Thiophosphate-Based Covalent Organic Framework (COF) or Porous Organic Polymer (POP)?
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Precursors 1 and 2
2.2. Synthesis of Material 3
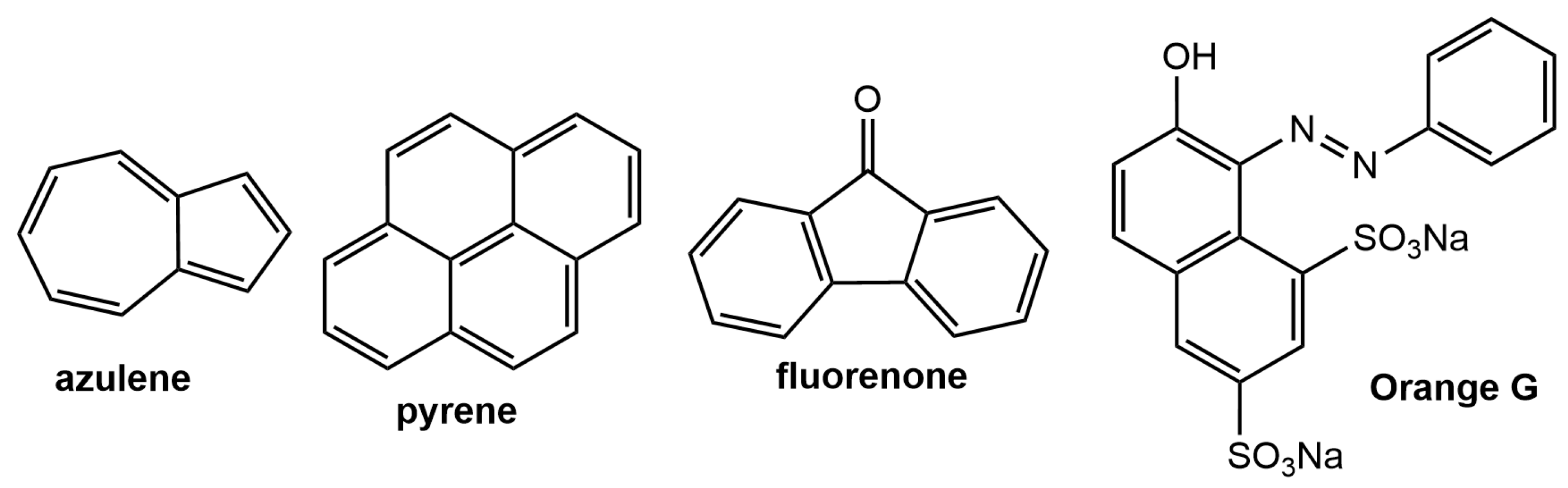
2.3. Attempted Trapping of Dyes
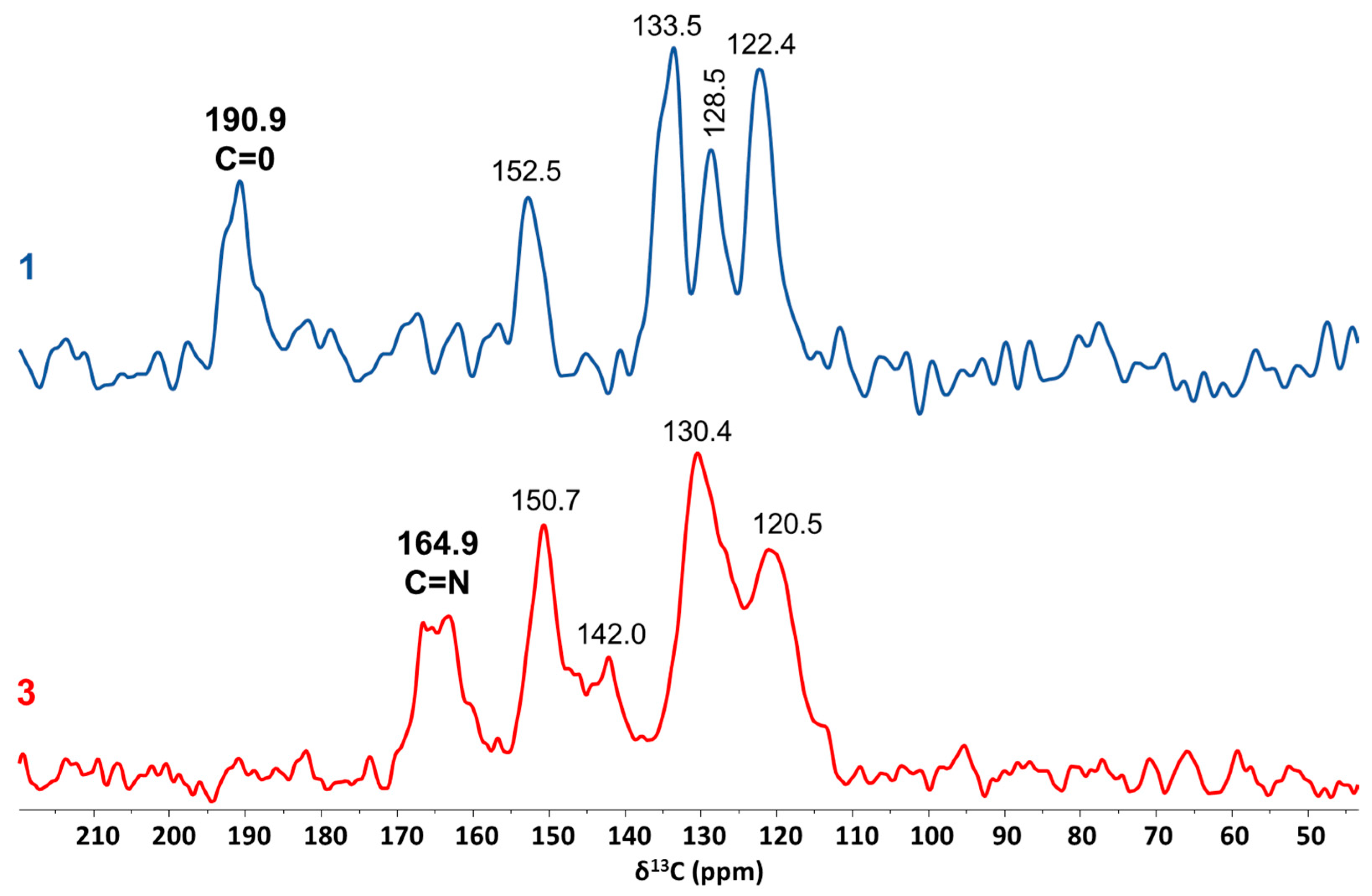
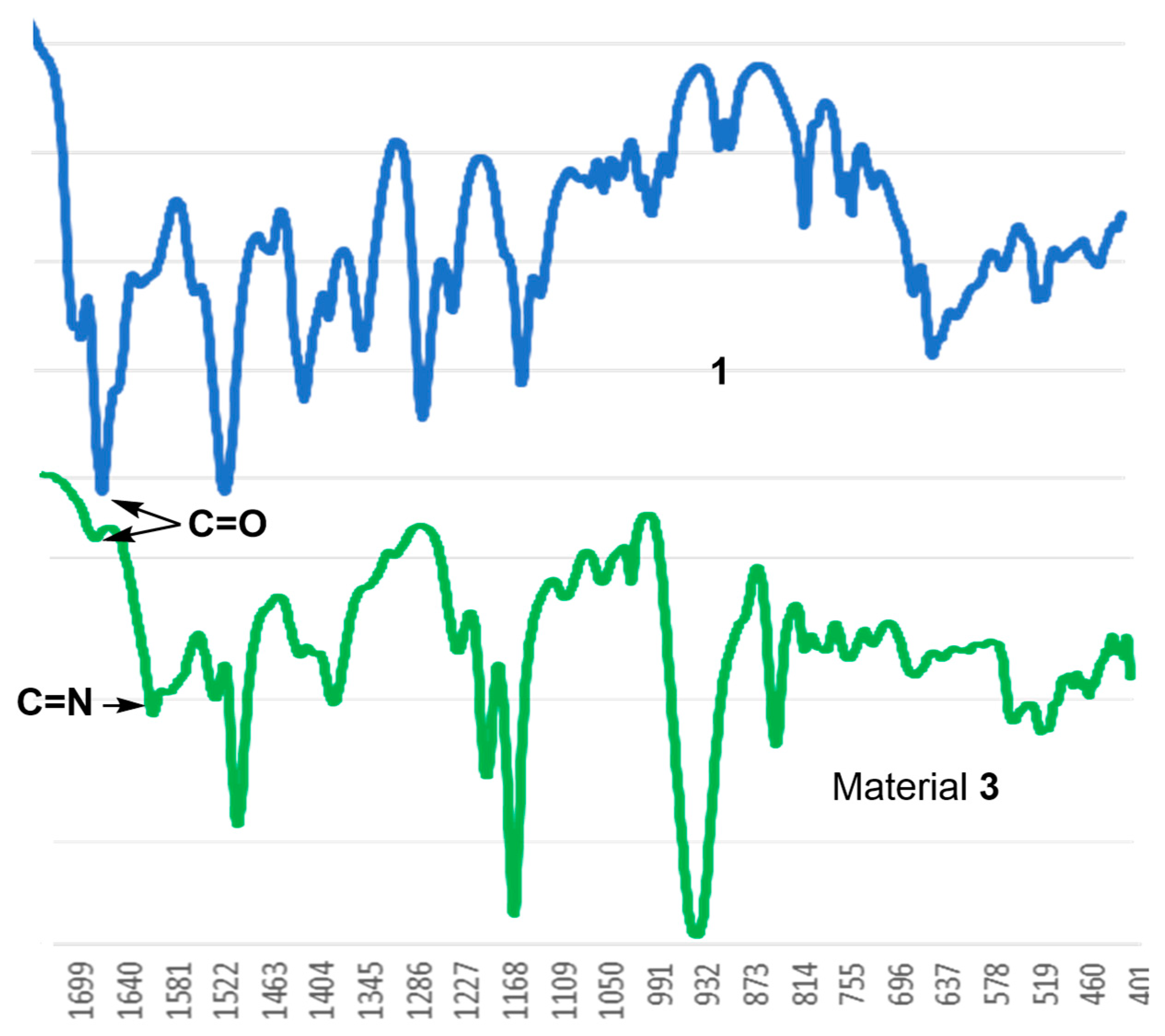
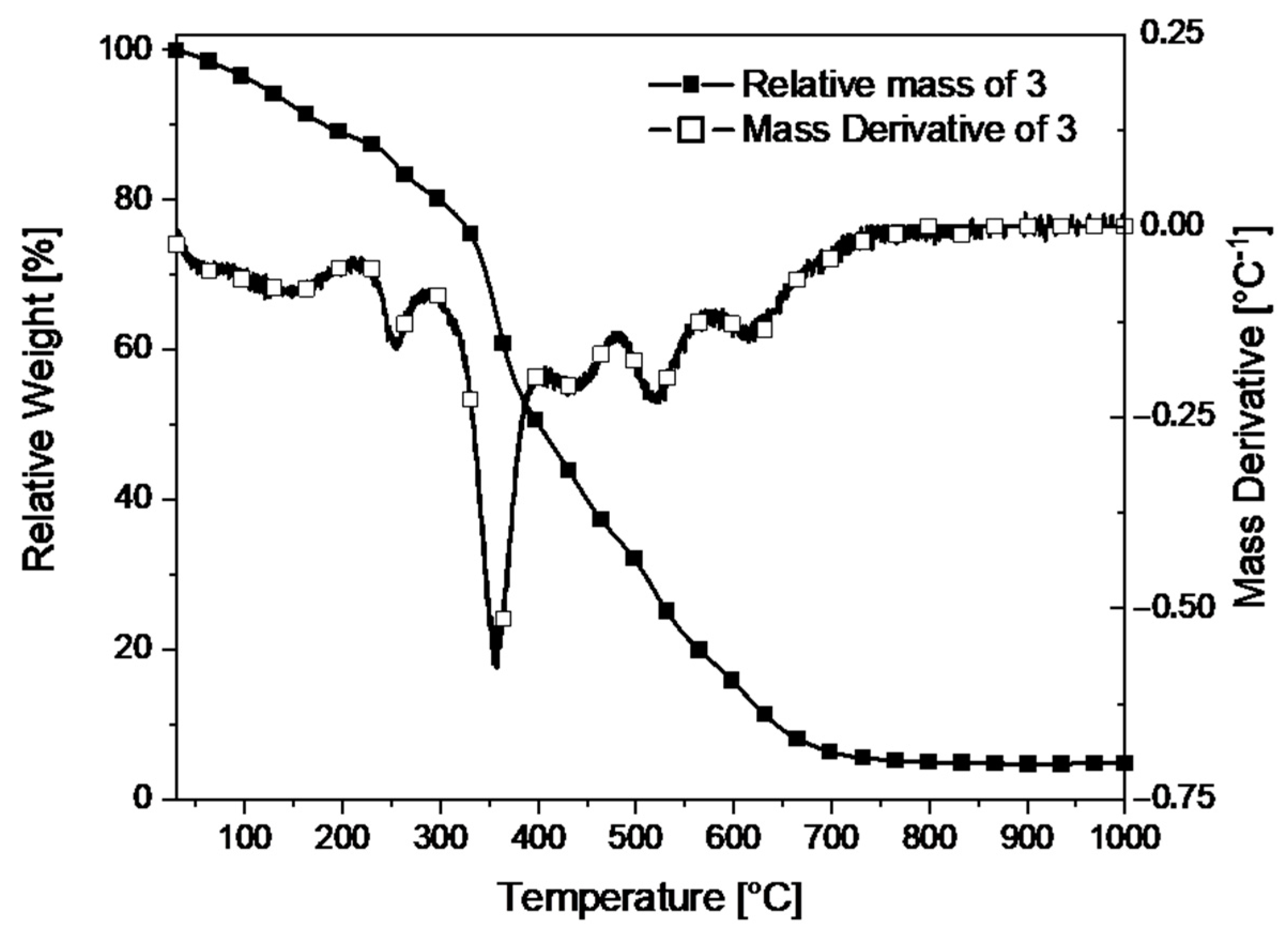
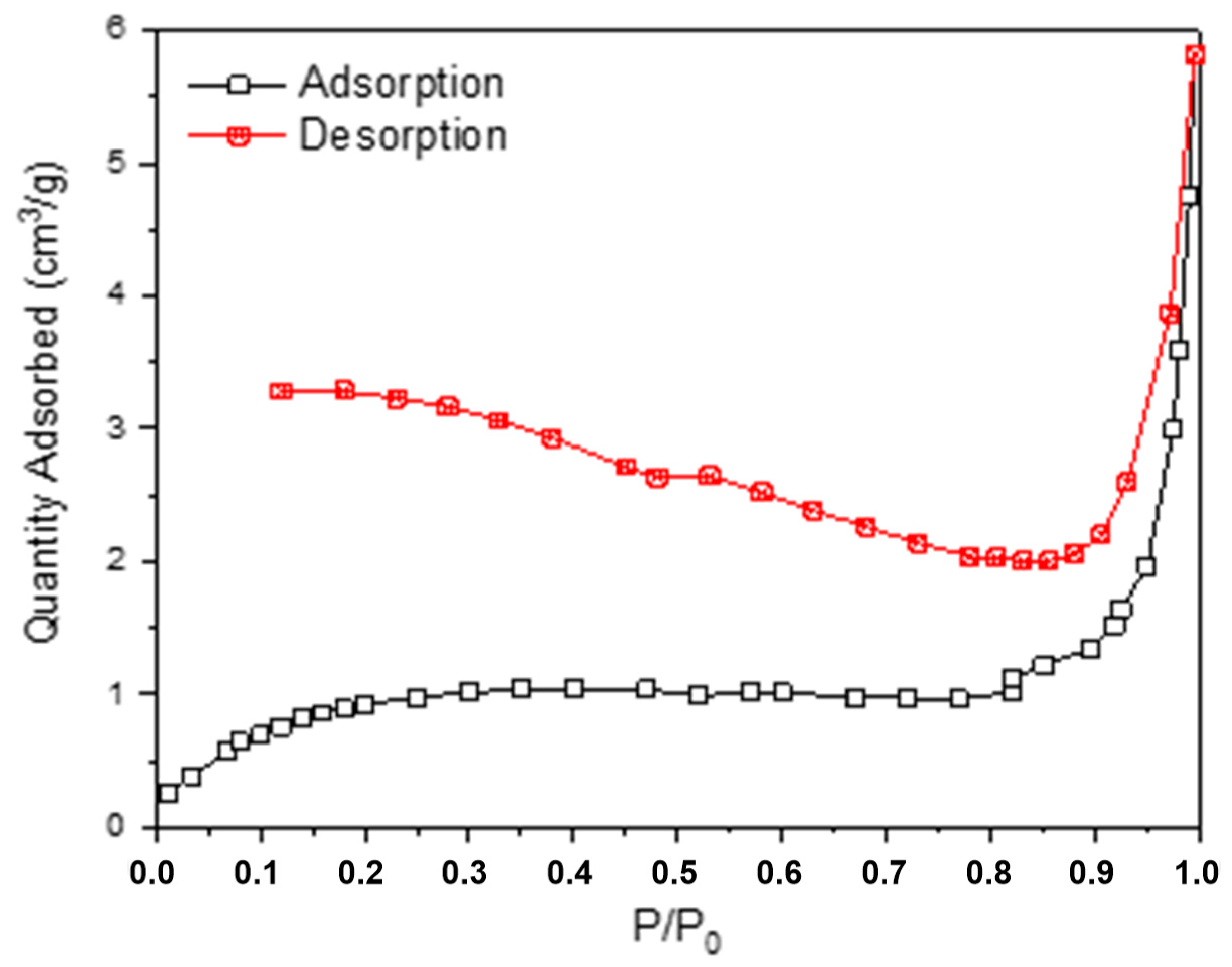
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Jhung, S.H. Covalent organic framework-based materials: Synthesis, modification, and application in environmental remediation. Coord. Chem. Rev. 2021, 441, 213989. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.L.; Dong, Y.B. Metalated covalent organic frameworks: From synthetic strategies to diverse applications. Chem. Soc. Rev. 2022, 51, 6307–6416. [Google Scholar] [CrossRef]
- Gong, Y.N.; Guan, X.; Jiang, H.L. Covalent organic frameworks for photocatalysis: Synthesis, structural features, fundamentals and performance. Coord. Chem. Rev. 2023, 475, 214889. [Google Scholar] [CrossRef]
- Iqbal, R.; Yasin, G.; Hamza, M.; Ibraheem, S.; Ullah, B.; Saleem, A.; Ali, S.; Hussain, S.; Tuan Anh, N.; Slimani, Y.; et al. State of the art two-dimensional covalent organic frameworks: Prospects from rational design and reactions to applications for advanced energy storage technologies. Coord. Chem. Rev. 2021, 447, 214152. [Google Scholar] [CrossRef]
