Abstract
Among numerous synthetic macromolecules, polyurethane in its different forms has proven its sheer dominance and established a reputation as a reliable and trusted material due to its proficiency in terms of superior properties, which include: high mechanical strength and abrasion resistance, good durability, good adhesion, good thermal stability, excellent chemical and weathering resistance. Synthetic polyurethane materials are non-biodegradable, poisonous, and use petrochemical-based raw materials, which are now depleting, leading to a surge in polyurethane production costs. Bio-based polyurethanes (PU) have been synthesized by researchers in recent decades and have mostly overtaken petrochemical-based PU in terms of challenges such as solid pollution, economic effectiveness, and availability of raw materials. Enormous kinds of available bio-renewable sources as predecessors for the production of polyols and isocyanates have been explored for the development of “greener” PU materials; these bio-based polyurethanes have significant potential to be used as future PU products, with a partial or total replacement of petroleum-based polyurethanes, due to increasing concern about the environment, their relatively low cost and biodegradability. This critical review concentrates on the possibilities of renewable sources to be used for polyurethane production and gives a clear perspective on the journey, utilization, and recent advancements in the field of different bio-based polyurethane polymers that have arisen over the last decade.
1. Introduction
Polyurethanes (PU) represent nearly 8% of the total plastics production and stand tall as the 6th most widely used polymer in the world, with total production reaching 18 MTon in 2016 [1] and still progressing. The superior properties of PU, such as flexibility, corrosion, resistance to chemicals, high automatic strength, low-temperature comfortability, adhesiveness, and chemical structure adaptability, etc. account for their considerable popularity among researchers. PU have urethane groups as major repeating units along their main backbone chains, which are derived by reacting hydroxyl (-OH) terminated chemical entities, with isocyanate (-NCO) terminated compounds, as represented by a generalized scheme shown in Figure 1. In addition, some other functional groups may also be present in the end constitutions of the PU chains, including ethers, esters, urea, biuret, and aromatic moieties [2].

Figure 1.
Formation of Urethane Linkage.
By altering the raw materials, additives, and the type of manufacturing process, the PUs can be tailor-made with a wide spectrum of product implications, including foams, fibres, adhesives, coatings, elastomers, and sealants, etc., Table 1 depicts the wide range of applications associated with polyurethanes. According to the latest report, the global polyurethane market will reach USD 88 million by 2026, with a compound annual growth rate (CGAR) of 6% [3]. The utilization of Polyurethanes in various spheres of life is represented in Figure 2.

Table 1.
Wide Range of Applications Associated with Polyurethanes.
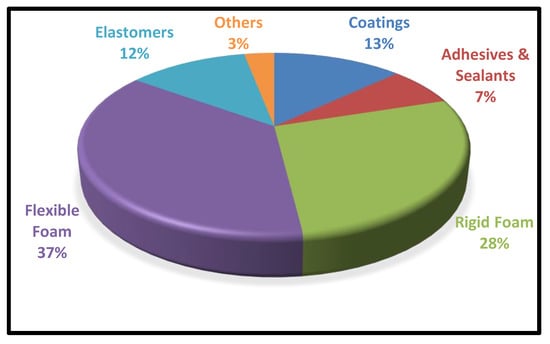
Figure 2.
Global Consumption of PUs in Different Sectors.
The physical properties of PU, such as superb adhesion, adaptability, superior chemical resistance, and high automotive strength make it a great alternative to the widely used polymers such as formaldehyde and epoxy resins; however, the drawbacks associated with the preparation and usage of polyurethanes poses a serious threat to the human health and environmental hazards, which includes (i) the use of dangerous phosgene gas for the conversion of amines into isocyanates, (ii) usage of CMR (Carcinogenic, Mutagenic, and Reprotoxic) labelled isocyanates for PU synthesis, namely Methylene Diphenyl Diisocyanate (MDI) and Toluene Diisocyanate (TDI), and the health risks associated with their long-term exposure, such as asthma, dermatitis conjunctivitis and acute poisoning (iii) post-consumption dumping (into landfills and dumping ground) or incineration of PU, leading to either hydrolytic degradation, that generates toxic amines which contaminates air and water bodies or the disintegration to yield hydrogen cyanide (HCN), during burning [4,5].
To address these issues, a new quest has already begun and gained popularity to produce non-isocyanate-based polyurethanes (NIPU), in which the common approach for the PU synthesis involves the reaction between cyclic carbonates and amines, resulting in a class of compounds called Polyhydroxyurethane (PHU) with hydroxyl groups [6,7]. This extensive review provides a deep insight into the recent research and developments related to polyurethanes prepared from bio-based materials being used in different segments, such as adhesives, coatings, elastomers, and foams for a broad range of practical applications. In addition, the new methodologies adopted in the field of NIPU, PU recycling, and PU biodegradation have been highlighted in detail.
1.1. Bio-Based Polyurethane
Synthetic polyurethane materials are non-biodegradable, expensive, poisonous, and have petrochemical-based raw materials, which are now depleting. Bio-based polyurethanes are one of today’s most flexible materials, which can play a significant role in fostering environmental goals of promoting waste reduction and sustainability. PU has seen a significant shift from traditional petroleum-based raw feedstock toward diverse renewable alternatives such as fatty acids [8], proteins [9], carbohydrates [10], vegetableoils [11], starch [12], polysaccharides [13], cellulose [14], and a variety of different agricultural products and by-products. Using renewable resources to produce polyurethane reduces adverse influences on the environment, for instance, greenhouse gas emissions. In the recent decade, diverse research groups have made rewarding efforts toward the synthesis of biopolyol-based polyurethanes [15,16]. Polyols behave as soft segments and transmit flexibility and exhibit elastomeric properties in the polyurethanes [17]. Polyols derived from vegetable oils have been the subject of extensive research. Hydroformylation [18], ozonolysis [19], amidation-esterification [20], metathesis [21], and epoxidation-ring opening [22], coupling of thiol-ene with mercaptoethanol [23] have all been used to achieve this, and all have successfully provided polyol with the desired properties; moreover, petrochemical-based isocyanates are replaced by bio-based isocyanate to remove toxicity such as partially bio-based aliphatic isocyanate. These impart water resistance, good mechanical strength, and flexibility to the PU network [24]. Hence, bio-based polyurethane materials were created to solve challenges such as solid pollution, economic effectiveness, and easy availability of raw materials. A comparison of the properties of bio-based PU and petro-based PU is given in Table 2.

Table 2.
Comparison of the Properties of Bio-Based Polyurethane and Petro-Based Polyurethane [16].
1.1.1. Vegetable Oil-Derived Monomers
Vegetable-oil-based polyols have reportedly been synthesized by ozonolysis, thiol-ene coupling process, photochemical oxidation, hydroformylation, ring-opening events, and epoxidation (Figure 3). Epoxidation is the major chemical pathway for making polyol, followed by epoxy group reactions with ring-opening reagents such as glycerol, alcohol, 1,2-propanediol, water, and acids.
