Magnetic Nanoparticles for Medical Applications: Updated Review
Abstract
:1. Introduction
2. Synthesis of Magnetic Nanoparticles
2.1. Precipitation and Coprecipitation
2.2. Thermal Decomposition
2.3. Microemulsion
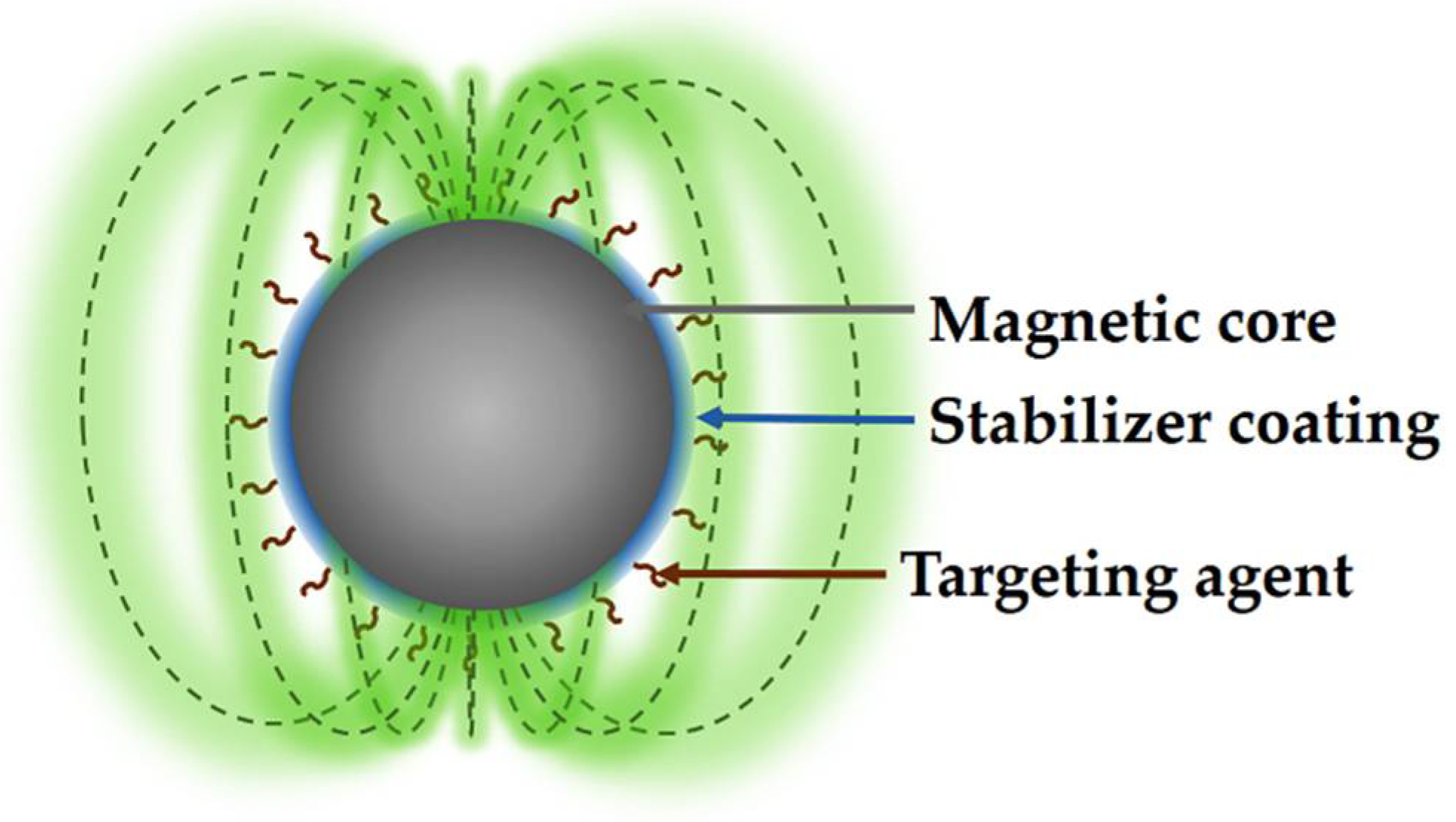
2.4. Coatings of MNPs
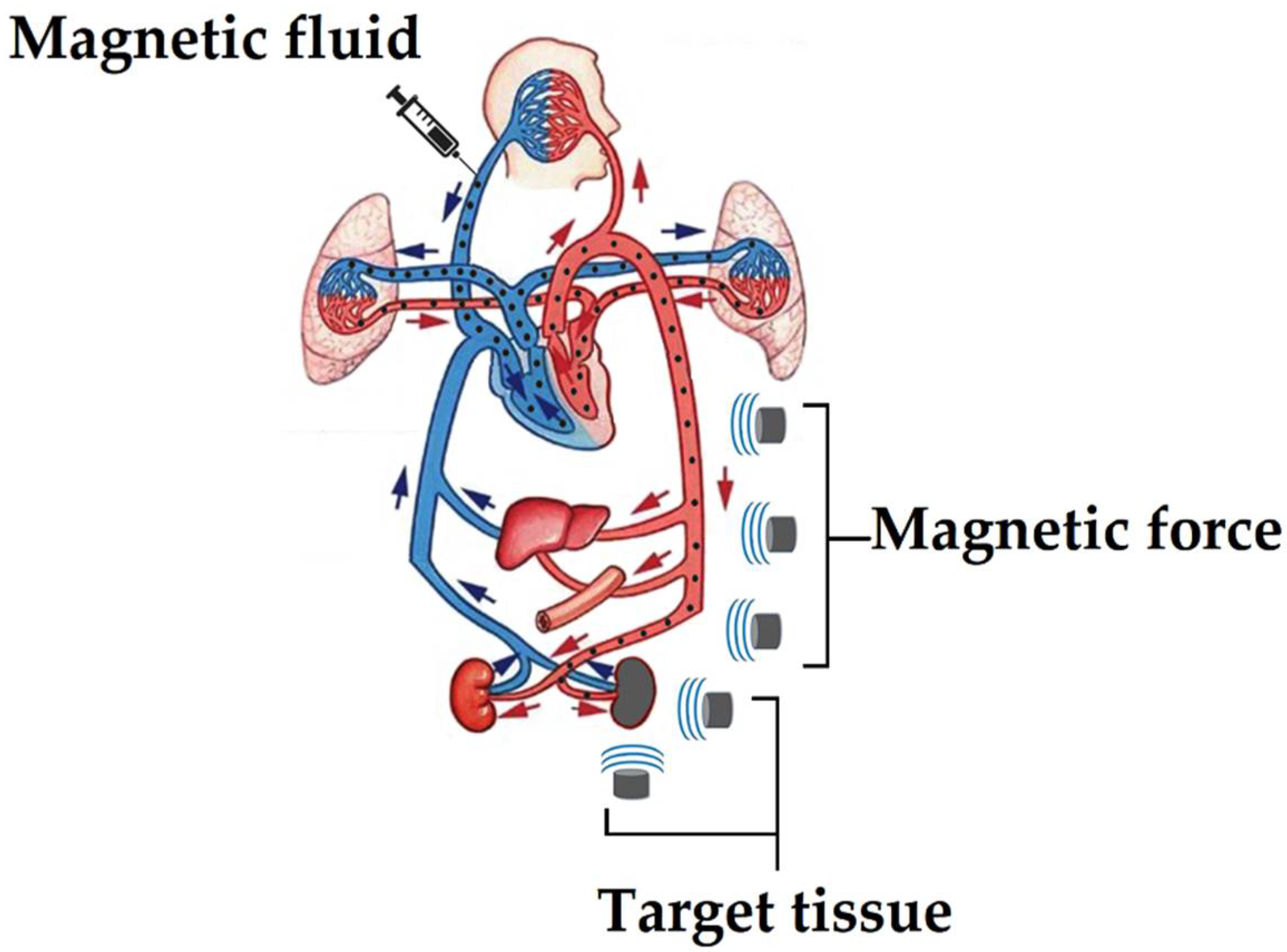
3. Magnetic Drug-Delivery System (MDDS)
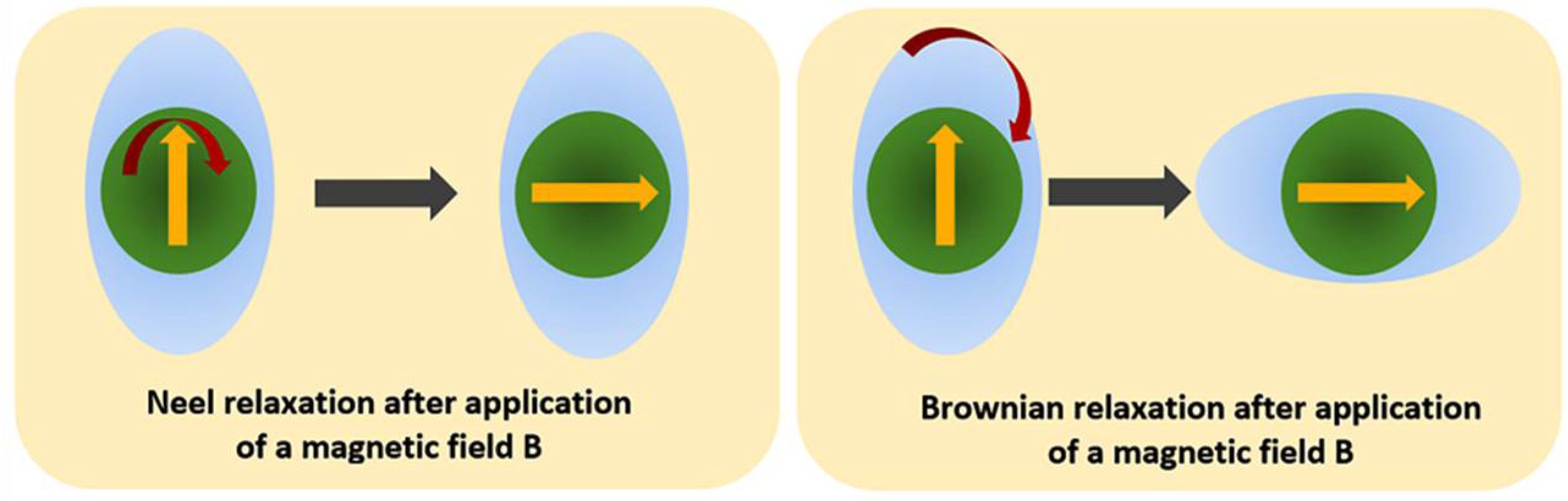
4. Magnetic Hyperthermia
- Local hyperthermia: consists of an increase in temperature in a specific area of the tissue.
- Regional hyperthermia: an increased temperature is applied over large tissue areas, such as an organ or limb.
