Microwave-Assisted Chemical Purification and Ultrasonication for Extraction of Nano-Fibrillated Cellulose from Potato Peel Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
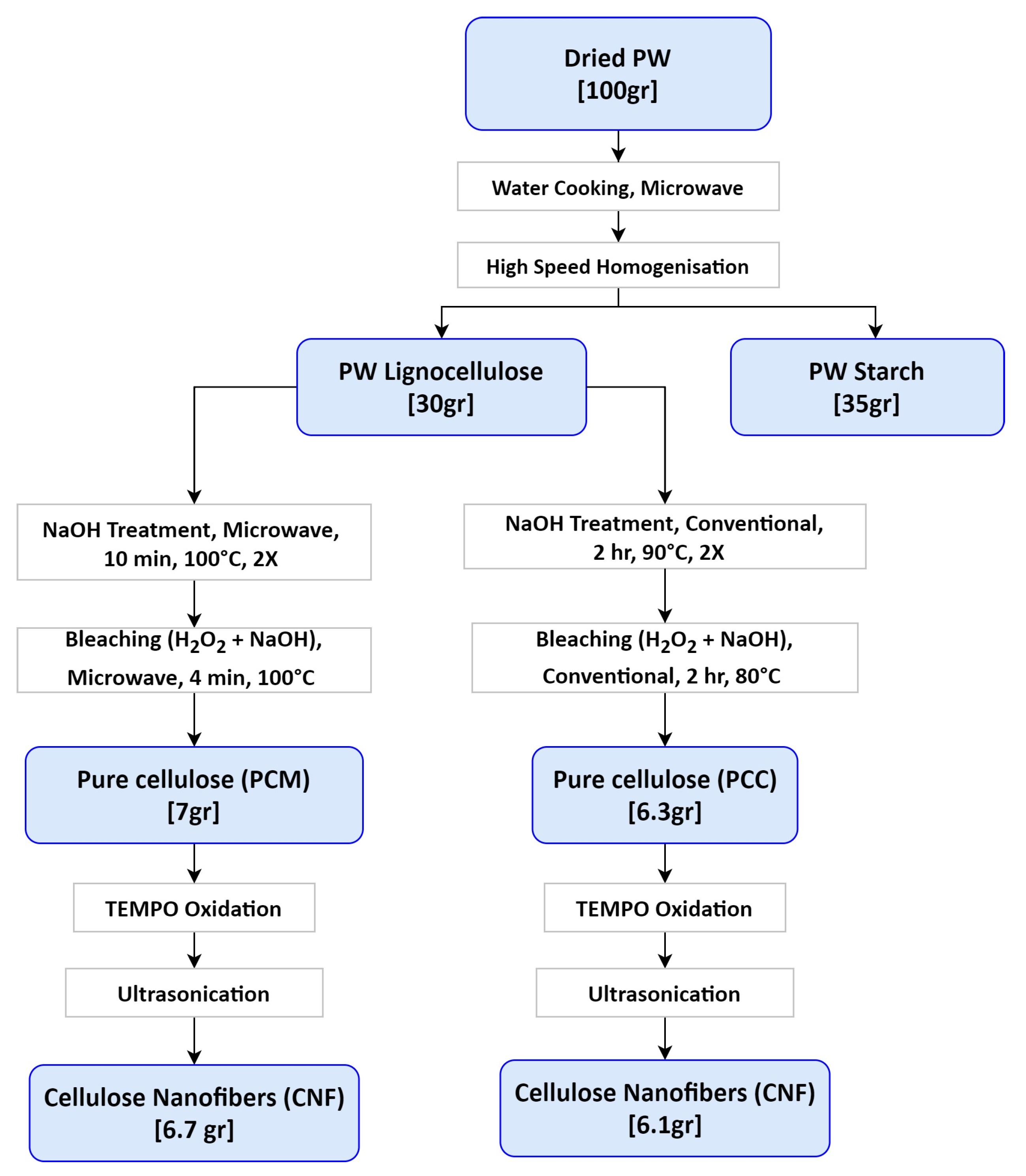
2.2. Extraction of Cellulose
2.3. Preparation of Cellulose
2.4. Characterization
2.4.1. Chemical Characterization
Hemicellulose (%) = NDF(%) − ADF (%)
Cellulose (%) = ADF (%) − ADL(%)
Lignin (%) = ADL (%)
2.4.2. FTIR
2.4.3. XRD
2.4.4. Transmission Electron Microscopy (TEM)
2.4.5. TGA
2.4.6. Dispersion Stability
3. Results and Discussion
3.1. Confirmation of Removal of Lignin and Hemicellulose
3.1.1. Fiber Chemical Analysis
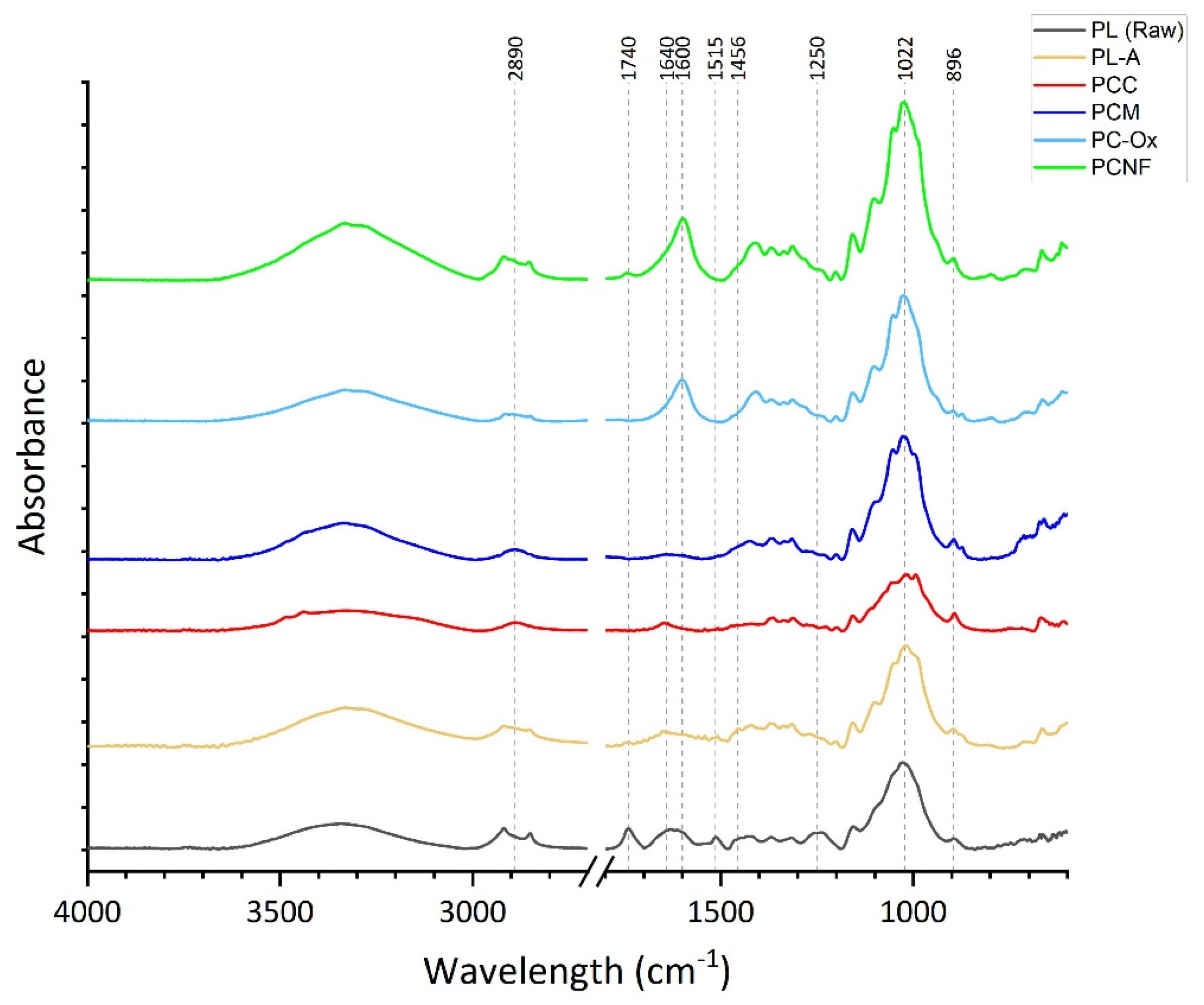
3.1.2. FTIR Spectroscopy
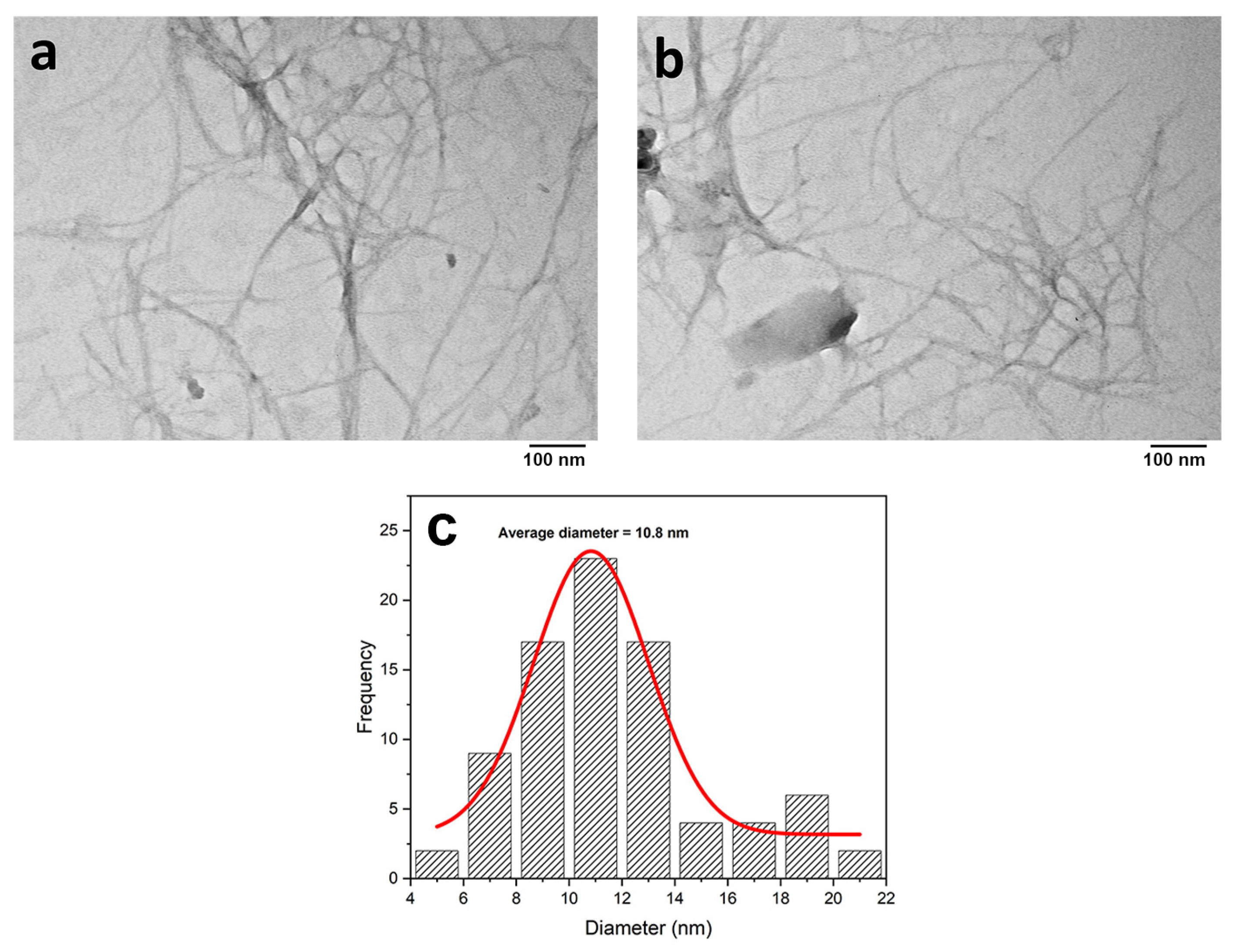
3.2. Structural Morphology
Morphological Observation of CNF (TEM)
3.3. Crystalline Properties (XRD)
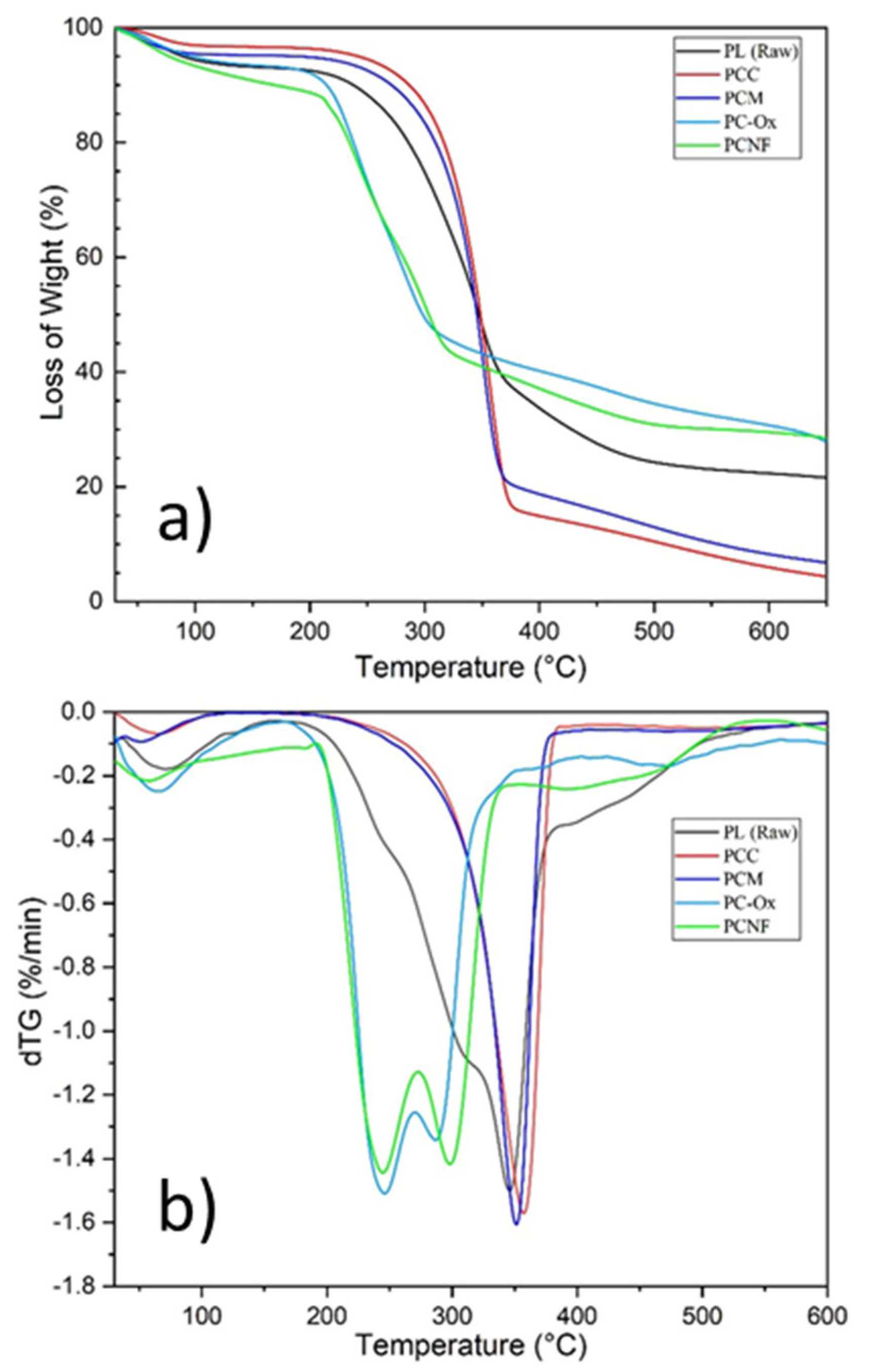
3.4. Thermal Properties of Nanofibers (TGA)
3.5. Dispersion Stability
4. Conclusions and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PW | Potato peel waste |
| PL | Potato peel lignocellulose |
| PCC | Potato peel cellulose-conventional extracted method |
| PCM | Potato peel cellulose-microwave extracted method |
| PC-Ox | Potato peel TEMPO-oxidized cellulose |
| PCNF | Potato peel cellulose nanofibers |
| CNF | Cellulose nanofibers |
| CNC | Cellulose nanocrystals |
| FTIR | Fourier-transform infrared spectroscopy |
| XRD | X-ray diffraction |
| TGA | Thermogravimetric analysis |
| TEM | Transmission electron microscopy |
References