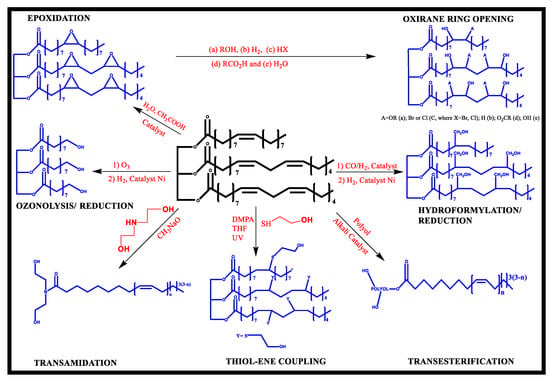
Figure 3.
Synthetic pathways for the synthesis of polyols based on vegetable oil [25].
Additionally, lactate polyols, prepared from 100% bio-renewable material are polyester polyols that incorporate lactic acid units as a component. Polyurethane obtained from lactate polyols is found to be biodegradable and biocompatible. Two methods are commonly being reported for the synthesis of polyols from polylactic acids: (1) ring-opening addition (lactide is added to hydroxyl groups to open the ring). (2) Different polyols are esterified using lactic acid or Transesterification with lactic acid esters [25].
1.1.2. Bio-Based Isocyanates
To boost the renewable content of PUs, partially or potentially bio-based di-, tri- or poly-isocyanates have been utilized. There are also reports on PU films made with two bio-based isocyanates—ethyl ester L-lysine diisocyanate (LLDI) and ethyl ester l-lysine triisocyanate (LLTI)—and one commercial isocyanate, isophorone diisocyanate (IPDI) [25]. Another study documents the preparation of bio-based polyurethane by utilizing isocyanates derived from palm oil, which was reported to exhibit low toxicity, low carbon emission, along with being cost-effective [26].
2. Polyurethanes: Types, Synthesis, and Utilities
2.1. Polyurethane Adhesives
Polyurethane (PU) adhesives are known for their remarkable adhesion, weathering resistance, excellent low-temperature performance, flexibility, and rate of curing, which can be speckled according to the need of the manufacturer [27,28]. PU adhesives have advantages over other adhesives being pressure-sensitive, having high tensile strength, compressive modulus, and rip strength, and being transparent at room temperature. Owing to a vast diversity among the raw materials, the properties of the PU adhesives can be accustomed to a vast extent as per the needs of the users. The high-strength PU adhesives can be utilized in both inelastic and permanently elastic applications [29].High penetration of the adhesive into the wood, limited resistance to exfoliation, and undesired gap-filling properties are the main drawbacks of PU adhesives [30]. Most of the commercially important PU adhesives can be categorized as one component or two components of adhesives, as depicted in Figure 4.
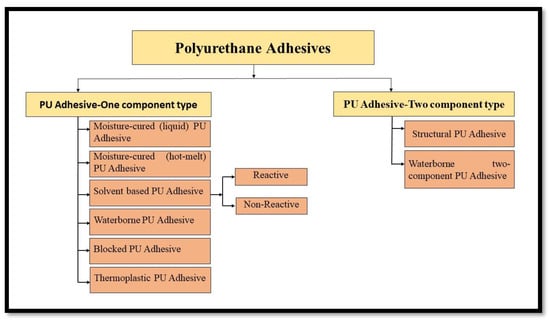
Figure 4.
Classification of Polyurethane Adhesives Based on Number of Constituents.
PU Adhesives—One-component Type: One component adhesives are self-curing adhesives that do not have to be mixed with any other ingredients (curing agent) before being applied to the substrate; they use an isocyanate-terminated pre-polymer that cures naturally in the presence of moisture. After applying to substrates, a mist of water is spurted on the adhesive to speed up the curing process [30].
Other types of one-component PU adhesives include solvent-based PU adhesives, which are further categorised as reactive solvent-borne PU adhesive and non-reactive solvent-borne PU adhesive [31]. The former involves high-molecular-weight oligomers bearing isocyanate functionality and the latter shows no further reaction after application. Solvents commonly used in these adhesives are toluene, ketones, ethyl acetate, butyl acetate, etc.
Additionally, Waterborne polyurethanes are a kind of system composed of a dispersion of binary colloidal particles within an aqueous medium that necessitate the addition of water-compatible stabilising groups (emulsifiers) due to their hydrophobicity [32]. Blocked PU Adhesives, as the term ‘blocked’ implies, are the one in which one of the reactants involved is blocked chemically to inhibit the reaction. Commercially available blocked isocyanates are mostly based on TDI, and derivatives of HMDI.
The thermoplastic PU adhesives are linear in structure with a functionality equal to 2 [33]. The desired molecular weight to synthesize thermoplastic PU adhesives is about 100,000 or higher; these are superior compared to hot melt adhesives, in terms of holding strength, attained by crystallization.
PU Adhesives—Two-Component Type: Two-component PU adhesives are applied where, a better flexible bond line is desired; these are commonly used for bonding two different kinds of substrate, which exhibits a wide difference in their thermal expansion coefficients. Due to their fast reaction rate, two-component polyurethane adhesives are generally prepared with components supplied in separate compartments. The two-component PU adhesive is broadly categorized as Structural PU adhesives and Waterborne Two-Component PU adhesives.
Structural PU Adhesive provides excellent mechanical strength by forming a chemical cross-link between the reactive components, thus leading to a three-dimensional polymer network, a necessity for structural performance [34]. Waterborne Two-Component PU Adhesive consists of two components, with one of the components as PU dispersions and the other one as a crosslinker [35].
Moreover, the addition of fillers can potentially change the physicochemical and mechanical properties of adhesives. Talc, calcium carbonate, silica, titanium oxide, carbon fibre, clay, and other fillers are commonly used in PU. The effect of nanosized TiO2 filler on various characteristics of PU glue was investigated in a recent study. In PU adhesives, TiO2 has been shown to improve surface adherence, solar reflectance, and crack resistance [36].
In general, synthetic adhesives are more resistant to moisture, chemicals, and biological organisms than natural adhesives. Bio-renewable sources such as vegetable oils, starch, protein, lignin, lactic acid etc. constitute a valuable source for the synthesis of polyols to produce ‘‘sustainable, environment friendly‘’ PU adhesives [37]. Bio-based polyurethane adhesives from various sources are discussed here:
2.1.1. Protein
Protein-based PU adhesives can be prepared from both animal as well as plant sources. The advantages and disadvantages of the different animal and plant protein-based adhesives have been reported in Table 3.

Table 3.
Different Animal and Plant Protein-based Adhesives [38].