- Tan, K.T.; Ghosh, S.; Wang, Z.; Wen, F.; Rodriguez-San-Miguel, D.; Feng, J.; Huang, N.; Wang, W.; Zamora, F.; Feng, X.; et al. Covalent organic frameworks. Nat. Rev. Methods Primers 2023, 3, 1. [Google Scholar] [CrossRef]
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of Chemical Stability and Crystallinity in Porphyrin-Containing Covalent Organic Frameworks by Intramolecular Hydrogen Bonds. Angew. Chem. Int. Ed. 2013, 52, 13052–13056. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Yang, Y.; Vitorica-Yrezabal, I.J.; Wang, H.; Tan, F.; Gong, L.; Li, Y.; Chen, P.; Dong, X.; et al. Growth of single-crystal imine-linked covalent organic frameworks using amphiphilic amino-acid derivatives in water. Nat. Chem. 2023, 15, 841–847. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheno, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Huang, S.; Chen, K.; Li, T.-T. Porphyrin and phthalocyanine based covalent organic frameworks for electrocatalysis. Coord. Chem. Rev. 2022, 464, 214563. [Google Scholar] [CrossRef]
- Gaumann, P.; Cartagenova, D.; Ranocchiari, M. Phosphine-Functionalized Porous Materials for Catalytic Organic Synthesis. Eur. J. Org. Chem. 2022, 2022, e202201006. [Google Scholar] [CrossRef]
