Influence of High-Pressure Processing and Microbial Transglutaminase on the Properties of Pea Protein Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Processing
2.3. Soluble Protein
2.4. Sulfhydryl Groups
2.5. Surface Hydrophobicity
2.6. Shear Viscosity
2.7. Statistical Analysis
3. Results and Discussion
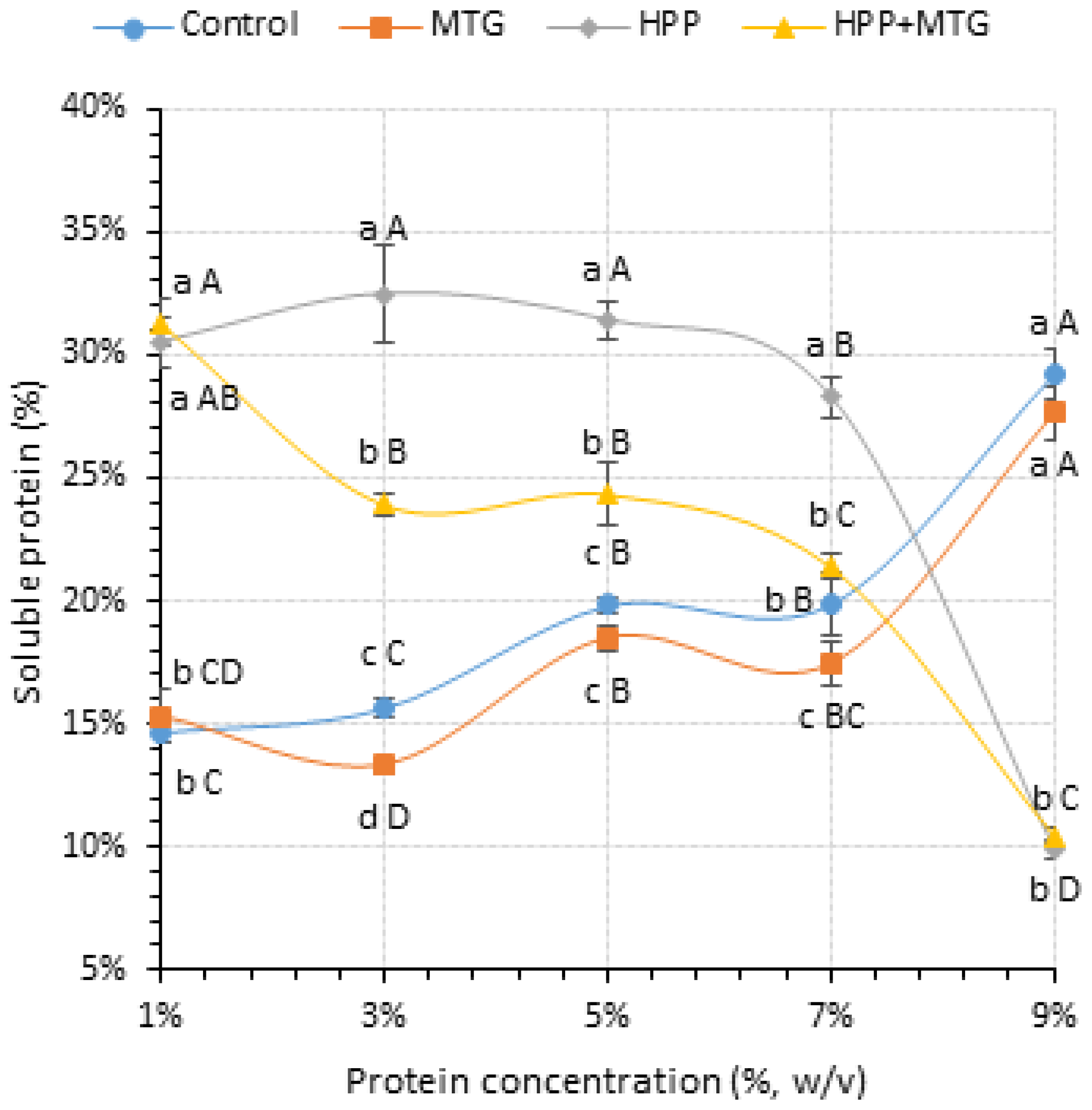
3.1. Effects of HPP and MTG on the Concentration of Dissolved Proteins
3.2. Effects of HPP and MTG on the Amount of Free Sulfhydryl Groups
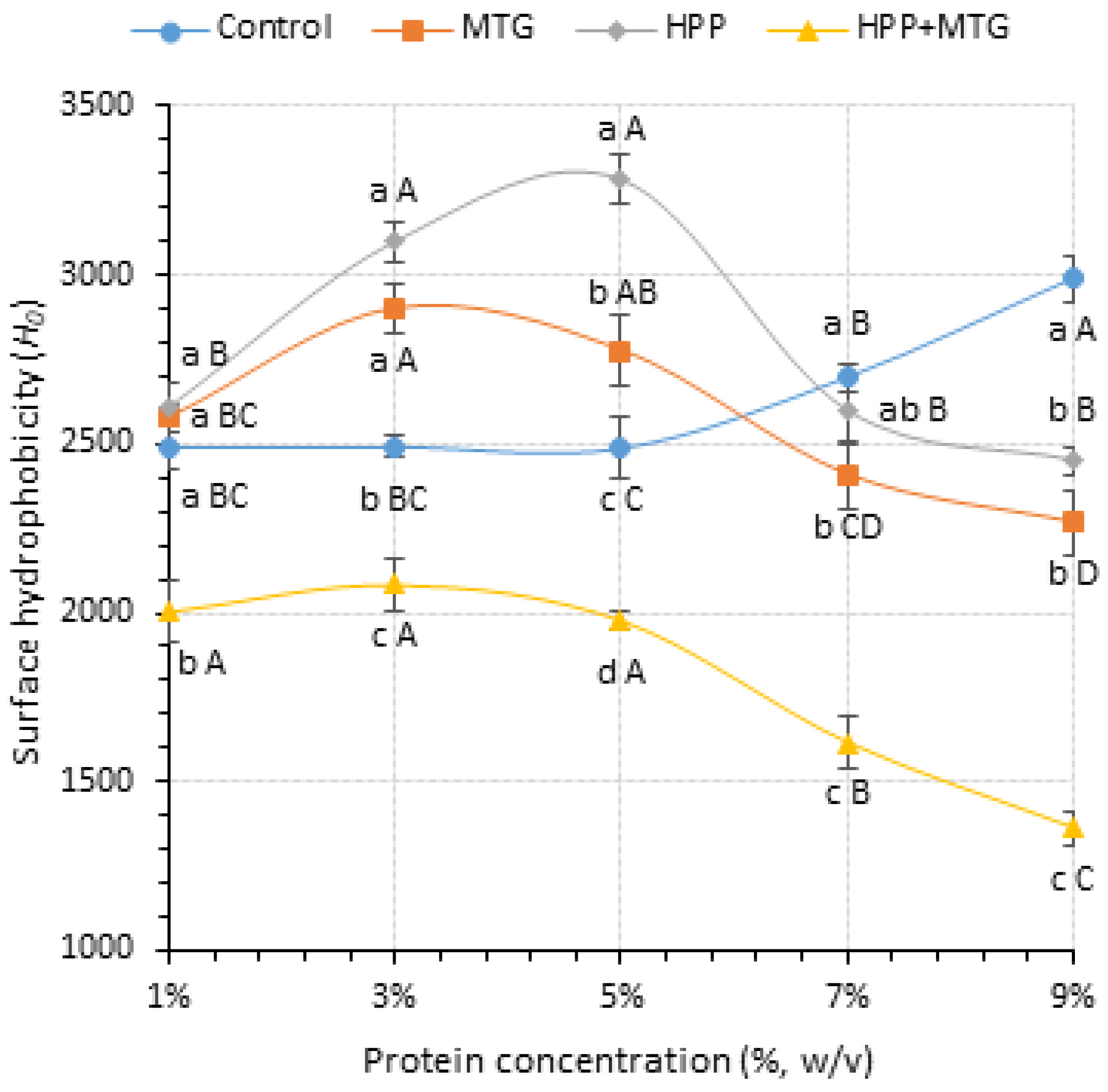
3.3. Effects of HPP and MTG on the Proteins’ Surface Hydrophobicity
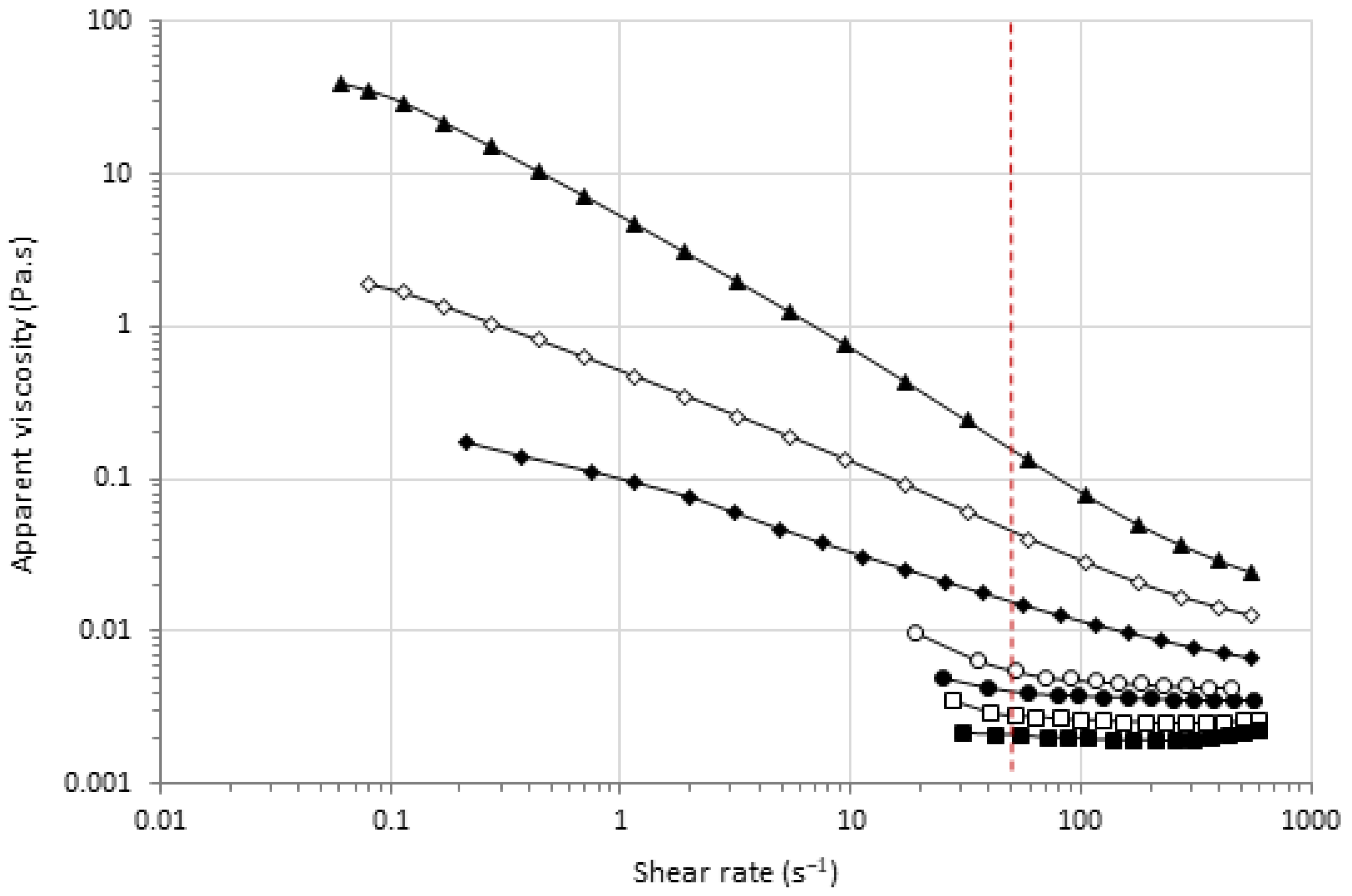
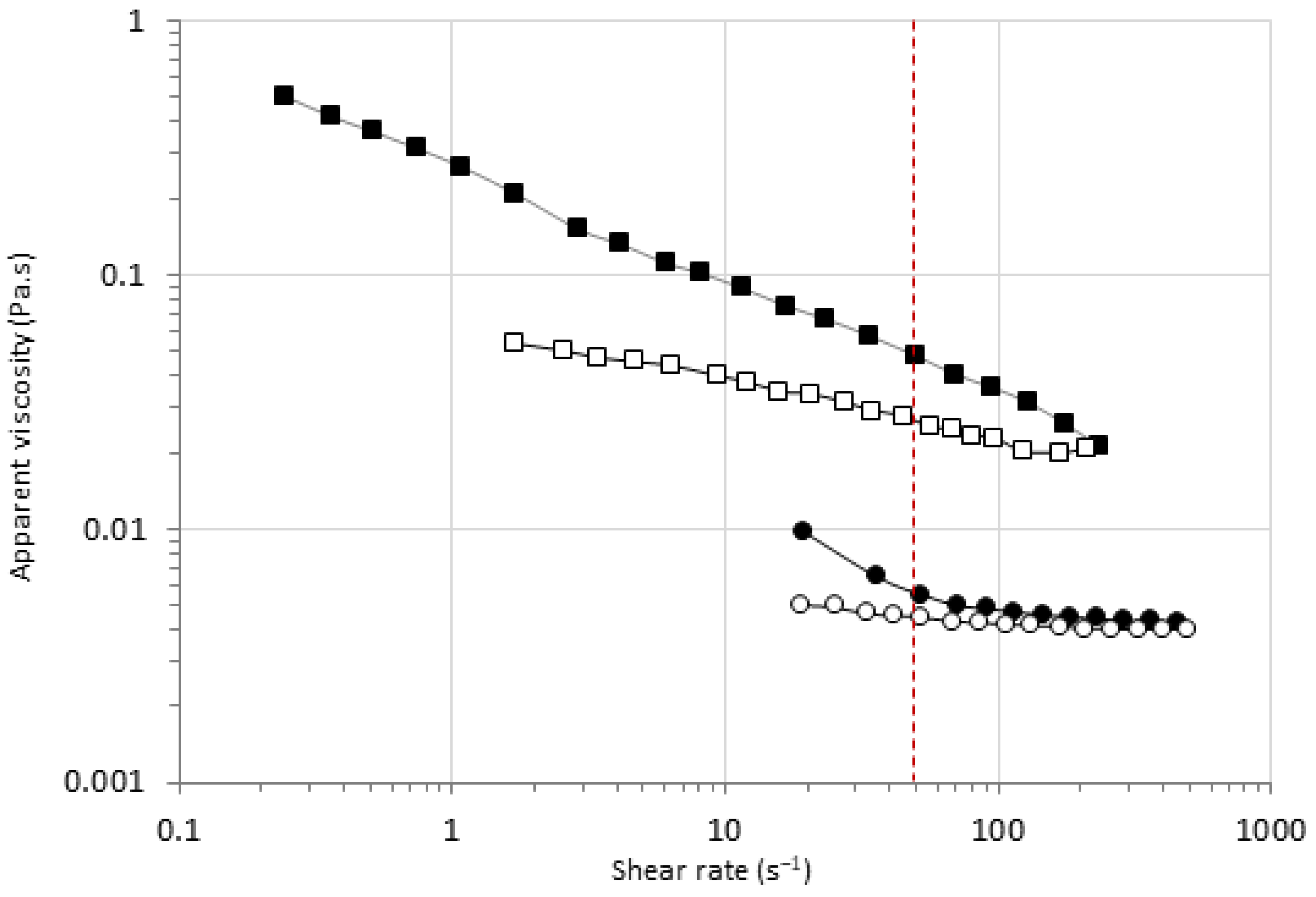
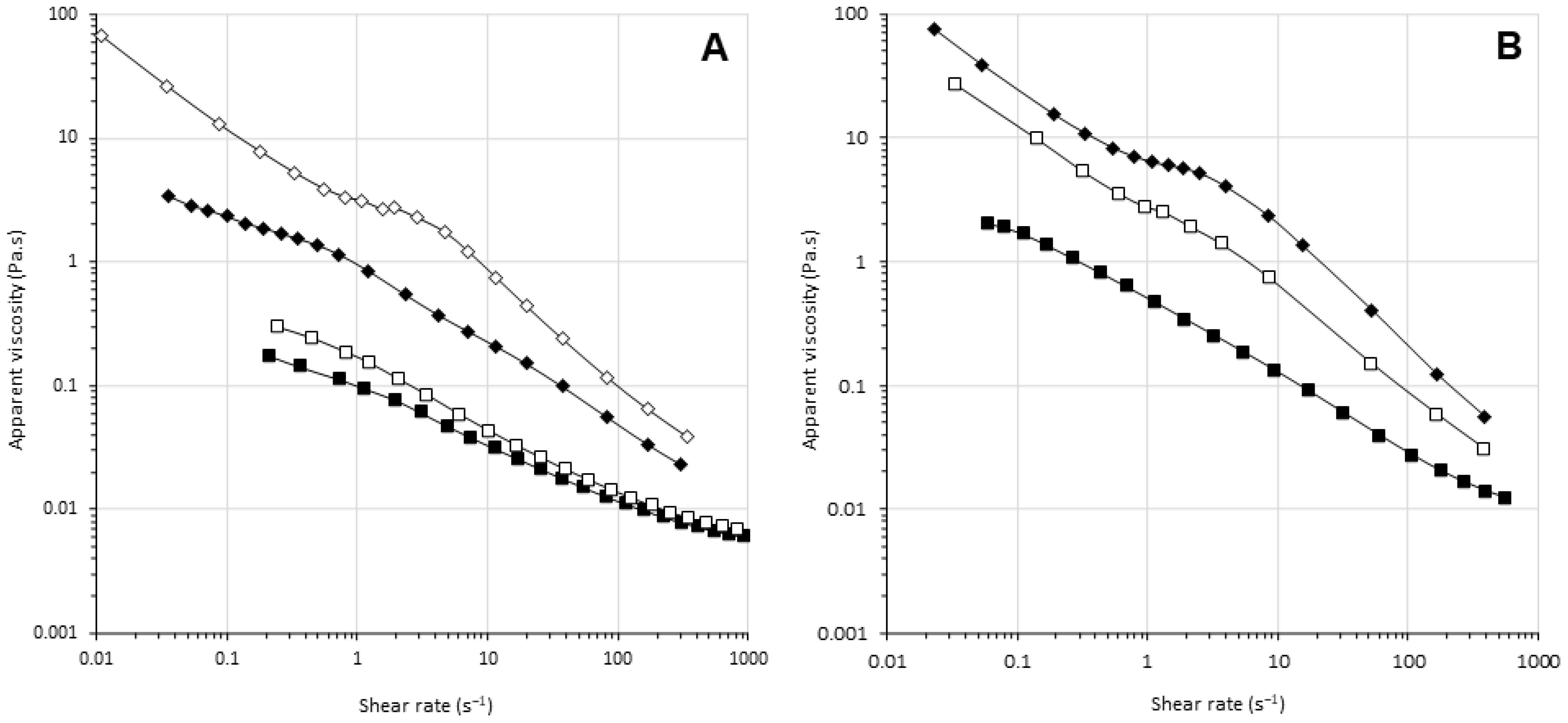
3.4. Effects of HPP and MTG on the Viscosity of Pea Protein Dispersions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent Food Proteins—Towards Sustainability, Health and Innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef]
- Day, L. Proteins from Land Plants—Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Zeeb, B.; McClements, D.J.; Weiss, J. Enzyme-Based Strategies for Structuring Foods for Improved Functionality. Annu. Rev. Food Sci. Technol. 2017, 8, 21–34. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring Structure and Technological Properties of Plant Proteins Using High Hydrostatic Pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef]