2.1.2. Vegetable Oils
Vegetable oils are an excellent raw material for the production of PU adhesives. The most important component of vegetable oil is triglycerides, which are glycerol esters with three long chains of fatty acids of varying composition depending on the source of the oil. As triglycerides hydrolyse to form a range of fatty acids and glycerols, polyols, and isocyanates, raw materials for the synthesis of polyurethane can be derived from them. Various vegetable oils that can be utilized for PU synthesis are Castor Oil, Palm Oil, Canola Oil, Soyabean Oil and Jatropha Oil.
Castor Oil
Of all the vegetable oils, castor oil is the favourite among the researchers and the most commonly used one because of its renewability, low cost, easy availability, and being non-edible. Castor oil contains ricinoleic acid, which is a monounsaturated fatty acid, bearing a hydroxyl functional group and thus, can be employed for PU synthesis [39]. Different compositions of PU adhesives derived from castor oil have been reported in Table 4. Being non-edible (due to the presence of toxic protein, i.e., ricin), there is no hesitation among the researchers to use it as a potential source of PU synthesis. The mechanical properties of the castor oil-based adhesive are highly dependent on the NCO/OH molar ratio, due to higher or lower cross-linking achieved during the reaction. Studies on solvent-less castor oil-based PU adhesives reported the impact of the NCO/OH molar ratio and the chemical characteristics of the substrates on the adhesion force [40]. As reported, the resulted PU adhesive foam joints displayed peeling strength values of 75% and wood joints showed lap shear strength values 20% higher than that of commercialized solvent-based adhesives.

Table 4.
Different Compositions of PU Adhesives Derived from Castor Oil [35].
Palm Oil
Palm oil and palm kernel oil are the two main forms of oil generated from the palm oil tree. Palm oil has been reportedly used by researchers for the production of PU adhesives, which shows higher hydrolytic stability and hydrophobicity. Patel et al. studied the ring-opening reaction of epoxidized palm olefin with phthalic acid, which is utilized to generate PU adhesives with, which contain palm oil-based polyester [41]. Khoon et al. studied polyurethane wood adhesive derived from palm oil-based polyester polyol, where glycerol was used as a crosslinker and DBTL as a catalyst [18]. TDI and MDI were employed as isocyanates in this study. Radojčićetal., explored the ring-opening polymerization reaction of palm oil-based polyol to synthesize polyester PU with low hydroxyl content, with high molecular weight exhibiting a longer gel time [42].
Canola Oil
Canola oil has unsaturated double bonds in its fatty acid chains as well as an ester group, which allows it to be employed in the manufacturing of a wide range of important goods, such as polyols, owing to its high protein content and hydrophobic properties. Canola oil-based PU adhesives have excellent attributes such as equivalent lap shear strength, chemical resistance, and hot water resistance, implying that they have a lot of potential in particleboard manufacture. Li et al. [20] studied the adhesion attributes of sodium bisulfite (NaHSO3)-modified canola protein. Protein was extracted from canola meal through alkali solubilization and acid precipitation methods and was further modified with different concentrations of NaHSO3 (0–15 g/L). With the increase in the concentration of NaHSO3, the purity of canola protein was found to be decreased. The isolated wet protein was used as an adhesive.
Jatropha Oil
Jatropha oil is a non-edible, renewable oil with gum that can be utilized in adhesives, with excellent adhesion and improved mechanical characteristics. Aung et al. reported jatropha oil-based PU adhesive bearing epoxy groups which enhanced adhesion [19]. Gadhave et al. reported jatropha oil-based polyurethane adhesive via epoxidation reaction of hydroxylated polyols with 2,4-toluene diisocyanates [43]. Saalah et al., synthesized a renewable jatropha oil-based polyol by polymerization of jatropha oil with isophorone diisocyanate and dimethylol propionic acid; the product is widely used as a binder for wood and decorative coatings [22].
Soybean Oil
Soybean-based adhesives have mostly been utilized in the production of adhesives as well as wood composites such as particleboard and medium-density fibre board. Dai et al. synthesized Soy oil-based PU adhesives by reacting BIOH polyols X-0002 and IPDI via maintaining NCO/OH ratio 1:4 and examined the variations in physical and mechanical properties with hydroxyl functionalities in soy-based polyols in PU films [37].
2.1.3. Lignin
Lignin is generated as a by-product from the paper and pulp industry. The glass transition temperature, aromatic content of the final polymer, and thermal stability of adhesives can all be improved by including lignin into the polyurethane matrix as a polyol substitute. Due to its aromatic crosslinked structure, lignin is somewhat steady at high temperatures and works as a thermosetting material. Because of its aromatic composition, lignin acts as a hard segment and replaces the soft segment of PU, i.e., polyol, and boosts the mechanical strength, thermal characteristics, and composite material properties of blends, copolymers, and composite materials. Lignin incorporation into PU matrix leads to enhancement in its thermal stability, delamination, and resistance to abrasion [44]. Alinejad et al., prepared lignin-based polyurethane adhesives by polymerization of lignin with toluene -2,4-diisocyanate (TDI) and partial polyethylene glycol (PEG). Partial polyethylene glycol was used but, it was further replaced by lignin to avoid the hard and brittle property. The final product was used as wood adhesives. The amount of lignin incorporated enhanced the glass transition temperature and thermal stability of the adhesive [29].
2.1.4. Starch
Starch is widely being used in many industrial applications, such as the food industry, pharmaceutical industry, paper industry, and printing industry because of its inherent biodegradability, abundance, and renewability [45]. The main sources of starch are wheat, rice, potato, corn, etc. Two natural resources are potato starch and edible or non-edible plant-derived oils that can be employed to prepare PU adhesives. Starch glycosylation followed by oil transesterification to generate polyol can be used to make starch-based PU adhesives.
2.1.5. Polylactic Acid
Thermoplastic polyester polylactic acid is noted for its biodegradability. Lactate polyols are polyester polyols that include lactic acid units as a component and can be prepared from 100% bio-renewable material. Gadhave et al., prepared high functionality polyester polyols containing lactate polyols suitable for rigid cast polyurethane, having high bio-based content and that exhibit biodegradability [43].
2.1.6. Applications of Bio-Based PU Adhesive
Owing to the high stickiness on a variety of substrates such as metals, wood, plastics, glass, etc. These bio-based polyurethane adhesives have a wide range of industrial applications. The applications of bio-based polyurethane adhesive in assorted sectors are described below:
In Construction: PU adhesives are widely used in wood flooring, heavy-duty rubber flooring, and parquet bonding due to their great strength and resistance. PU adhesives are suitable for ceramic tiles, mirrors, bath surrounds, and concrete due to their ability to bond wet/frozen timber, as well as their high green strength and shear strength [27].
In Packaging: Snack food bags, printed films, and shopping bags commonly use one-component PU adhesives for adherence. In addition, for medical applications, a tailored two-component PU adhesive is used to adhere polyvinyl chloride to aluminium sheets. Hot melt PU adhesives are suited for wrapping applications due to their low viscosity and low application temperature.
Transportation: Polyurethane adhesives are used in a variety of industries, including automotive, trucks, and railways. Polyurethane adhesives are highly appreciated due to their short cure time and ability to generate strong flexible bonds [46].