- Whole-body hyperthermia: is mainly used to treat pathogenic tissue spread throughout the body, such as metastatic cancer.
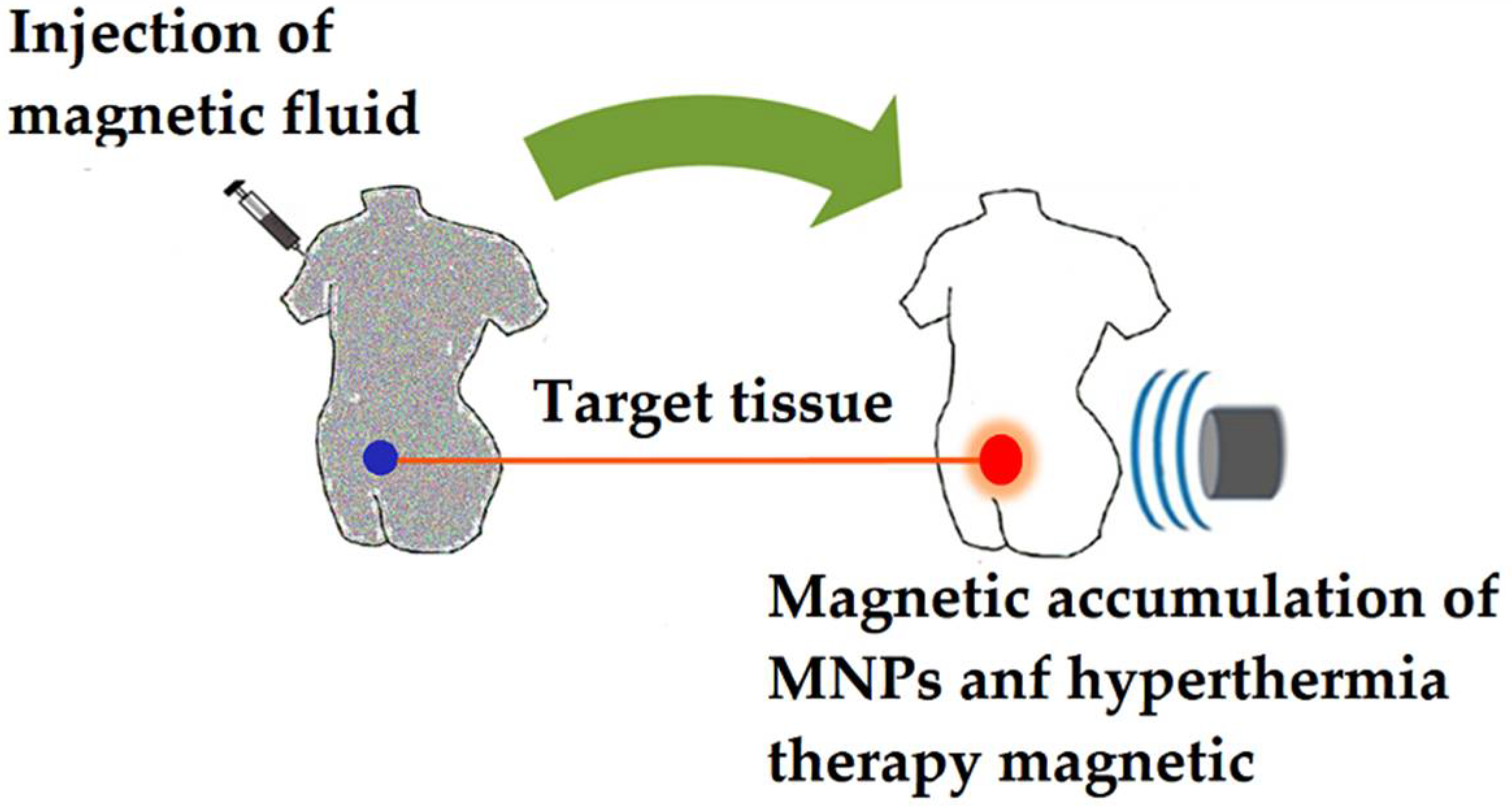
- Direct injection: a magnetic fluid is injected directly into the cancerous tissue, with the NPs localizing mainly in the interstitial space. Thus, heat is mainly generated outside the cells when the magnetic pulses are applied. In addition, direct injection of functionalized MNPs with cancer-specific antibodies can be applied [102], decreasing uptake by normal cells (differential endocytosis) and increasing retention of MNPs in cancer tissue.