- Ma, H.-C.; Sun, Y.-N.; Chen, G.-J.; Dong, Y.-B. A BINOL-phosphoric acid and metalloporphyrin derived chiral covalent organic framework for enantioselective α-benzylation of aldehydes. Chem. Sci. 2022, 13, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Gropp, C.; Ma, T.; Hanikel, N.; Yaghi, O.M. Design of higher valency in covalent organic frameworks. Science 2020, 370, eabd6406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Y.; Bai, C.; Guo, X.; Han, J.; Hu, S.; Jiang, H.; Tan, W.; Li, S.; Ma, L. Synthesis of Microporous Covalent Phosphazene-Based Frameworks for Selective Separation of Uranium in Highly Acidic Media Based on Size-Matching Effect. ACS Appl. Mater. Interfaces 2018, 10, 28936–28947. [Google Scholar] [CrossRef]
- Peng, H.; Mao, Y.; Wang, D.; Fu, S. B-N-P-linked covalent organic frameworks for efficient flame retarding and toxic smoke suppression of polyacrylonitrile composite fiber. Chem. Eng. J. 2022, 430, 133120. [Google Scholar] [CrossRef]
- Kumar, P.; Das, A.; Maji, B. Phosphorus containing porous organic polymers: Synthetic techniques and applications in organic synthesis and catalysis. Org. Biomol. Chem. 2021, 19, 4174–4192. [Google Scholar] [CrossRef]