Electronics: PU adhesives are good for bonding copper foil, electronic coil fabrication, surface fix conductive adhesion, and bonding die-stamped printed circuit boards due to their properties of curing at room temperature, endurance, and great resistance to thermal shocks and vibrations.
Footwear: The shoe industry requires PU adhesive to join waterproof insole materials and in the sole attachment because of its excellent water resistance, substantial heat resistance, flexibility in cold rapid settings, and good initial strength [47].
Biomedical applications: Tissue adhesives have gained popularity in recent decades, owing to an increase in the number of surgical procedures performed around the world. Depending on the adhesive purpose, these tissue adhesives are categorised into three basic types: (i) Hemostats, which prevent blood loss by interfering with the coagulation cascade, generally have weak mechanical characteristics and are employed in cases of blood loss owing to sutures tissue injury; (ii) glues that cling strongly to tissues and (iii) Sealants, which create a physical barrier to stop air/gas or blood seeping [48]; they usually provide mid-range tissue adhesion and are utilised in conjunction with sutures [34], and these systems, on the other hand, perform better in a dry atmosphere.
2.2. Polyurethane Coatings
PU coatings are used to enhance mechanical strength and to induce desired barrier properties in the components of aircraft, automotive, container ships, oil tankers, industrial machines, residential refrigerators, etc., [49]. In addition, these organic coatings shield the materials from intrusive environmental conditions such as humidity, biological deterioration, chemical and automated destruction, and radiation and impart colour and gloss [50]. Different types of PU coatings of industrial importance are discussed in the subsequent section.
High solids polyurethane coatings: High solids coatings have narrow molecular weight distribution and low solvent emissions; they have a suitable spray viscosity, which in combination with their low molecular weight, is responsible for their drying behaviour, without compromising the performance of the material [51].
Ultraviolet (UV) curable polyurethane coatings: PU curing polyurethane coatings have become popular in recent years. Polymerization and thermal curing processes, which involve solvents and catalysts, are used to fix traditional polyurethane coatings [52]. UV-curing technology is distinguished from thermal curing by the 5E abbreviation, which stands for Eco-Friendly, Economical, Efficiency, Enabling, and Energy Saving, making it “clean and green” [53]. Polyurethane acrylate-based UV-curable coatings are excellent in imparting abrasion resistance, impact resistance, scratch resistance, and optical transparency [53].
Hyperbranched polyurethane coatings: The three main types of dendritic polymers are dendrimers, hyperbranched polymers, and dendrigrafts. Because of their unique structural properties such as no entanglement and high surface functionalities, hyperbranched polymers and dendrimers emerged as an important class of polymeric materials in the recent years; they also have enhanced cross-link density, excellent reactivity, and reduced viscosity. Hyperbranched polymers, on the other hand, are preferred over dendrimers due to their one-step synthesis and fewer purification processes. Hyperbranched structures outperform linear structures in terms of characteristics [54]. Hyperbranched poly (ester amide) was also shown to be biodegradable, which might be considered a green advantage in advanced surface coating technology [55].
Water-borne polyurethane coatings: WPU material has received a lot of attention in the past few decades due to its many advantages, such as good flexibility, stiffness, impact and abrasion resistance, gloss, chemical resistance, lesser flammability, high adhesive strength, durability, low viscosity, easy cleaning and weather-ability, as well as zero or low volatile organic chemical compound emissions [56]. Because the viscosity of a WPU dispersion is independent of the polymer’s molecular weight, high solid content WPU dispersions with high-molecular-weight films can be produced with outstanding quality simply by physical drying [57]. A binary colloidal system in which PU particles are continually disseminated in aqueous solutions is known as PU dispersions. Due to ionic groups (PU ionomers) in the linear thermoplastic PU backbone, WPUs are dispersible in water. Polyurethanes, on the other hand, are not water-dispersible due to hydrophobic isocyanates (which also react with water) [58]. A PU ionomer is a copolymer with a PU backbone and repeat units that include pendant acid groups that are neutralised completely or partially to form salts. Internal emulsifiers include cationic-like quaternary ammonium groups, anionic-like carboxylated or sulfonated groups, and non-ionic groups such as polyols containing ethylene oxide end groups. Micelles are formed in water by cationic and anionic PU ionomers, respectively. The types of ionomers, isocyanates, and polyols utilised all have an impact on the performance of the resulting WPU dispersions.
UV curable WPU coatings: UV stable coatings cure quickly when exposed to ultraviolet light, resulting in outstanding performance. UV-curable waterborne polyurethane combines the advantages of UV equipment with those of aqueous coatings. UV-curable WPU coatings had been widely investigated because of their flexibility, non-toxic behaviour, chemical resistance, and remarkable mechanical properties. UV-WPU coatings, despite their many advantages, have worse solvent resistance and thermal stability than typical solvent-based polyurethane coatings, and the tensile strength is significantly reduced at temperatures above 80 °C. Due to their lower VOC emissions than solvent-based PU coatings, environmentally friendly waterborne polyurethane (WPU) coatings are widely employed [58]. The UV-cured WPU coatings are a significant class of green and environmentally friendly coatings with excellent mechanical qualities and a quick curing procedure. Due to numerous end groups, compact molecular structure, and reducing chain entanglement, hyperbranched polyurethanes (PUs) have intriguing features such as high solubility, reactivity, and good rheological behaviour.
Hyperbranched WPU coatings: Because of their distinct molecular architectures, such as compact molecular nature, numerous end groups, and decreased chain entanglement, hyperbranched polymers differ significantly from their linear counterparts. Branched-chain polymers have the most exciting properties, such as good rheological behaviour, strong reactivity, and solubility, and so have a wide range of applications [59]. The step-growth polycondensation technique is used to make polycarbonate, polyphenylene, polyesters, poly(ether ketone), polyamides, poly(4-chloromethylstyrene), polyurethanes, and other hyperbranched polymers. The number of generations, which is a statistical measure of the total number of monomers in a hyperbranched polymer, influences the compatibility of hyperbranched WPUs with nanofillers.
Nanocomposite WPU coatings: Waterborne surface coating materials with low volatile organic compounds (VOCs) are environmentally safe and, as a result, the most sought-after materials in today’s society. Waterborne polyurethane (WPU) coatings offer excellent stiffness and elasticity, but they have much lower mechanical strength and toughness than solvent-based coatings. Nano-sized inorganic fillers have been added to WPU dispersions to create nanostructured coatings, demonstrating that this is a potential method for increasing WPU properties. The manufactured WPU nanocomposites have a lot of potential as an environmentally friendly transparent surface coating material with low VOC content.