- In situ implant formation: this strategy uses in situ gelation systems, which are injected forming gels directly in the cancerous tissue, trapping the MNPs and consequently improving the focal concentration [103].
- Active targeting: the method consists of the surface functionalization of the MNPs with specific antibodies against cancer cells [95], which are injected into the bloodstream, and subsequently accumulated in the desired area through the attraction exerted by an external magnetic field, where the functionalized MNPs end up binding to cancer cells through cancer-cell-specific receptors and antibodies present on MNPs. However, despite these efforts in the active targeting of MNPs with antibodies, excellent results have not been achieved to date in the accumulation of MNPs in the damaged area, preventing successful hyperthermic treatment.
5. MNPs in MRI
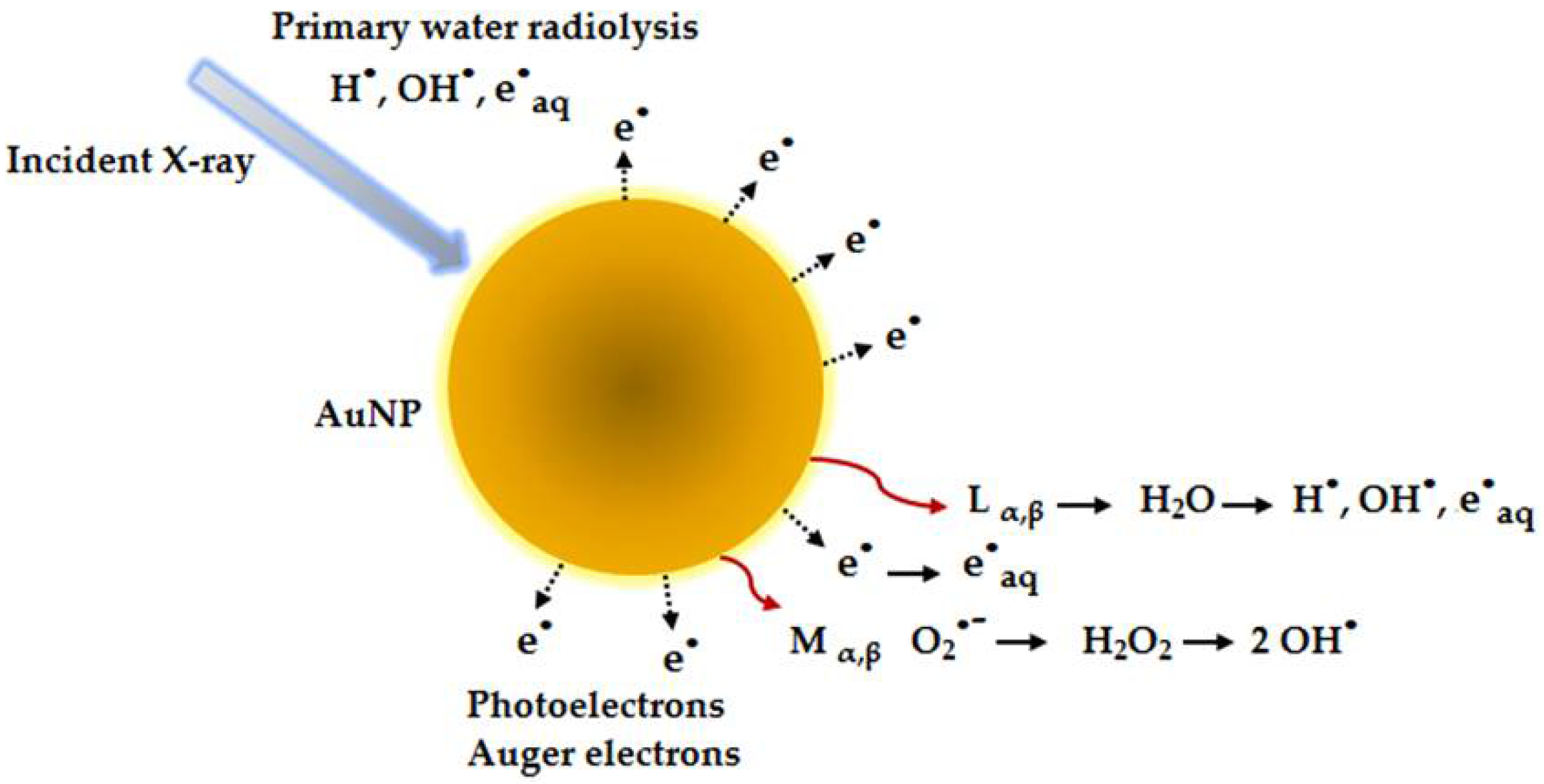
6. NPs in Radioterapy Treatment
7. MNPs in Magnetoencephalography (MEG)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Högemann, D.; Ntziachristos, V.; Josephson, L.; Weissleder, R. High throughput magnetic resonance imaging for evaluating targeted nanoparticle probes. Bioconjug. Chem. 2002, 13, 116–121. [Google Scholar] [CrossRef]
- Fortin, J.P.; Gazeau, F.; Wilhelm, C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2008, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Billotey, C.; Wilhelm, C.; Devaud, M.; Bacri, J.C.; Bittoun, J.; Gazeau, F. Cell internalization of anionic maghemite nanoparticles: Quantitative effect on magnetic resonance imaging. Magn. Reson. Med. 2003, 49, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Moroz, P.; Jones, S.K.; Gray, B.N. Magnetically mediated hyperthermia: Current status and future directions. Int. J. Hyperth. 2002, 18, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Dürr, S.; Janko, C.; Lyer, S.; Tripal, P.; Schwarz, M.; Zaloga, J.; Tietze, R.; Alexiou, C. Magnetic nanoparticles for cancer therapy. Nanotechnol. Rev. 2013, 2, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Chin, A.B.; Yaacob, I.I. Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart’s procedure. J. Mater. Process. Technol. 2007, 191, 235–237. [Google Scholar] [CrossRef]
- Wan, J.; Chen, X.; Wang, Z.; Yang, X.; Qian, Y. A soft-template-assisted hydrothermal approach to single-crystal Fe3O4 nanorods. J. Cryst. Growth 2005, 276, 571–576. [Google Scholar] [CrossRef]
- Kimata, M.; Nakagawa, D.; Hasegawa, M. Preparation of monodisperse magnetic particles by hydrolysis of iron alkoxide. Powder Technol. 2003, 132, 112–118. [Google Scholar] [CrossRef]
- Suslick, K.S.; Fang, M.; Hyeon, T. Sonochemical synthesis of iron colloids. J. Am. Chem. Soc. 1996, 118, 11960–11961. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal decomposition synthesis of iron oxide nanoparticles with diminished magnetic dead layer by controlled addition of oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Zarrintaj, P.; Ganjali, M.R.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Ghaffari, M.; Saeb, M.R. Curing epoxy with electrochemically synthesized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105245. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Muhammed, M.; Zagorodni, A.A. Novel flow injection synthesis of iron oxide nanoparticles with narrow size distribution. Chem. Eng. Sci. 2006, 61, 4625–4633. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.G.; Meléndez-Ortiz, H.I.; Morfín-Gutierrez, A.; Luna-Straffon, M.A.; Bucio, E. Stimuli-responsive nanomaterials for drug delivery. In Characterization and Biology of Nanomaterials for Drug Delivery. Nanoscience and Nanotechnology in Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–424. [Google Scholar]
- Basak, S.; Chen, D.R.; Biswas, P. Electrospray of ionic precursor solutions to synthesize iron oxide nanoparticles: Modified scaling law. Chem. Eng. Sci. 2007, 62, 1263–1268. [Google Scholar] [CrossRef]
- Andrade, Â.L.; Valente, M.A.; Ferreira, J.M.F.; Fabris, J.D. Preparation of size-controlled nanoparticles of magnetite. J. Magn. Magn. Mater. 2012, 324, 1753–1757. [Google Scholar] [CrossRef] [Green Version]
- Naous, M.; García-Gómez, D.; López-Jiménez, F.J.; Bouanani, F.; Lunar, M.L.; Rubio, S. Multicore magnetic nanoparticles coated with oligomeric micelles: Characterization and potential for the extraction of contaminants over a wide polarity range. Anal. Chem. 2017, 89, 1353–1361. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Bucio, E. Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: A short review. Radiat. Phys. Chem. 2020, 169, 107962. [Google Scholar] [CrossRef]
