.; Ho, Y.-H. Samarangenin B from Limonium sinense Suppresses Herpes simplex Virus Type 1 Replication in Vero Cells by Regulation of Viral Macromolecular Synthesis. Antimicrob. Agents Chemother. 2002, 46, 2854–2864. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses Herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Song, Y.; He, P.; Rodrigues, A.L.; Datta, P.; Tandon, R.; Bates, J.T.; Bierdeman, M.A.; Chen, C.; Dordick, J.; Zhang, F.; et al. Anti-SARS-CoV-2 Activity of Rhamnan Sulfate from Monostroma nitidum. Mar. Drugs 2021, 19, 685. [Google Scholar] [CrossRef]
- Rohilla, D.; Srivastava, A.K.; Singh, R.P.; Yadav, P.; Singh, S.K.; Kumar, D.; Bhardwaj, N.; Kesawat, M.S.; Pandey, K.D.; Kumar, A. Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV2. Stresses 2023, 3, 555–569. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Bataglioli, R.A.; Rocha Neto, J.B.M.; Calais, G.B.; Lopes, L.M.; Tsukamoto, J.; de Moraes, A.P.; Arns, C.W.; Beppu, M.M. Hybrid alginate–copper sulfate textile coating for coronavirus inactivation. J. Am. Ceram. Soc. 2022, 105, 1748–1752. [Google Scholar] [CrossRef]
- Sangtani, R.; Ghosh, A.; Jha, H.C.; Parmar, H.S.; Bala, K. Potential of algal metabolites for the development of broad-spectrum antiviral therapeutics: Possible implications in COVID-19 therapy. Phytother. Res. 2021, 35, 2296–2316. [Google Scholar] [CrossRef]
- Mohammed Ali, H.S.H.; Altayb, H.N.; Bayoumi, A.A.M.; El Omri, A.; Firoz, A.; Chaieb, K. In silico screening of the effectiveness of natural compounds from algae as SARS-CoV-2 inhibitors: Molecular docking, ADMT profile and molecular dynamic studies. J. Biomol. Struct. Dyn. 2023, 41, 3129–3144. [Google Scholar] [CrossRef]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef]
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 307. [Google Scholar] [CrossRef]
- Yang, C.; Li, D.; Wang, S.; Xu, M.; Wang, D.; Li, X.; Xu, X.; Li, C. Inhibitory activities of alginate phosphate and sulfate derivatives against SARS-CoV-2 in vitro. Int. J. Biol. Macromol. 2023, 227, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pei, R.; Li, M.; Su, H.; Sun, H.; Ding, Y.; Su, M.; Huang, C.; Chen, X.; Du, Z.; et al. Cocktail polysaccharides isolated from Ecklonia kurome against the SARS-CoV-2 infection. Carbohydr. Polym. 2022, 275, 118779. [Google Scholar] [CrossRef]
- Wang, H.; Ooi, E.V.; Ang, P.O. Antiviral activities of extracts from Hong Kong seaweeds. J. Zhejiang Univ. Sci. B 2008, 9, 969–976. [Google Scholar] [CrossRef]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Ceña-Díez, R.; Jiménez, J.L.; Ángeles Muñoz-Fernández, M. Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides as Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections. Med. Res. Rev. 2017, 37, 149–179. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulfated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef] [PubMed]


| Seaweed | pH | EC | TDS | Viscosity (cP) |
|---|---|---|---|---|
| Padina boergesenii | 4.21 | 118 | 46 | 3.9 |
| Sargassum euryphyllum | 3.81 | 200 | 108 | 3.6 |
| Reference Wave Number (cm−1) | Bound | Wave Number Observed (cm−1) | |
|---|---|---|---|
| Padina Boergesenii | Sargassum Euryphyllum | ||
| 1730–1710 | Carboxylic acid ester (C=O) | +++ | +++ |