2.2.1. Vegetable Oil-Based Polyurethane Coatings
Polyurethane coatings based on vegetable oil have proven their worth by exhibiting significant properties such as abrasion and chemical resistance, remarkable toughness, corrosion resistance, low-temperature flexibility, and prospering industrial applications, as well as lowering or eliminating the use of volatile organic compounds (VOCs). The type of PU coating depends on the curing procedure and solvent employed. In addition to dispersing conventional polyurethane using petrochemical solvents, extensive research has been done on water-based PU extracts that use water as a solvent. Dispersions of waterborne PU have an inherent benefit over traditional in that they are environmentally friendly and safe to use [54]. PU treatment can occur at room temperature or below room temperature. UV curing is a type of thermal curing that uses UV radiation to provide the energy needed for the curing operation. To improve the properties of conventional PU coatings to fulfil the industrial norms, extensive investigation has been done on high-solid contents, nanocomposites, and hyperbranched coatings of PU, which are dependent on the respective properties and compatibility of distinct components [49]. Because it takes less time, uses less energy, and has a better curing action than other techniques, this method has proven to be more efficient than others. A sustainable alternative to mitigating failures from degradative corrosion is the appropriate utilization of domestically leftover resources such as vegetable oils (Figure 5).
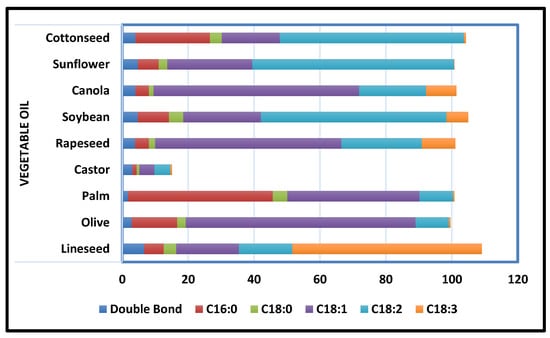
Figure 5.
Common Vegetable Oils’ Degree of Unsaturation and Fatty Acid Composition [55].
2.2.2. PU Coatings Based on Cashew Nut Shell Liquid (CNSL)
In addition to vegetable oils, CNSL, an agricultural by-product of the cashew industry, has been utilized as binders for coatings material [60,61,62]. Meta substituted phenolic compounds identified in CNSL, a reddish-brown phenolic lipid, include 2-methyl cardol, cardol, cardinal, and anacardic acid. High vacuum distillation of CNSL yielded cardanol, a crucial precursor for a variety of oligomers and polymers [62,63]. For polymer curing, the meta-position of an unsaturated alkyl chain (C15H31-2n, n = 0–3) can be used as an autoxidizable site [64]. CNSL (cashew nut shell liquid) is a naturally occurring substituted phenol that can be used in place of phenol, with similar or superior outcomes. It is a low-cost and renewable material that finds use in Insecticidal, fungicidal, anti-termite, and therapeutic applications. In addition, it is also used as an additive in many plastic compositions. Cardanol-based polyols are considerably modified (the most common modification site is the phenolic hydroxyl group), resulting in autoxidizable bio-based polyurethane coatings with isocyanates [64]. All cardanol-based polyols have a 15-carbon side chain at the meta position, which may affect the polymers’ thermal and mechanical properties, as all changes are made to these unsaturation sites. Friction materials, cars, surface coatings, adhesives, laminates, rubber compounding, and a range of other applications use CNSL resins. The chemical structure of different cardanol-based polyols (CAP) from aromatic cardanol is depicted in Figure 6.
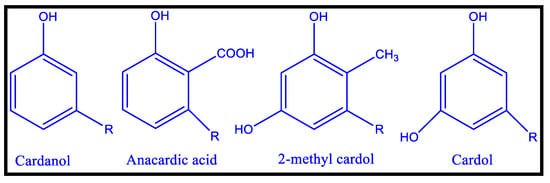
Figure 6.
Chemical Structures of CNSL’s Major Components [63].
Because of the aromatic ring of cardanol, the PU backbone is particularly rigid, and the PU coating has a firm adherence to the metal, resulting in improved stiffness and hardness. Aside from CNLS-based polyol, CNLS-based cyclic carbonate (CC) was efficiently used as a precursor for non-isocyanate polyurethane preparation (NIPUs) [64]. For the synthesis of NIPU, di-glycidyl ether of cardanol (NC-514), a CNSL-derived product and epoxy formulation modification, was employed.
2.2.3. Polyurethane Coatings Based on Terpene
Terpene-based polyols with strong impact strength, water resistance, adhesion, flexibility, and pencil hardness can be crosslinked with poly-isocyanate to produce PU coatings (Figure 7). Terpenoids are good bio-based raw ingredients that have a high value in polymer applications [65].
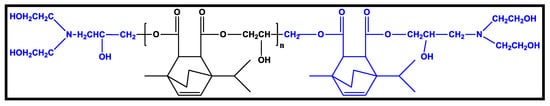
Figure 7.
Chemical structure of polyols based on terpene [65].
2.2.4. Polyurethane Coatings Based on Eucalyptus Tar
Eucalyptus tar is a complicated combination primarily made up of phenols, particularly guaiacyl and syringyl derivatives [66]. A solid residue known as bio-pitch is created during the distillation of bio-oil, and it makes up a considerable component of the final product (approximately 50%). To make a tar-based varnish and a bio-pitch-based black paint, eucalyptus tar derivatives are used as a source of hydroxyl groups. Eucalyptus tar was distilled to produce heavy oil and bio-pitch, which were then mixed with castor oil to create a renewable polyol [67].
2.3. Polyurethane Foams
Among most polymers, the actual kingpin of the cellular structure kingdom is Polyurethane (PU) foams, which account for 67% of the global PU consumption and represents 50% of the polymer foam market [68]. PU foams hold a good reputation for several applications such as thermal insulation, acoustic insulation, automotive seating, mattresses, etc. Based on their density they are classified as rigid, semi-rigid, and flexible foams.
Bio-based polyurethane foams: Bio-based building blocks such as alginate and other carbohydrates have been successfully integrated into PU foam networks via their hydroxyl groups (-OH) for improved biocompatibility and breakdown kinetics.