- Gaspar, A.L.C.; de Góes-Favoni, S.P. Action of Microbial Transglutaminase (MTGase) in the Modification of Food Proteins: A Review. Food Chem. 2015, 171C, 315–322. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and Application of High Pressure–Based Technologies in the Food Industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef]
- González-Angulo, M.; Serment-Moreno, V.; Queirós, R.P.; Tonello-Samson, C. Food and Beverage Commercial Applications of High Pressure Processing. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 39–73. [Google Scholar]
- Suthar, P.; Paul, A.A.; Bhatt, K.; Barthwal, R. High-Pressure Processing of Plant-Based Proteins. In Novel Plant Protein Processing; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-336979-0. [Google Scholar]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High Pressure Enhancement of Enzymes: A Review. Enzym. Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Moreira, S.A.; Ormando, P.; Pinto, C.A.; Queirós, R.P.; Saraiva, J.A. Chapter 6—High-Pressure Processing Associated with Other Technologies to Change Enzyme Activity. In Effect of High-Pressure Technologies on Enzymes; de Castro Leite Júnior, B.R., Tribst, A.A.L., Eds.; Foundations and Frontiers in Enzymology; Academic Press: Cambridge, MA, USA, 2023; pp. 141–168. ISBN 978-0-323-98386-0. [Google Scholar]