- Cherubin, M.R.; Oliveira, D.M.d.S.; Feigl, B.J.; Pimentel, L.G.; Lisboa, I.P.; Gmach, M.R.; Varanda, L.L.; Morais, M.C.; Satiro, L.S.; Popin, G.V. Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Sci. Agric. 2018, 75, 255–272. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J. Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: A review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Ncobela, C.N.; Kanengoni, A.T.; Hlatini, V.A.; Thomas, R.S.; Chimonyo, M. A review of the utility of potato by-products as a feed resource for smallholder pig production. Anim. Feed Sci. Technol. 2017, 227, 107–117. [Google Scholar] [CrossRef]
- Sepelev, I.; Galoburda, R. Industrial potato peel waste application in food production: A review. Res. Rural Dev. 2015, 1, 130–136. [Google Scholar]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef]
- Othman, S.H.; Edwal, S.A.M.; Risyon, N.P.; Basha, R.K.; A TALIB, R. Water sorption and water permeability properties of edible film made from potato peel waste. Food Sci. Technol. 2017, 37, 63–70. [Google Scholar] [CrossRef]
- Liang, S.; McDonald, A.G. Anaerobic digestion of pre-fermented potato peel wastes for methane production. Waste Manag. 2015, 46, 197–200. [Google Scholar] [CrossRef]
- Martinez-Fernandez, J.S.; Seker, A.; Davaritouchaee, M.; Gu, X.; Chen, S. Recovering valuable bioactive compounds from potato peels with sequential hydrothermal extraction. Waste Biomass Valorization 2021, 12, 1465–1481. [Google Scholar] [CrossRef]
- Al-Weshahy, A.; Rao, A.V. Isolation and characterization of functional components from peel samples of six potatoes varieties growing in Ontario. Food Res. Int. 2009, 42, 1062–1066. [Google Scholar] [CrossRef]