- Duong, G.V.; Turtelli, R.S.; Nunes, W.C.; Schafler, E.; Hanh, N.; Grössinger, R.; Knobel, M. Ultrafine Co1-xZnxFe2O4 particles synthesized by hydrolysis: Effect of thermal treatment and its relationship with magnetic properties. J. Non. Cryst. Solids 2007, 353, 805–807. [Google Scholar] [CrossRef]
- Rossi, L.M.; Silva, F.P.; Vono, L.L.R.; Kiyohara, P.K.; Duarte, E.L.; Itri, R.; Landers, R.; Machado, G. Superparamagnetic nanoparticle-supported palladium: A highly stable magnetically recoverable and reusable catalyst for hydrogenation reactions. Green Chem. 2007, 9, 379. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, P.S.; Yadav, M.S.; Pant, R.P. Effect of Gd3+ ion distribution on structural and magnetic properties in nano-sized Mn–Zn ferrite particles. Ceram. Int. 2015, 41, 1297–1302. [Google Scholar] [CrossRef]
- De Silva, C.R.; Smith, S.; Shim, I.; Pyun, J.; Gutu, T.; Jiao, J.; Zheng, Z. Lanthanide(III)-doped magnetite nanoparticles. J. Am. Chem. Soc. 2009, 131, 6336–6337. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, Y.; Miyaji, F.; Kokubo, T.; Ohura, K.; Nakamura, T. Bioactivity of ferrimagnetic glass-ceramics in the system FeO-Fe2O3-CaO-SiO2. Biomaterials 1997, 18, 1277–1284. [Google Scholar] [CrossRef]
- Hariani, P.L.; Faizal, M.; Setiabudidaya, D. Synthesis and properties of Fe3O4 nanoparticles by Co-precipitation method to removal procion dye. Int. J. Environ. Sci. Dev. 2013, 4, 336–340. [Google Scholar] [CrossRef]
- Samad, A.; Beg, S.; Nazish, I. Liposomal Delivery Systems: Advances and Challenges; Samad, A., Beg, S., Nazish, I., Eds.; Future Science Ltd.: London, UK; Panacea Biotec Ltd.: New Delhi, India, 2015; Volume 1, ISBN 978-1-910419-05-2. [Google Scholar]
- Sivakumar, M.; Kanagesan, S.; Chinnaraj, K.; Suresh Babu, R.; Nithiyanantham, S. Synthesis, characterization and effects of citric acid and PVA on magnetic properties of CoFe2O4. J. Inorg. Organomet. Polym. Mater. 2013, 23, 439–445. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.E.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Jeraal, M.I.; Roberts, K.J.; McRobbie, I.; Harbottle, D. Assessment of the thermal degradation of sodium lauroyl isethionate using predictive isoconversional kinetics and a temperature-resolved analysis of evolved gases. Ind. Eng. Chem. Res. 2019, 58, 8112–8122. [Google Scholar] [CrossRef] [Green Version]
- Stojanovic, B.; Dzunuzovic, A.; Ilic, N. Review of methods for the preparation of magnetic metal oxides. In Magnetic, Ferroelectric, and Multiferroic Metal Oxides; Stojanovic, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 333–359. [Google Scholar]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M=Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Rogach, A.L.; Kornowski, A.; Haase, M.; Weller, H. Colloidal synthesis and self-assembly of CoPt3 nanocrystals. J. Am. Chem. Soc. 2002, 124, 11480–11485. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef]
- Yamada, Y.; Suzuki, T.; Abarra, E.N. Magnetic properties of electron beam evaporated CoPt alloy thin films. IEEE Trans. Magn. 1998, 34, 343–345. [Google Scholar] [CrossRef]
- Jana, N.R.; Chen, Y.; Peng, X. Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach. Chem. Mater. 2004, 16, 3931–3935. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Q.; Gao, M. Preparation of water-soluble magnetite nanocrystals from hydrated ferric salts in 2-pyrrolidone: Mechanism leading to Fe3O4. Angew. Chemie Int. Ed. 2005, 44, 123–126. [Google Scholar] [CrossRef]
- Hu, F.; Wei, L.; Zhou, Z.; Ran, Y.; Li, Z.; Gao, M. Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Adv. Mater. 2006, 18, 2553–2556. [Google Scholar] [CrossRef]
- Butter, K.; Philipse, A.P.; Vroege, G.J. Synthesis and properties of iron ferrofluids. J. Magn. Magn. Mater. 2002, 252, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.K.; Moulik, S.P. Uses and applications of microemulsions Uses and applications of microemulsions. Curr. Sci. 2001, 80, 990–1001. [Google Scholar]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 2000, 104, 1143–1145. [Google Scholar] [CrossRef]
- Moumen, N.; Pileni, M.P. Control of the size of cobalt ferrite magnetic fluid. J. Phys. Chem. 1996, 100, 1867–1873. [Google Scholar] [CrossRef]
- Poornima Vijayan, P.; Somadas Radhamany, A.; Ereath Beeran, A.; Jouyandeh, M.; Reza Saeb, M. Magnetic nanoparticles-based coatings. In Nanotechnology in the Automotive Industry; Song, H., Nguyen, T.A., Yasin, G., Singh, N.B., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 317–343. [Google Scholar]