2.3.1. Vegetable Oil-Based Polyurethane Foams
In recent years, interest in the synthesis of vegetable oil-based polyurethane foams has increased significantly. Lubczak et al. [69] produced rigid polyurethane foams based on polyol derived from hydroxyalkylation of cellulose with glycidol and ethylene carbonate. These foams exhibited several inherent properties equivalent to conventional rigid polyurethane foams including apparent density (60.5 kg/m3), low shrinkage (~3%), and low water absorption percentage of 6.6%. The thermal Conductivity of these foams turned out to be 0.0338 W/m K. Lin et al. [70] produced bio-based polyurethanes utilizing polyols consisting of lipids derived from enzymatic hydrolysis of food wastes, comprising a mixture of rice, noodles, meat, and vegetables, etc. The polyols required for foam synthesis were developed by trans-amidation with diethanolamine and epoxidation followed by epoxy ring-opening with trimethylolpropane. Takemura et al. [71] tried to reduce the reliance on petroleum-based polyol by using four different sets of agricultural waste liquefied polyols including Oil seed Rape Straw (OS), Rice Straw (RS), Wheat Straw (WS), and Corn Stover (CS) respectively and consequently studied the influence of NCO/OH on the foam properties such as density, compressive strength, elastic modulus, morphological structure, storage modulus (E’), glass transition temperature (Tg), water absorption ability, etc. respectively. It was observed that density, compressive strength, and elastic modulus first decrease then held stable, and finally improve with increasing NCO/OH ratio; moreover, the glass transition temperature values shift to the higher temperature range at a high NCO/OH ratio. Chittapun et al. [72] investigated the usage of cellulose extracted in a high yield percentage of ~37.2% from Kai algae composed of Cladophora sp. and Microspora sp. by the alkalization of macroalgae having incorporated in varying dosages as a reinforcing filler for polyurethane foam composites. The addition of algal cellulose led to open cells and softer foam with reduced cell size along with an increase in apparent density, however, a decline in compressive modulus values was observed. The presence of filler at 1% (w/v) concentration has caused increased stiffness and transition temperature but a drop in loss modulus.
Hu et al. [73] successfully developed flame retardant and self-extinguishing flexible polyurethane foam by coating the foam surface through a layer-by-layer assembly of up to 9 bilayers of alginate, alginate, chitosan, and hydroxyapatite particles; they studied the effect of the number of bilayers deposited on the foam surface on the flame-retardant and the mechanical properties of the resultant foams. PU Foams coated with 9 bilayers exhibited self-extinguishing characteristics at 32 s during exposure to a butane torch. The incorporation of 9 bilayers of FR coating to the flexible foam remarkably led to a decline in both peak heat release rate and smoke production rate by 77.7% and 53.8% respectively. The introduction of hydroxyapatite particles as reinforcement having a rigid surface resulted in an enhancement in tensile strength value by 56% against uncoated foams [74]. Senthil et al. [75] prepared reinforced biodegradable polyurethane foams and strips in a one-shot process by reacting a trifunctional polyol-polycaprolactonetriol as a soft segment and isophorone diisocyanate (IPDI) as a hard segment while incorporating fullerenes as a reinforcing agent in different loadings into the cellular network structure. It has been observed that the density and the hardness values of the fabricated foams tend to increase and vary as a function of C60 concentrations. Leszczyńskaa et al. [76] investigated the combined role of renewable resources on the individual properties of cellular foams during the fabrication of rigid polyurethane foams; they utilized different concentrations (10–50 wt.%) of rapeseed oil-based polyol (RO) and egg shell (ES) waste in a fixed amount of 20 php as reinforcing filler for the production of rigid foams. The obtained foams appeared light-yellow in colour with apparent density ranging from 85 to 93 kg/m3. The presence of RO polyol and ES filler induced lowering of glass transition temperature in the final foams owing to the plasticizing effect of fatty acid hydrocarbon chains of vegetable oil-based polyol. The compressive strength values of obtained foams were also found to be decreased with the addition of RO in the formulation recipe. The incorporation of vegetable-based polyol in higher concentrations has also compensated for the rise in foam friability and water absorption characteristics due to the presence of filler powder while improving the dimensional stability of the resultant foams.
2.3.2. Application of Bio-Based Polyurethane Foams
Polyurethane foams are one of the most versatile materials used today in different engineering and household applications, which include:
Insulation: Polyurethanes are inexpensive, long-lasting, and reduce carbon emissions [77]. Polyurethanes provide insulation that reduces heat loss in buildings, automobiles and refrigerators, thus saves energy. Polyurethane insulations are popular because of their durability and less maintenance and easy installation [78].
Furniture and Bedding: Polyurethanes are widely employed in furniture and beddings [79]. Flexible polyurethane foams are generally used for cushioning purposes, as their density can be adjusted according to the requirement of the users.
Footwear: Polyurethanes allow designers to make footwear that should be restful, durable and fit for use [80]. Polyurethanes with good abrasion-resistant properties are used to make shoe soles of high strength.
Packing Foams: Packing foams provides protection and cushioning to packaged products [81]. Polyurethane foams are available in convoluted, flat and custom-cut shapes and are often used to pack highly sensitive equipment such as electronics and circuit boards.
Rafting Boats: Raft manufacturers use RPUFs to produce inflatable boats and surfboards, as it provides strength, buoyancy and sounds deadening [82].
Biomedical applications of PU foams: There are various advantages to using PU foams for wound dressing purposes; they can provide acceptable gas and moisture mobility while having little adhesion to the wound spot due to their tunable porosity and pore diameters [83]. Silver nanoparticles for asiaticoside and antibacterial activity, a recognised herbal wound curing agent, were used to enhance bioactive characteristics. High polysaccharide components contributed to a powdery surface and reduced mechanical characteristics, so foams with middle polysaccharide components were analysed for their experimental efficacy in human patients with distressing dermal burns or abrasion wounds [84]. Outcomes demonstrated non-infected and accelerated wound healing as compared to usual treatments. Silver nanoparticles display cytotoxicity at high amounts, an approach to tackle this concern is by nanoparticle encapsulation with phenolated lignin before its incorporation into the PU foam [85].
2.4. Polyurethanes Elastomers
Polyurethane is an extensively used synthetic elastomer in biomedical applications due to its outstanding hemocompatibility and biocompatibility. Amending its chemical structure can affect its mechanical attributes such as responsiveness, fatigue resistance, elasticity, tolerance, or durability in the body during healing. Additionally, adjustment of chemical groups for PU structure, allows for an alteration of surface and bulk, by introducing biomolecules such as biorecognizable groups or anticoagulants, as well as hydrophilic/hydrophobic balance. Such variations have been developed to improve implant acceptability. As a consequence, traditional solvent-based polyurethanes have become the industry standard for high-performance systems, and are widely employed in medical devices such as tubing, dressings, antimicrobial membranes, catheters, and total artificial hearts, among others [86]. Figure 8 briefly displays the different applications of polyurethane elastomers in the biomedical field.
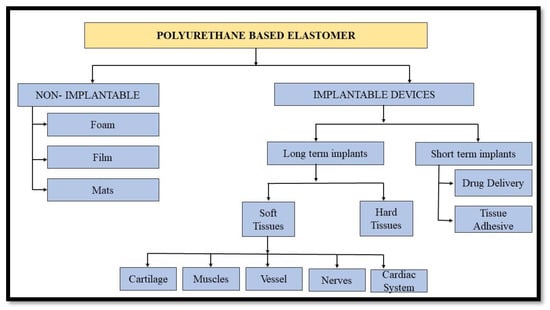
Figure 8.
Classification of Polyurethane Elastomers in Biomedical Field.