| 1610–1600 | Asymmetric stretching vibration of carboxyl groups (COOH) | + | + |
| 1428–1400 | Symmetric stretching vibration of carboxyl groups (COOH) | + | + |
| 1280–1230 | Sulfated esters (S=O) | +++ | +++ |
| 1030–1025 | Alginic acid (C-O group) | +++++ | +++++ |
| 950–930 | C-O stretching vibration of uronic acids | ++ | ++ |
| 806 | Guluronic acid residues | + | + |
| 788 | Mannuronic acid residues | ++ | ++ |
| Antiviral Compound | CC50 µg/mL | IC50 µg/mL | Selectivity Index (SI) (CC50/IC50) |
|---|---|---|---|
| Padina boergesenii polysaccharide | 587.7 | 24.2 | 24.3 |
| Sargassum euryphyllum polysaccharide | 396.4 | 29.3 | 13.5 |
| Standard antiviral drug (Remdesivir) | 351.8 | 2.34 5 | 150 |
| Mechanism | Conc. µg/mL | Virus Control (PFU/mL) | Viral Count Following Treatment (PFU/mL) | Viral Inhibition % |
|---|---|---|---|---|
| Viral adsorption | 575 | 3.5 × 105 | 1.50 × 105 | 57.1% ± 1.4 |
| 550 | 1.60 × 105 | 54.3% ± 1.3 | ||
| 500 | 1.75 × 105 | 50.0% ± 0.6 | ||
| Viral replication | 575 | 3.5 × 105 | 2.20 × 105 | 37.2% ± 3.0 |
| 550 | 2.30 × 105 | 34.3% ± 1.7 | ||
| 500 | 2.40 × 105 | 31.5% ± 6.7 | ||
| Virucidal | 575 | 3.5 × 105 | 1.10 × 105 | 68.6% ± 0.8 |
| 550 | 1.25 × 105 | 64.3% ± 0.7 | ||
| 500 | 1.45 × 105 | 58.6% ± 0.8 |
| Mechanism | Conc. µg/mL | Virus Control (PFU/mL) | Viral Count Following Treatment (PFU/mL) | Viral Inhibition % |
|---|---|---|---|---|
| Viral adsorption | 350 | 3.5 × 105 | 3.15 × 105 | 27.1% ± 1.40 |
| 300 | 2.80 × 105 | 20.0% ± 2.10 | ||
| 250 | 2.55 × 105 | 10.0% ± 1.10 | ||
| Viral replication | 350 | 3.5 × 105 | 0.45 × 105 | 87.1% ± 0.87 |
| 300 | 0.65 × 105 | 81.4% ± 0.73 | ||
| 250 | 0.87 × 105 | 75.1% ± 0.95 | ||
| Virucidal | 350 | 3.5 × 105 | 2.85 × 105 | 42.9% ± 1.90 |
| 300 | 2.50 × 105 | 33.3% ± 1.20 | ||
| 250 | 2.00 × 105 | 18.6% ± 2.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheda, S.; Karkour, A.M.; Shafay, S.E.; GabAllah, M.; Cotas, J.; Pereira, L. Polysaccharides from Brown Seaweeds (Padina boergesenii and Sargassum euryphyllum) as Promising Inhibitors of SARS-CoV-2: Characterization, Mechanisms, and Therapeutic Potential. Macromol 2025, 5, 18. https://doi.org/10.3390/macromol5020018
Gheda S, Karkour AM, Shafay SE, GabAllah M, Cotas J, Pereira L. Polysaccharides from Brown Seaweeds (Padina boergesenii and Sargassum euryphyllum) as Promising Inhibitors of SARS-CoV-2: Characterization, Mechanisms, and Therapeutic Potential. Macromol. 2025; 5(2):18. https://doi.org/10.3390/macromol5020018
Chicago/Turabian StyleGheda, Saly, Ali M. Karkour, Shimaa El Shafay, Mohamed GabAllah, João Cotas, and Leonel Pereira. 2025. "Polysaccharides from Brown Seaweeds (Padina boergesenii and Sargassum euryphyllum) as Promising Inhibitors of SARS-CoV-2: Characterization, Mechanisms, and Therapeutic Potential" Macromol 5, no. 2: 18. https://doi.org/10.3390/macromol5020018
APA StyleGheda, S., Karkour, A. M., Shafay, S. E., GabAllah, M., Cotas, J., & Pereira, L. (2025). Polysaccharides from Brown Seaweeds (Padina boergesenii and Sargassum euryphyllum) as Promising Inhibitors of SARS-CoV-2: Characterization, Mechanisms, and Therapeutic Potential. Macromol, 5(2), 18. https://doi.org/10.3390/macromol5020018