- Ando, H.; Adachi, M.; Umeda, K.; Matsuura, A.; Nonaka, M.; Uchio, R.; Tanaka, H.; Motoki, M. Purification and Characteristics of a Novel Transglutaminase Derived from Microorganisms. Agric. Biol. Chem. 1989, 53, 2613–2617. [Google Scholar] [CrossRef]
- Yokoyama, K.; Nio, N.; Kikuchi, Y. Properties and Applications of Microbial Transglutaminase. Appl. Microbiol. Biotechnol. 2004, 64, 447–454. [Google Scholar] [CrossRef]
- De Jong, G.H.A.; Koppelman, S.J. Transglutaminase Catalyzed Reactions: Impact on Food Applications. J. Food Sci. 2002, 67, 2798–2806. [Google Scholar] [CrossRef]
- Queirós, R.P.; Gouveia, S.; Saraiva, J.A.; Lopes-da-Silva, J.A. Impact of pH on the High-Pressure Inactivation of Microbial Transglutaminase. Food Res. Int. 2019, 115, 73–82. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Roohinejad, S.; George, S.; Barba, F.J.; Greiner, R.; Barbosa-Cánovas, G.V.; Mallikarjunan, K. Innovative Food Processing Technologies on the Transglutaminase Functionality in Protein-Based Food Products: Trends, Opportunities and Drawbacks. Trends Food Sci. Technol. 2018, 75, 194–205. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.J.; Nakai, S. Determination of SH- and SS-groups in some food proteins using Ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity Determined by a Fluorescence Probe Method and Its Correlation with Surface Properties of Proteins. Biochim. Biophys. Acta (BBA)-Protein Struct. 