2.4.1. Non-Implantable Devices
Wound Dressing
Skin healing takes place in different time spans, depending on the extent of the case, according to the wound kind, infection degree, origin, depth, and health of the patient (diabetes, cancer). Wound exfoliation (removal of infected tissues), swabbing for infection, washing, and ultimately dressing with an acceptable system are all part of the chronic wound care standard; this stage is especially delicate, as an inappropriate substance might induce wound dryness or over-moisture, which can lead to bacterial infection [87].
Films as Wound Dressing Applications
Bio-based PU wound dressing membranes have been developed from vegetable oils. For example, a ricinoleic methyl ester-based prepolymer and methoxysilane (Si–OMe)-terminated castor oil prepolymer were used to make hybrid PU/siloxanes [88]. The introduction of a synthesised aniline tetramer resulted in significant electroactivity, antioxidant, and antibacterial properties [89].
Fibrous Mat for Wound Dressing Applications
Blending TPUs with biopolymers such as gelatin and/or chitosan before electrospinning has significantly improved the attributes such as biocompatibility, flexibility, and hydrophilicity [90] of wound dressing mats. Another method relies on the creation of a bi-layered mat prepared using gelatin electrospinning and electrospun TPU [91]. With AgNO3, for example, antibacterial TPU-gelatin-chitosan blends have been created, where, the mechanical support was provided by PU, with gelatin and chitosan as supplementary components.
2.4.2. Implantable Devices
Long Term Implants
Soft Tissues
Cardiac system: The production of biomimetic cardiac patches with elastomeric qualities while preserving mechanical support for the tissue is the topic of investigation for cardiac system applications. Due to a dearth of effective solutions, the demand for bioactive and biocompatible cardiac patches for improved cell proliferation and adhesion is increasing. Many polycaprolactone (PCL)-based PUs are used in cardiac patches and cardiac scaffolds containing urethane and urea groups for better mechanical attributes, derived from castor oil-based polyols [92]. To promote cell electroactivity and proliferation, oligomeric aniline subunits were added [93]. The generated scaffolds in this case had increased conductivity as the aniline level increased. The integration of manufactured gold nanowires/nanotubes in a castor oil-based PU also led to a fruitful electrical stimulation of cardiomyocyte cells for improved proliferation, adhesion, and subsequent heart tissue regeneration [94].
Cartilage: Cartilage tissues need better mechanical strength and modulus. Supramolecular ionic bonds from alginate-based PU elastomers with configurable elevated mechanical performance have been developed for this application [93]. The Young moduli ranged from 14 to 93 MPa. In vivo, the scaffolds degraded slowly, which was equivalent to PCL-based scaffolds. The preparation of water-based PU micelles has also been tried in conjunction with the synthesis of biodegradable PU in the form of porous scaffolds or ionic nanoparticles [95]. Researchers proceeded further by adding chondrogenic induction factors and blending the PU with hyaluronan solution for improved cartilage healing [96].
Muscles: For muscular applications, biobased TPU films based on biobased polyesters such as polylactic acid (PLA) [97] or polyhydroxyalkanoates (PHA) derivates have lately been prepared. Novel polyurethanes made from Hexamethylene Diamine Diisocyanate (HMDI), poly (3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4 H B) diol, and PLA diol were produced in a recent work [88]. Owing to a more regular structure compared to arbitrary block polymerization, porous scaffolds from PULA-alt-3/4 H B exhibit superior hemocompatibility, and shape recovery hydrophilicity, in vivo and in vitro cytocompatibility, which makes it a better candidate for muscle tissue scaffolds [88].
Nerves: Treatment options for peripherical nerve injuries is end-to-end interconnection if the space is less than 2–3 cm, and autologous graft for a longer gap. Biobased and biodegradable nerve conduit scaffolds have now been developed to direct the nerve’s repair through a tubular graft to replace autologous grafts. Owing to their excellent biocompatibility, tunable mechanical strength, biodegradability, and flexibility, polyurethanes are the most competitive materials. WPU films and a porous polyurethane network were prepared from a reported formulated [95] and employed in various biological applications. The inner, outer surfaces (9 and 42 m, respectively) and cross-section (23 m) of a porous PU scaffold showed pore interconnectivity and unequal pore diameters, which are reportedly found to be more effective than symmetrical pores, at draining wound inflammation waste during the early stages of neuron regeneration [98], thus, resulting in faster nerve regeneration. Porous networks showed superior permeability of a model bovine serum albumin (BSA) solution compared to PU films [99], implying effective mass transport for improved nerve healing.
Vessels: Currently, autogenous grafts are the most common procedure for vascular replacement, much as they are for hard tissue repair. Some individuals, however, lack sufficient autogenous tissues, necessitating the use of synthetic vascular grafts. Nonetheless, the development of tiny vessel scaffolds is required for improved compatibility and tissue regeneration [100]. A lysine triisocyanate PEG prepolymer was combined with an earlier synthesised polyester triol composed of glycolide, glycerol, D, L lactide, and caprolactone to create biobased PUs networks [101].
Hard Tissues
Even though the physicochemical and mechanical qualities of scaffolds are acceptable for bone tissue growth, and bone tissue regeneration; using PU scaffolds can have drawbacks such as inadequate cellular adhesion, differentiation, and biomineralization; this could be due to some breakdown products causing large pH changes in the scaffold microenvironment. It has been documented that a pH of around 7.40, which is around the physiological value, promotes osteoblast proliferation and differentiation [102]. Another disadvantage of PUs is their overall lack of bioactivity in specific tissue applications such as bone tissue regeneration. The scaffold prepared from glycerol-modified CO had better mechanical properties than the CO-based scaffold, with an elastic modulus of up to 165.36 MPa, probably due to a reduced number of hanging and unreacted end-groups. Good biocompatibility and osteogenic differentiation were achieved, as well as excellent bone matrix and trabecula regeneration. Another method is to graft bone ECM components to boost cell affinity and, as a result, promote high bone tissue regeneration [103].
Short-Term Implants
Drug delivery: Bio-based drug delivery systems have been developed with the goal of enhancing medication release by accelerating PU breakdown. It was accomplished by incorporating PLA [96] or PLGA [25] macromers into the PU backbone. By altering the quantity of PLA or molar mass in the final polyurethane, tailored degradation rates can be obtained [104]. Biodegradable PU with various soft segments are reported as efficient carriers of epirubicin [105]. Another method involves grafting PU onto biopolymers such as chitosan to create injectable hydrogels with controlled drug release, such as tetracycline hydrochloride [106]. The swellingbehaviour of pure Chitosan shows very high swelling (6000% of pure Chitosan), and it can be tuned nicely up to 200% by controlling its degree of substitution in PU or cross-link density through grafting. The first stimulus (such as temperature) normally allows these drug-loaded micelles to enter cancer cells, and the second stimulus (here, enzymatic attack) causes micelles to disassemble and release the drug. Other degradation stimuli have been investigated, with the goal of developing enzymatic intracellular sensitive bio-based NIPU micelles for the release of anticancer drugs [107]. From L-tyrosine amino acid resources, few amphiphilic poly-(ester-urethane)s were synthesized, and their self-assembled nanoparticles were used as drug delivery carriers for cancer therapy [108].