- Islam, H.B.M.Z.; Susan, M.A.B.H.; Imran, A.B. Effects of plasticizers and clays on the physical, chemical, mechanical, thermal, and morphological properties of potato starch-based nanocomposite films. ACS Omega 2020, 5, 17543–17552. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- Gujral, H.; Sinhmar, A.; Nehra, M.; Nain, V.; Thory, R.; Pathera, A.K.; Chavan, P. Synthesis, characterization, and utilization of potato starch nanoparticles as a filler in nanocomposite films. Int. J. Biol. Macromol. 2021, 186, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S. Simple, economic, ecofriendly method to extract starch nanoparticles from potato peel waste for biological applications. Starch-Stärke 2021, 73, 2100055. [Google Scholar] [CrossRef]
- Raigond, P.; Raigond, B.; Kochhar, T.; Sood, A.; Singh, B. Conversion of Potato Starch and Peel Waste to High Value Nanocrystals. Potato Res. 2018, 61, 341–351. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef]
- Bruce, D.; Hobson, R.; Farrent, J.; Hepworth, D. High-performance composites from low-cost plant primary cell walls. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1486–1493. [Google Scholar] [CrossRef]
- Heidarian, P.; Behzad, T.; Sadeghi, M. Investigation of cross-linked PVA/starch biocomposites reinforced by cellulose nanofibrils isolated from aspen wood sawdust. Cellulose 2017, 24, 3323–3339. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Zeng, J.; Zeng, Z.; Cheng, Z.; Wang, Y.; Wang, X.; Wang, B.; Gao, W. Cellulose nanofibrils manufactured by various methods with application as paper strength additives. Sci. Rep. 2021, 11, 11918. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, N.; Munagala, M.; Rajkhowa, R.; Aallardyce, B.; Shastri, Y.; Agrawal, R. Nanocellulose: Resources, physio-chemical properties, current uses and future applications. Front. Nanotechnol. 2021, 3, 747329. [Google Scholar] [CrossRef]
- Yadav, C.; Saini, A.; Zhang, W.; You, X.; Chauhan, I.; Mohanty, P.; Li, X. Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting. Int. J. Biol. Macromol. 2021, 166, 1586–1616. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Radhakrishnan, P. Cellulose nanoparticles from agro-industrial waste for the development of active packaging. Appl. Surf. Sci. 2019, 484, 1274–1281. [Google Scholar] [CrossRef]
- Liu, X.; Sun, H.; Mu, T.; Fauconnier, M.L.; Li, M. Preparation of cellulose nanofibers from potato residues by ultrasonication combined with high-pressure homogenization. Food Chem. 2023, 413, 135675. [Google Scholar] [CrossRef]
- Impoolsup, T.; Chiewchan, N.; Devahastin, S. On the use of microwave pretreatment to assist zero-waste chemical-free production process of nanofibrillated cellulose from lime residue. Carbohydr. Polym. 2020, 230, 115630. [Google Scholar] [CrossRef]
- Sidana, A.; Yadav, S.K. Recent developments in lignocellulosic biomass pretreatment with a focus on eco-friendly, non-conventional methods. J. Clean. Prod. 2022, 335, 130286. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafan-Vidales, H.I.; Longoria, A.; Sebastian, P.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Tohamy, H.-A.S. Preparation and infrared study of cellulose based amphiphilic materials. Cellul. Chem. Technol. 2018, 52, 193–200. [Google Scholar]