- Lee, H.; Lee, E.; Kim, D.K.; Jang, N.K.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J. Am. Chem. Soc. 2006, 128, 7383–7389. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Aitchison, G.; Curtis, A.S.G. Cell response to dextran-derivatised iron oxide nanoparticles post internalisation. Biomaterials 2004, 25, 5405–5413. [Google Scholar] [CrossRef]
- Jodin, L.; Dupuis, A.C.; Rouvière, E.; Reiss, P. Influence of the catalyst type on the growth of carbon nanotubes via methane chemical vapor deposition. J. Phys. Chem. B 2006, 110, 7328–7333. [Google Scholar] [CrossRef] [PubMed]
- Nitin, N.; LaConte, L.E.W.; Zurkiya, O.; Hu, X.; Bao, G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. JBIC J. Biol. Inorg. Chem. 2004, 9, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Mi, K.Y.; Park, S.; Moon, S.; Jung, J.M.; Yong, Y.J.; Kang, H.W.; Jon, S. Thermally cross-linked superparamagnetic iron oxide nanoparticles: Synthesis and application as a dual imaging probe for cancer in vivo. J. Am. Chem. Soc. 2007, 129, 12739–12745. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Imai, Y.; Nakamura, T.; Yamasaki, Y.; Sekino, M.; Ueno, S.; Hanaoka, K.; Kikuchi, K.; Nagano, T.; Kaneko, E.; et al. Iron hydroxide nanoparticles coated with poly(ethylene glycol)-poly(aspartic acid) block copolymer as novel magnetic resonance contrast agents for in vivo cancer imaging. Colloids Surf. B Biointerfaces 2007, 56, 174–181. [Google Scholar] [CrossRef]
- LaConte, L.E.W.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O’Connor, C.J.; Hu, X.; Bao, G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J. Magn. Reson. Imaging 2007, 26, 1634–1641. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [Green Version]
- Lartigue, L.; Coupeau, M.; Lesault, M. Luminophore and magnetic multicore nanoassemblies for dual-mode MRI and fluorescence imaging. Nanomaterials 2019, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Liu, C.; Yuan, Y.; Tao, X.; Yang, F.; Shan, X.; Zhou, H.; Xu, F. Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials 2009, 30, 2340–2348. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Wang, L.; Wang, M.; Gao, F. One-pot synthesis of water-soluble superparamagnetic iron oxide nanoparticles and their MRI contrast effects in the mouse brains. Mater. Sci. Eng. C 2015, 48, 416–423. [Google Scholar] [CrossRef]
- Wiseman, J.W.; Goddard, C.A.; McLelland, D.; Colledge, W.H. A comparison of linear and branched polyethylenimine (PEI) with DCChol/DOPE liposomes for gene delivery to epithelial cells in vitro and in vivo. Gene Ther. 2003, 10, 1654–1662. [Google Scholar] [CrossRef]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Karimi Zade, A.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef] [Green Version]
- Vicennati, P.; Giuliano, A.; Ortaggi, G.; Masotti, A. Polyethylenimine in medicinal chemistry. Curr. Med. Chem. 2008, 15, 2826–2839. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Li, M.; Xia, N.; Huang, Q.; Do, H.; Liu, Y.N.; Zhou, F. Carboxymethylated dextran-coated magnetic iron oxide nanoparticles for regenerable bioseparation. J. Nanosci. Nanotechnol. 2011, 11, 10187–10192. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Thorat, N.D.; Phadatare, M.R.; Sathish, C.I.; Dhawale, D.S.; Pawar, S.H. Polyvinyl alcohol functionalized cobalt ferrite nanoparticles for biomedical applications. Appl. Surf. Sci. 2013, 264, 598–604. [Google Scholar] [CrossRef]
- Vu-Quang, H.; Muthiah, M.; Lee, H.J.; Kim, Y.K.; Rhee, J.H.; Lee, J.H.; Cho, C.S.; Choi, Y.J.; Jeong, Y.Y.; Park, I.K. Immune cell-specific delivery of beta-glucan-coated iron oxide nanoparticles for diagnosing liver metastasis by MR imaging. Carbohydr. Polym. 2012, 87, 1159–1168. [Google Scholar] [CrossRef]
- Patel, A.; Asik, D.; Snyder, E.M.; Dilillo, A.E.; Cullen, P.J.; Morrow, J.R. Binding and release of FeIII complexes from glucan particles for the delivery of T1 MRI contrast agents. ChemMedChem 2020, 15, 1050–1057. [Google Scholar] [CrossRef]
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan particles for macrophage argeted delivery of nanoparticles. J. Drug Deliv. 2012, 2012, 143524. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Son, J.; Seo, Y.; Jo, Y.; Lee, K.; Lee, D.; Khan, M.S.; Chavan, S.; Park, C.; Sharma, A.; et al. Functional silica nanoparticles conjugated with beta-glucan to deliver anti-tuberculosis drug molecules. J. Ind. Eng. Chem. 2018, 58, 376–385. [Google Scholar] [CrossRef]
- Su, Y.; Chen, L.; Yang, F.; Cheung, P.C.K. Beta-d-glucan-based drug delivery system and its potential application in targeting tumor associated macrophages. Carbohydr. Polym. 2021, 253, 117258. [Google Scholar] [CrossRef]