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Queirós, R.P.N.; Pinto, C.A.C.; Lopes-da-Silva, J.A.; Saraiva, J.M.A. Effects of High-Pressure and Transglutaminase, Individually and Simultaneously Applied, on Pea and Soy Protein Isolates. Sustain. Food Technol. 2023, 1, 696–708. [Google Scholar] [CrossRef]
- Yaputri, B.P.; Feyzi, S.; Ismail, B.P. Transglutaminase-Induced Polymerization of Pea and Chickpea Protein to Enhance Functionality. Gels 2024, 10, 11. [Google Scholar] [CrossRef]
- Queirós, R.; Ferreira, R.; Saraiva, J.A.; Lopes-da-Silva, J.A. High-Pressure Effects on Selected Properties of Pea and Soy Protein Isolates. Appl. Sci. 2023, 13, 2359. [Google Scholar] [CrossRef]
- Manassero, C.A.; Vaudagna, S.R.; Añón, M.C.; Speroni, F. High Hydrostatic Pressure Improves Protein Solubility and Dispersion Stability of Mineral-Added Soybean Protein Isolate. Food Hydrocoll. 2015, 43, 629–635. [Google Scholar] [CrossRef]
- Achouri, A.; Boye, J.I. Thermal Processing, Salt and High Pressure Treatment Effects on Molecular Structure and Antigenicity of Sesame Protein Isolate. Food Res. Int. 2013, 53, 240–251. [Google Scholar] [CrossRef]
- Yin, S.-W.; Tang, C.-H.; Wen, Q.-B.; Yang, X.-Q.; Li, L. Functional Properties and in Vitro Trypsin Digestibility of Red Kidney Bean (Phaseolus vulgaris L.) Protein Isolate: Effect of High-Pressure Treatment. Food Chem. 2008, 110, 938–945. [Google Scholar] [CrossRef]