- Bennedsen, N.R.; Kramer, S.; Kegnaes, S. A chiral porous organic polymer as a heterogeneous ligand for enantioselective Pd-catalyzed C(sp3)−H functionalization. Catal. Sci. Technol. 2020, 10, 7697–7705. [Google Scholar] [CrossRef]
- Wang, T.; Lyu, Y.; Xiong, K.; Wang, W.; Zheng, H.; Zhan, Z.; Jiang, Z.; Ding, Y. Chiral BINAP-based hierarchical porous polymers as platforms for efficient heterogeneous asymmetric catalysis. Chin. J. Catal. 2017, 38, 890–898. [Google Scholar] [CrossRef]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent triazine frameworks: Synthesis and applications. J. Mater. Chem. A 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Jeromenok, J.; Thomas, A.; Antonietti, M. Rational Extension of the Family of Layered, Covalent, Triazine-Based Frameworks with Regular Porosity. Adv. Mater. 2010, 22, 2202–2205. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; Liu, X.; Li, P.; Sun, L.; Yang, R.; Wang, S.; Wu, Z.-S.; Bao, X.; Deng, W.-Q. Conductive Microporous Covalent Triazine-Based Framework for High-Performance Electrochemical Capacitive Energy Storage. Angew. Chem. Int. Ed. 2018, 57, 7992–7996. [Google Scholar] [CrossRef] [PubMed]
- Iranpoor, N.; Panahi, F.; Roozbin, F.; Rahimi, S.; Haghighi, M.G. Immobilized copper iodide on a porous organic polymer bearing P,N-ligation sites: A highly efficient heterogeneous catalyst for C-O bond formation reaction. Mol. Catal. 2017, 438, 214–223. [Google Scholar] [CrossRef]