- Jiang, J.; Wang, X. Adsorption of Hg (II) ions from aqueous solution by thiosemicarbazide-modified cellulose adsorbent. BioResources 2019, 14, 4670–4695. [Google Scholar] [CrossRef]
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr. Polym. 2018, 201, 329–339. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Abd Hamid, S.B. Preparation and characterization of nanocrystalline cellulose using ultrasonication combined with a microwave-assisted pretreatment process. BioResources 2016, 11, 3397–3415. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kandhola, G.; Djioleu, A.; Rajan, K.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.-W. Maximizing production of cellulose nanocrystals and nanofibers from pre-extracted loblolly pine kraft pulp: A response surface approach. Bioresour. Bioprocess. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Kasyapi, N.; Chaudhary, V.; Bhowmick, A.K. Bionanowhiskers from jute: Preparation and characterization. Carbohydr. Polym. 2013, 92, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Homogenous and transparent nanocellulosic films from carrot. Ind. Crops Prod. 2018, 118, 53–64. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. A combined homogenization-high intensity ultrasonication process for individualizaion of cellulose micro-nano fibers from rice straw. Cellulose 2019, 26, 5831–5849. [Google Scholar] [CrossRef]
- Hassan, M.L.; Mathew, A.P.; Hassan, E.A.; Oksman, K. Effect of pretreatment of bagasse pulp on properties of isolated nanofibers and nanopaper sheets. Wood Fiber Sci. 2010, 42, 362–376. [Google Scholar]
- Kim, U.-J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.; Deng, Y.; Ragauskas, A.J. Characterization of cellulose nanofibrillation by micro grinding. J. Nanoparticle Res. 2014, 16, 2349. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Effects of cellulose nanocrystals and cellulose nanofibers on the structure and properties of polyhydroxybutyrate nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef]
- González, K.; Retegi, A.; González, A.; Eceiza, A.; Gabilondo, N. Starch and cellulose nanocrystals together into thermoplastic starch bionanocomposites. Carbohydr. Polym. 2015, 117, 83–90. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Soni, B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef]
- Pacaphol, K.; Aht-Ong, D. Preparation of hemp nanofibers from agricultural waste by mechanical defibrillation in water. J. Clean. Prod. 2017, 142, 1283–1295. [Google Scholar] [CrossRef]
- Masruchin, N.; Kurniawan, Y.; Kusumah, S.; Amanda, P.; Suryanegara, L.; Nuryawan, A. TEMPO-mediated oxidation cellulose pulp modified with Monosodium Glutamate (MSG). Proc. IOP Conf. Ser. Earth Environ. Sci. 2019, 374, 012010. [Google Scholar] [CrossRef]
- Rohaizu, R.; Wanrosli, W. Sono-assisted TEMPO oxidation of oil palm lignocellulosic biomass for isolation of nanocrystalline cellulose. Ultrason. Sonochemistry 2017, 34, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Yamato, K.; Yoshida, Y.; Kumamoto, Y.; Isogai, A. Surface modification of TEMPO-oxidized cellulose nanofibers, and properties of their acrylate and epoxy resin composite films. Cellulose 2022, 29, 2839–2853. [Google Scholar] [CrossRef]