- Condés, M.C.; Speroni, F.; Mauri, A.; Añón, M.C. Physicochemical and Structural Properties of Amaranth Protein Isolates Treated with High Pressure. Innov. Food Sci. Emerg. Technol. 2012, 14, 11–17. [Google Scholar] [CrossRef]
- Menéndez, O.M.P. Stability of Microbial Transglutaminase and Its Reactions with Individual Caseins under Atmospheric and High Pressure; Technische Universität Dresden: Dresden, Germany, 2006. [Google Scholar]
- Lauber, S.; Krause, I.; Klostermeyer, H.; Henle, T. Microbial Transglutaminase Crosslinks β-Casein and β-Lactoglobulin to Heterologous Oligomers under High Pressure. Eur. Food Res. Technol. 2003, 216, 15–17. [Google Scholar] [CrossRef]
- Bulaj, G. Formation of Disulfide Bonds in Proteins and Peptides. Biotechnol. Adv. 2005, 23, 87–92. [Google Scholar] [CrossRef]
- He, R.; He, H.-Y.; Chao, D.; Ju, X.; Aluko, R. Effects of High Pressure and Heat Treatments on Physicochemical and Gelation Properties of Rapeseed Protein Isolate. Food Bioprocess Technol. 2013, 7, 1344–1353. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, T.; Zhang, Y.; Ni, Y.; Li, Q. Optimization of Arachin Extraction from Defatted Peanut (Arachis hypogaea) Cakes and Effects of Ultra-High Pressure (UHP) Treatment on Physiochemical Properties of Arachin. Food Bioprod. Process. 2015, 95, 38–46. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, C.; Ye, J.; Chen, H.; Tao, R.; Cao, F. Effects of High Hydrostatic Pressure Treatment on Structural, Allergenicity, and Functional Properties of Proteins from Ginkgo Seeds. Innov. Food Sci. Emerg. Technol. 