- Colombo, D.; Caminade, A.M.; Majoral, J.P. Functionalized macrocycles incorporating P-N and P-O bonds—Strategies of synthesis. Inorg. Chem. 1991, 30, 3365–3367. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem. Int. Edit. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Dinari, M.; Hatami, M. Novel N-riched crystalline covalent organic framework as a highly porous adsorbent for effective cadmium removal. J. Environ. Chem. Eng. 2019, 7, 102907. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Guo, W.; Ding, Y. Construction of novel nitrogen-rich covalent organic frameworks for highly efficient La(III) adsorption. Sep. Purif. Technol. 2022, 303, 122210. [Google Scholar] [CrossRef]
- Turrin, C.O.; Maraval, V.; Leclaire, J.; Dantras, E.; Lacabanne, C.; Caminade, A.M.; Majoral, J.P. Surface, core, and structure modifications of phosphorus-containing dendrimers. Influence on the thermal stability. Tetrahedron 2003, 59, 3965–3973. [Google Scholar] [CrossRef]
- Beraa, A.; Hajjaji, M.; Laurent, R.; Caminade, A.M. Dendrimers-containing organoclays: Characterisation and interaction with methylene blue. Appl. Clay Sci. 2017, 136, 142–151. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/FR/en/sds/aldrich/f1506?userType=anonymous (accessed on 22 January 2025).