- Langereis, S.; Geelen, T.; Grüll, H.; Strijkers, G.J.; Nicolay, K. Paramagnetic liposomes for molecular MRI and MRI-guided drug delivery. NMR Biomed. 2013, 26, 728–744. [Google Scholar] [CrossRef]
- Kamaly, N.; Miller, A.D. Paramagnetic liposome nanoparticles for cellular and tumour imaging. Int. J. Mol. Sci. 2010, 11, 1759–1776. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Mikac, U.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Psakhye, I.; Babes, L.; Reinheckel, T.; Peters, C.; Zeiser, R.; et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat. Nanotechnol. 2011, 6, 594–602. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017, 29, 1700996. [Google Scholar] [CrossRef]
- Safari, A.; Sarikhani, A.; Shahbazi-Gahrouei, D.; Alamzadeh, Z.; Beik, J.; Dezfuli, A.S.; Mahabadi, V.P.; Tohfeh, M.; Shakeri-Zadeh, A. Optimal scheduling of the nanoparticle-mediated cancer photo-thermo-radiotherapy. Photodiagn. Photodyn. Ther. 2020, 32, 102061. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Zhou, Z.; Bai, D.; Zhang, Q.; Ai, X.; Gao, W.; Zhang, L. White blood cell membrane-coated nanoparticles: Recent development and medical applications. Adv. Healthc. Mater. 2022, 11, 2101349. [Google Scholar] [CrossRef]
- Narain, A.; Asawa, S.; Chhabria, V.; Patil-Sen, Y. Cell membrane coated nanoparticles: Next-generation therapeutics. Nanomedicine 2017, 12, 2677–2692. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Fukui, S.; Fujimoto, S.; Mishima, F.; Takeda, S.; Izumi, Y.; Ohtani, S.; Fujitani, Y.; Nishijima, S. Ex Vivo investigation of magnetically targeted drug delivery system. J. Magn. Magn. Mater. 2007, 310, 2880–2882. [Google Scholar] [CrossRef]
- Mishima, F.; Takeda, S.I.; Izumi, Y.; Nishijima, S. Three dimensional motion control system of ferromagnetic particles for magnetically targeted drug delivery systems. IEEE Trans. Appl. Supercond. 2006, 16, 1539–1542. [Google Scholar] [CrossRef]
- Alexiou, C.; Schmid, R.J.; Jurgons, R.; Kremer, M.; Wanner, G.; Bergemann, C.; Huenges, E.; Nawroth, T.; Arnold, W.; Parak, F.G. Targeting cancer cells: Magnetic nanoparticles as drug carriers. Eur. Biophys. J. 2006, 35, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Morishita, N.; Nakagami, H.; Morishita, R.; Takeda, S.I.; Mishima, F.; Terazono, B.; Nishijima, S.; Kaneda, Y.; Tanaka, N. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem. Biophys. Res. Commun. 2005, 334, 1121–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednorz, J.G.; Takashige, M.; Müller, K.A. Susceptibility measurements support high-Tcsuperconductivity in the Ba-La-Cu-O system. Prop. Perovskites Other Oxides 2010, 193, 555–565. [Google Scholar] [CrossRef]
- Baker, I. Magnetic nanoparticle synthesis. In Nanobiomaterials; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–229. ISBN 9780081007167. [Google Scholar]
- Barnsley, L.C.; Carugo, D.; Owen, J.; Stride, E. Halbach arrays consisting of cubic elements optimised for high field gradients in magnetic drug targeting applications. Phys. Med. Biol. 2015, 60, 8303–8327. [Google Scholar] [CrossRef]
- Owen, J.; Rademeyer, P.; Chung, D.; Cheng, Q.; Holroyd, D.; Coussios, C.; Friend, P.; Pankhurst, Q.A.; Stride, E. Magnetic targeting of microbubbles against physiologically relevant flow conditions. Interface Focus 2015, 5, 20150001. [Google Scholar] [CrossRef]
- Nishijima, S.; Takeda, S.I.; Mishima, F.; Tabata, Y.; Yamamoto, M.; Joh, J.I.; Iseki, H.; Muragaki, Y.; Sasaki, A.; Kubota, J.; et al. A study of magnetic drug delivery system using bulk high temperature superconducting magnet. IEEE Trans. Appl. Supercond. 2008, 18, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Mishima, F.; Akiyama, Y.; Nishijima, S. Study on magnetic drug delivery system using HTS bulk magnet. IEEE Trans. Appl. Supercond. 2012, 22, 4–7. [Google Scholar] [CrossRef]
- Kamihara, Y.; Watanabe, T.; Hirano, M.; Hosono, H. Iron-based layered superconductor La [O1−x Fx] FeAs (x = 0.05–0.12) with Tc = 26 K. J. Am. Chem. Soc. 2008, 130, 3296–3297. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.K.T.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef] [Green Version]
- Nariki, S.; Sakai, N.; Murakami, M. Melt-processed Gd-Ba-Cu-O superconductor with trapped field of 3 T at 77 K. Supercond. Sci. Technol. 2005, 18, S126. [Google Scholar] [CrossRef]
- Ganin, A.Y.; Takabayashi, Y.; Khimyak, Y.Z.; Margadonna, S.; Tamai, A.; Rosseinsky, M.J.; Prassides, K. Bulk superconductivity at 38 K in a molecular system. Nat. Mater. 2008, 7, 367–371. [Google Scholar] [CrossRef]