2016, 34, 187–195. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, T.; Zhang, M.; Richel, A. Effects of High Hydrostatic Pressure and Microbial Transglutaminase Treatment on Structure and Gelation Properties of Sweet Potato Protein. LWT—Food Sci. Technol. 2019, 115, 108436. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, M.; Sun, W.; Zhao, G.; Ren, J. Effects of Microfluidization Treatment and Transglutaminase Cross-Linking on Physicochemical, Functional, and Conformational Properties of Peanut Protein Isolate. J. Agric. Food Chem. 2011, 59, 8886–8894. [Google Scholar] [CrossRef]
- Agyare, K.K.; Xiong, Y.L.; Addo, K. Influence of Salt and pH on the Solubility and Structural Characteristics of Transglutaminase-Treated Wheat Gluten Hydrolysate. Food Chem. 2008, 107, 1131–1137. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of High-Pressure Treatment on Some Physicochemical and Functional Properties of Soy Protein Isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Khan, N.M.; Mu, T.-H.; Zhang, M.; Chen, J.-W. Effects of High Hydrostatic Pressure on the Physicochemical and Emulsifying Properties of Sweet Potato Protein. Int. J. Food Sci. Technol. 2013, 48, 1260–1268. [Google Scholar] [CrossRef]
- Lakshmanan, R.; de Lamballerie, M.; Jung, S. Effect of Soybean-to-Water Ratio and pH on Pressurized Soymilk Properties. J. Food Sci. 2006, 71, E384–E391. [Google Scholar] [CrossRef]