| Cellulose | Hemicellulose | Lignin | Extractible | |
|---|---|---|---|---|
| PL (Raw) | 32.27 ± 0.3 | 42.23 ± 1.7 | 19.08 ± 0.34 | 5.12 ± 1.6 |
| PCC | 87.36 ± 0.36 | 4.09 ± 0.22 | 0.43 ± 0.22 | 6.67 ± 0.2 |
| PCM | 84.44 ± 0.77 | 5.2 ± 0.61 | 1.48 ± 0.27 | 7.13 ± 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi-Shapourabadi, M.; Elkoun, S.; Robert, M. Microwave-Assisted Chemical Purification and Ultrasonication for Extraction of Nano-Fibrillated Cellulose from Potato Peel Waste. Macromol 2023, 3, 766-781. https://doi.org/10.3390/macromol3040044
Sadeghi-Shapourabadi M, Elkoun S, Robert M. Microwave-Assisted Chemical Purification and Ultrasonication for Extraction of Nano-Fibrillated Cellulose from Potato Peel Waste. Macromol. 2023; 3(4):766-781. https://doi.org/10.3390/macromol3040044
Chicago/Turabian StyleSadeghi-Shapourabadi, Mohsen, Said Elkoun, and Mathieu Robert. 2023. "Microwave-Assisted Chemical Purification and Ultrasonication for Extraction of Nano-Fibrillated Cellulose from Potato Peel Waste" Macromol 3, no. 4: 766-781. https://doi.org/10.3390/macromol3040044
APA StyleSadeghi-Shapourabadi, M., Elkoun, S., & Robert, M. (2023). Microwave-Assisted Chemical Purification and Ultrasonication for Extraction of Nano-Fibrillated Cellulose from Potato Peel Waste. Macromol, 3(4), 766-781. https://doi.org/10.3390/macromol3040044