- Dietmar Drung High-Tc and low-Tc dc SQUID electronics. Supercond. Sci. Technol. 2003, 16, 1320. [CrossRef]
- Huang, H.S.; Hainfeld, J.F. IJN-43770-intravenous-magnetic-nanoparticle-hyperthermia. Int. J. Nanomed. 2013, 8, 2521–2532. [Google Scholar]
- Foster, K.R. Thermal and nonthermal mechanisms of interaction of radio-frequency energy with biological systems. IEEE Trans. Plasma Sci. 2000, 28, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qin, J.; Zhang, Y.; Ma, J. Stimuli-responsive self-regulating magnetic-thermal materials for selective magnetic hyperthermia therapy. OpenNano 2022, 7, 100052. [Google Scholar] [CrossRef]
- Jordan, A.; Wust, P.; Fähling, H.; John, W.; Hinz, A.; Felix, R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int. J. Hyperth. 2009, 25, 499–511. [Google Scholar] [CrossRef]
- Mortezaee, K.; Narmani, A.; Salehi, M.; Bagheri, H.; Farhood, B.; Haghi-Aminjan, H.; Najafi, M. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 2021, 269, 119020. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nandhini, G.; Shobana, M.K. Role of ferrite nanoparticles in hyperthermia applications. J. Magn. Magn. Mater. 2022, 552, 169236. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Van den Berg, C.A.T.; Bartels, L.W.; De Leeuw, A.A.C.; Lagendijk, J.J.W.; Van de Kamer, J.B. Experimental validation of hyperthermia SAR treatment planning using MR B1+ imaging. Phys. Med. Biol. 2004, 49, 5029–5042. [Google Scholar] [CrossRef]
- Tang, Y.-D.; Zou, J.; Flesch, R.C.C.; Jin, T. Effect of injection strategy for nanofluid transport on thermal damage behavior inside biological tissue during magnetic hyperthermia. Int. Commun. Heat Mass Transf. 2022, 133, 105979. [Google Scholar] [CrossRef]
- Peng, X.H.; Qian, X.; Mao, H.; Wang, A.Y.; Chen, Z.G.; Nie, S.; Shin, D.M. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed. 2008, 3, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Le Renard, P.E.; Jordan, O.; Faes, A.; Petri-Fink, A.; Hofmann, H.; Rüfenacht, D.; Bosman, F.; Buchegger, F.; Doelker, E. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials 2010, 31, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.; Hricak, H. Molecular MR imaging in oncology. Magn. Reson. Imaging Clin. N. Am. 2005, 13, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.J.; Basu, P. Factors regulating macrophage endocytosis of nanoparticles: Implications for targeted magnetic resonance plaque imaging. Atherosclerosis 2005, 178, 67–73. [Google Scholar] [CrossRef]
- Montet, X.; Montet-Abou, K.; Reynolds, F.; Weissleder, R.; Josephson, L. Nanoparticle imaging of integrins on tumor cells. Neoplasia 2006, 8, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Ng, Q.K.T.; Su, H.; Armijo, A.L.; Czernin, J.; Radu, C.G.; Segura, T. Clustered Arg-Gly-Asp peptides enhances tumor targeting of nonviral vectors. ChemMedChem 2011, 6, 623–627. [Google Scholar] [CrossRef]
- Lazaro-Carrillo, A.; Filice, M.; Guillén, M.J.; Amaro, R.; Viñambres, M.; Tabero, A.; Paredes, K.O.; Villanueva, A.; Calvo, P.; del Puerto Morales, M.; et al. Tailor-made PEG coated iron oxide nanoparticles as contrast agents for long lasting magnetic resonance molecular imaging of solid cancers. Mater. Sci. Eng. C 2020, 107, 110262. [Google Scholar] [CrossRef]
- Sun, C.; Veiseh, O.; Gunn, J.; Fang, C.; Hansen, S.; Lee, D.; Sze, R.; Ellenbogen, R.G.; Olson, J.; Zhang, M. In Vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small 2008, 4, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Veiseh, O.; Sun, C.; Gunn, J.; Kohler, N.; Gabikian, P.; Lee, D.; Bhattarai, N.; Ellenbogen, R.; Sze, R.; Hallahan, A.; et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005, 5, 1003–1008. [Google Scholar] [CrossRef]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 135, 536–539. [Google Scholar] [CrossRef]
- Chen, H.J.; Zhang, Z.H.; Luo, L.J.; Yao, S.Z. Surface-imprinted chitosan-coated magnetic nanoparticles modified multi-walled carbon nanotubes biosensor for detection of bovine serum albumin. Sens. Actuators B Chem. 2012, 163, 76–83. [Google Scholar] [CrossRef]
- Chee, H.L.; Gan, C.R.R.; Ng, M.; Low, L.; Fernig, D.G.; Bhakoo, K.K.; Paramelle, D. Biocompatible peptide-coated ultrasmall superparamagnetic iron oxide nanoparticles for in vivo contrast-enhanced magnetic resonance imaging. ACS Nano 2018, 12, 6480–6491. [Google Scholar] [CrossRef]
- Geng, H.; Zhou, M.; Li, B.; Liu, L.; Yang, X.; Wen, Y.; Yu, H.; Wang, H.; Chen, J.; Chen, L. Metal-drug nanoparticles-mediated osteolytic microenvironment regulation for enhanced radiotherapy of orthotopic osteosarcoma. Chem. Eng. J. 2021, 417, 128103. [Google Scholar] [CrossRef]
- Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 2010, 7, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Schüller, A.; Heinrich, S.; Fouillade, C.; Subiel, A.; De Marzi, L.; Romano, F.; Peier, P.; Trachsel, M.; Fleta, C.; Kranzer, R.; et al. The european joint research project UHDpulse—Metrology for advanced radiotherapy using particle beams with ultra-high pulse dose rates. Phys. Medica 2020, 80, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Brero, F.; Albino, M.; Antoccia, A.; Arosio, P.; Avolio, M.; Berardinelli, F.; Bettega, D.; Calzolari, P.; Ciocca, M.; Corti, M.; et al. Hadron therapy, magnetic nanoparticles and hyperthermia: A promising combined tool for pancreatic cancer treatment. Nanomaterials 2020, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Smirnov, V.; Vorozhtsov, S. Superconducting cyclotron for flash therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 986, 164742. [Google Scholar] [CrossRef]
- Cooper, D.R.; Bekah, D.; Nadeau, J.L. Gold nanoparticles and their alternatives for radiation therapy enhancement. Front. Chem. 2014, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, X.; Liu, S.; Yang, W.; Pan, F.; Yang, X.Y.; Du, B.; Qin, L.; Pan, Y. Gold nanoparticles attenuate metastasis by tumor vasculature normalization and epithelial-mesenchymal transition inhibition. Int. J. Nanomed. 2017, 12, 3509–3520. [Google Scholar] [CrossRef] [Green Version]
- Schuemann, J.; Berbeco, R.; Chithrani, D.B.; Cho, S.H.; Kumar, R.; McMahon, S.J.; Sridhar, S.; Krishnan, S. Roadmap to clinical use of gold nanoparticles for radiation sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef] [Green Version]
- Detappe, A.; Kunjachan, S.; Rottmann, J.; Robar, J.; Tsiamas, P.; Korideck, H.; Tillement, O.; Berbeco, R. AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer Nanotechnol. 2015, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Popovtzer, A.; Mizrachi, A.; Motiei, M.; Bragilovski, D.; Lubimov, L.; Levi, M.; Hilly, O.; Ben-Aharon, I.; Popovtzer, R. Actively targeted gold nanoparticles as novel radiosensitizer agents: An in vivo head and neck cancer model. Nanoscale 2016, 8, 2678–2685. [Google Scholar] [CrossRef]
- Schneiderman, J.F. Information content with low- vs. high-Tc SQUID arrays in MEG recordings: The case for high-Tc SQUID-based MEG. J. Neurosci. Methods 2014, 222, 42–46. [Google Scholar] [CrossRef]
- Maestú, F.; Cuesta, P.; Hasan, O.; Fernandéz, A.; Funke, M.; Schulz, P.E. The importance of the validation of M/EEG with current biomarkers in Alzheimer’s disease. Front. Hum. Neurosci. 2019, 13, 17. [Google Scholar] [CrossRef]
- Andersen, L.M.; Pfeiffer, C.; Ruffieux, S.; Riaz, B.; Winkler, D.; Schneiderman, J.F.; Lundqvist, D. On-scalp MEG SQUIDs are sensitive to early somatosensory activity unseen by conventional MEG. Neuroimage 2020, 221, 117157. [Google Scholar] [CrossRef]


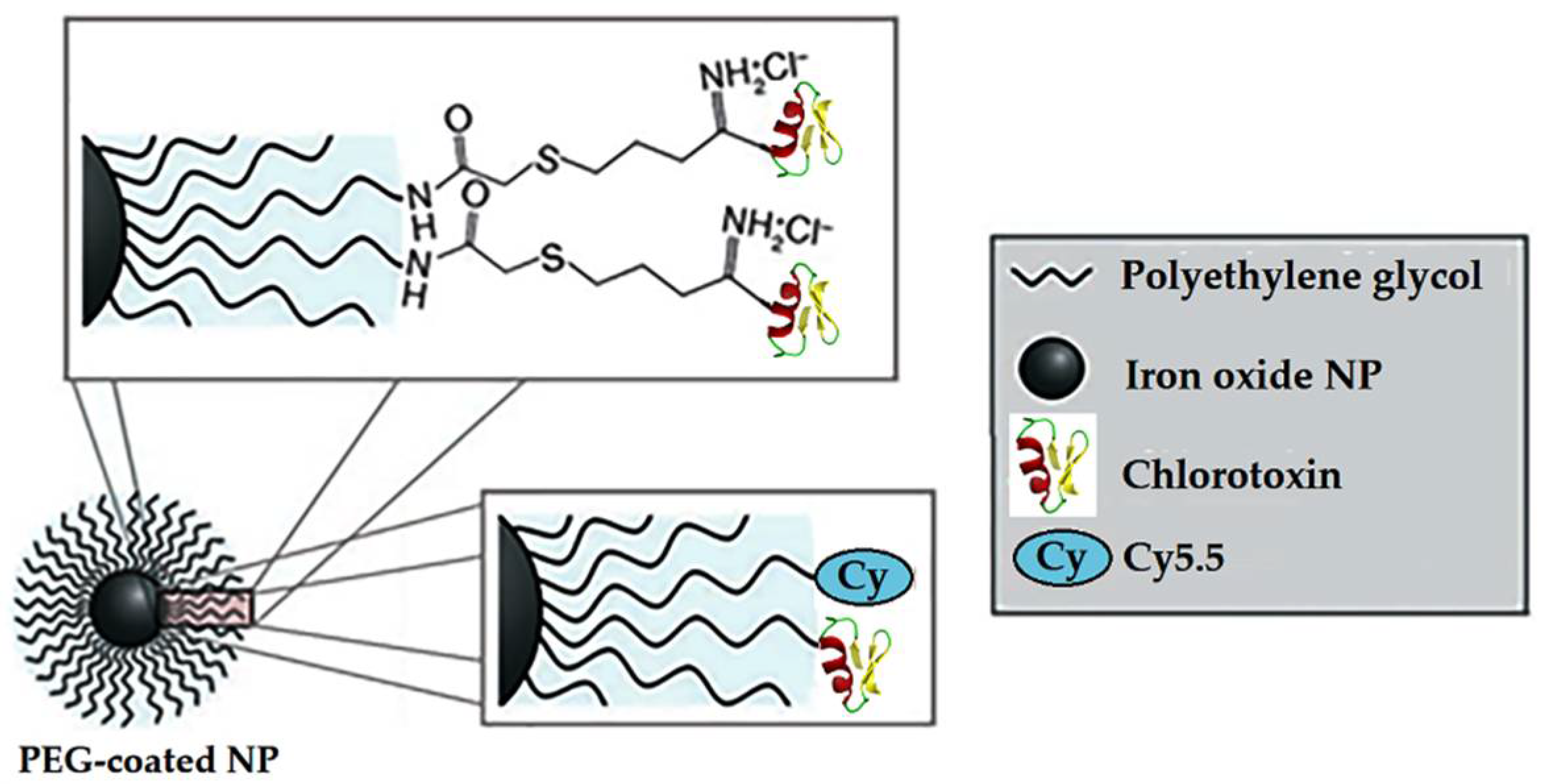
| Coating | Advantages | Medical Application |
|---|---|---|
| PEG [52,53] | Enhanced water solubility, reduced phagocytosis, and increased blood circulation time | MRI, tumor diagnosis, and treatment |
| Polyethylenimine [54,55,56] | Good biocompatibility | Gene and vectors |
| PVA [57,58,59] | Elevated stability, reducing the particle aggregation | MRI, vectors, and bioseparation |
| Glucan [60,61,62,63,64] | Excellent stability and extended blood circulation time | Vectors, MRI |
| Liposome [65,66,67,68] | Good biocompatibility | Tumor treatment, thermotherapy, and MRI |
| Chitosan [69,70,71] | Good biocompatibility, essential small-molecule vitamin for the human body | Vector, thermotherapy, and radiotherapy |
| White blood cells [72,73] | Biomimetic properties, excellent biocompatibility | Vector, nanovaccines, and treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol 2022, 2, 374-390. https://doi.org/10.3390/macromol2030024
Flores-Rojas GG, López-Saucedo F, Vera-Graziano R, Mendizabal E, Bucio E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol. 2022; 2(3):374-390. https://doi.org/10.3390/macromol2030024
Chicago/Turabian StyleFlores-Rojas, Guadalupe Gabriel, Felipe López-Saucedo, Ricardo Vera-Graziano, Eduardo Mendizabal, and Emilio Bucio. 2022. "Magnetic Nanoparticles for Medical Applications: Updated Review" Macromol 2, no. 3: 374-390. https://doi.org/10.3390/macromol2030024
APA StyleFlores-Rojas, G. G., López-Saucedo, F., Vera-Graziano, R., Mendizabal, E., & Bucio, E. (2022). Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol, 2(3), 374-390. https://doi.org/10.3390/macromol2030024