- Zhang, S.; Han, J.; Chen, L. Fabrication of Pea Protein Gels with Modulated Rheological Properties Using High Pressure Processing. Food Hydrocoll. 2023, 144, 109002. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queirós, R.P.; Moreira, N.; Pinto, C.A.; Fidalgo, L.G.; Saraiva, J.A.; Lopes-da-Silva, J.A. Influence of High-Pressure Processing and Microbial Transglutaminase on the Properties of Pea Protein Isolates. Macromol 2024, 4, 213-226. https://doi.org/10.3390/macromol4020011
Queirós RP, Moreira N, Pinto CA, Fidalgo LG, Saraiva JA, Lopes-da-Silva JA. Influence of High-Pressure Processing and Microbial Transglutaminase on the Properties of Pea Protein Isolates. Macromol. 2024; 4(2):213-226. https://doi.org/10.3390/macromol4020011
Chicago/Turabian StyleQueirós, Rui P., Nicole Moreira, Carlos A. Pinto, Liliana G. Fidalgo, Jorge A. Saraiva, and José A. Lopes-da-Silva. 2024. "Influence of High-Pressure Processing and Microbial Transglutaminase on the Properties of Pea Protein Isolates" Macromol 4, no. 2: 213-226. https://doi.org/10.3390/macromol4020011
APA StyleQueirós, R. P., Moreira, N., Pinto, C. A., Fidalgo, L. G., Saraiva, J. A., & Lopes-da-Silva, J. A. (2024). Influence of High-Pressure Processing and Microbial Transglutaminase on the Properties of Pea Protein Isolates. Macromol, 4(2), 213-226. https://doi.org/10.3390/macromol4020011