- Available online: https://www.sigmaaldrich.com/FR/en/sds/aldrich/a97203?userType=anonymous (accessed on 22 January 2025).
- Available online: https://www.sigmaaldrich.com/FR/en/sds/aldrich/185515?userType=anonymous (accessed on 22 January 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menendez, C.; Coppel, Y.; Martin, B.; Caminade, A.-M. Thiophosphate-Based Covalent Organic Framework (COF) or Porous Organic Polymer (POP)? Macromol 2025, 5, 10. https://doi.org/10.3390/macromol5010010
Menendez C, Coppel Y, Martin B, Caminade A-M. Thiophosphate-Based Covalent Organic Framework (COF) or Porous Organic Polymer (POP)? Macromol. 2025; 5(1):10. https://doi.org/10.3390/macromol5010010
Chicago/Turabian StyleMenendez, Christophe, Yannick Coppel, Baptiste Martin, and Anne-Marie Caminade. 2025. "Thiophosphate-Based Covalent Organic Framework (COF) or Porous Organic Polymer (POP)?" Macromol 5, no. 1: 10. https://doi.org/10.3390/macromol5010010
APA StyleMenendez, C., Coppel, Y., Martin, B., & Caminade, A.-M. (2025). Thiophosphate-Based Covalent Organic Framework (COF) or Porous Organic Polymer (POP)? Macromol, 5(1), 10. https://doi.org/10.3390/macromol5010010