3. Non-Isocyanate Polyurethanes (NIPUs)
The isocyanates, as raw material in PU synthesis, are produced from hazardous and toxic phosgene by the process called phosgenation. Phosgene is a subtle toxin as the smell may not be observed and symptoms may be slow to arrive. It is known to be a widely used chemical weapon during the first world war accounting for a significant majority of fatalities. Moreover, the isocyanates themselves are toxic and moisture sensitive and cannot be prepared without sophisticated safety devices and massive investments.
PU foam production involves the generation of gas, usually, carbon dioxide, which may promote the release of isocyanates as vapours or aerosols, whose exposure may result in detrimental health effects such as asthma and skin irritation. The Bhopal Gas Tragedy of December 1984 in India was one of the deadliest industrial accidents in history that resulted in the deaths of thousands of people due to the methyl isocyanate gas leak; this chemical disaster attracted worldwide attention and demonstrated the immediate and hazardous effects of isocyanates [109]. Isocyanates enter the body primarily through inhalation or skin exposure. The most extensively used isocyanates for polyurethane production; MDI (methylene diisocyanate) and TDI (toluene diisocyanate) are labeled as CMR (carcinogenic, mutagen, reprotoxic) and are categorized as “very harmful” chemicals by the European Community. Perpetual exposure to MDI and TDI vapors leads to irritation in the skin, eyes, and respiratory tract. Additionally, during the storage and formation processes of moisture curing polyurethanes, the reaction between the isocyanate and water is troublesome, as it may cause moisture curing. In such systems, carefulseparation of these materials from the water becomes exceptionally essential to avoid an irreversible reaction between the polyisocyanate component and water, forming CO2 and urea, thus, resulting in an unusable, hardened product [109] (Figure 9).
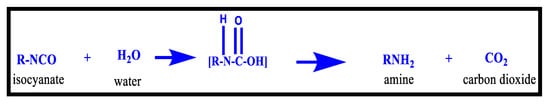
Figure 9.
Reaction of Isocyanate with water [109].
Thus, modern researchers have recognized the need to eliminate the use of isocyanates and are looking for ways to employ environment-friendly alternatives. One such alternative is the synthesis of Non-isocyanate polyurethanes (NIPU), a new type of PU that is safer and more promising than conventional PUs, and is becoming a viable substitute for conventional PUs.
One example of the isocyanate-free routes of PU production is the synthesis of hydroxyurethane in the presence of cyclic carbonate and amines [110], which is regarded as the best alternative method to synthesize Non-isocyanate polyurethanes (NIPU) as shown in Figure 10. There are two isomers formed in the reaction, one containing a secondary hydroxyl group, the other having a methylol group, each containing urethane, and a hydroxyl group. These hydroxyl entities can develop intermolecular hydrogen bonds with the urethane group [109].
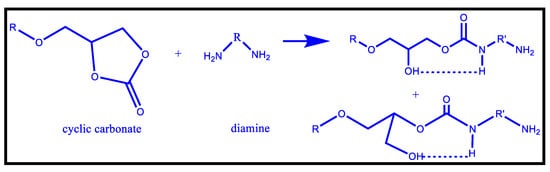
Figure 10.
Reaction of Cyclic Carbonates and Amines [110].
Another important method of NIPU synthesis is Aminolysis, which is mainly a two-step procedure involving, (i). the development of the cyclic carbonate oligomers and then (ii) treating it with polyamines or amines resulting in the production of NIPU; as depicted in Figure 11. Step 1 includes the development of cyclic carbonate, i.e., one of the most efficient ways of producing cyclic carbonate, by introducing the CO2 within the epoxide group using a suitable catalyst, followed by Step 2, i.e., Treating this cyclic carbonate oligomer with a primary amine to provide NIPU, via an addition reaction after ring-opening. Thus, if the cyclocarbonate oligomer has only cyclocarbonate, then it is called NIPU. Moreover, if something more than cyclocarbonate is present, i.e., hydroxyl groups or epoxy groups, etc., then it is termed HNIPU, which has different properties than that acquired by NIPU [109]. This is the most broadly used technique for the synthesis of isocyanate-free polyurethanes; the procedure comprises the ring opening of a cyclic carbonate via polyamine, leading to the formation of hydroxypolyurethanes.
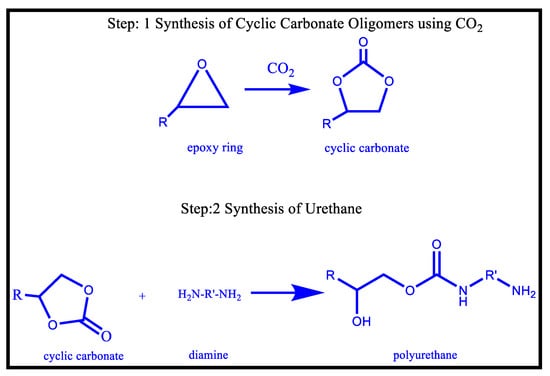
Figure 11.
Aminolysis for the synthesis of NIPU [109].
Merits of NIPU over PU: PU finds remarkable applications in paints, coatings, adhesives, and inks; for this, they are generally prepared in organic solvents. Consequently, large concentrations of hazardous air pollutants (HAPs) and volatile organic compounds (VOCs) evaporate in the atmosphere. Exposure to VOCs can cause health effects such as dizziness, headaches, cancer, irritation, etc. NIPU has many advantages over conventional polyurethanes. Primarily, the elimination of the use of isocyanates. Besides this, the cyclic carbonates are derived from the natural sources available through epoxy resins and CO2. Carbon dioxide is a cheap starting material and its utilization for PUs production would also definitely help to trim down the Global Warming, by reducing CO2, which is a Greenhouse gas. Thus, the two problems can be solved at once- firstly the shortage of oil resources, and secondly, the greenhouse effect. So overall it stands by the laws for environmental protection [111].
Applications of NIPU
NIPU Coatings and Adhesives: The applications of NIPU include especially chemical-resistant coatings and adhesives. In addition to the numerous applications of PUR in the production of coatings and in polymer industries. In addition to thermosetting coatings and UV-curable coatings, NIPU systems are capable of being used for monolithic flooring. Bio-sourced NIPU coatings and adhesives from mono(glucose) and disaccharides (sucrose) have been reported. It was also observed that the glucose-based NIPU displayed good surface coating and thermosetting joint adhesives [110].
Fire-resistant hybrid coatings made from inorganic material and PHUs have also been reported to have better properties, such as chemical resistance or fire resistance, or processability [112]. NIPU technology can also be employed in gasoline-stable and hydrolysis-stable sealants for safeguarding electronic devices and their associated machinery in rocket construction and aircraft and for civil engineering applications; glues with high adhesion strength and longevity. Lately, a UV curable PU resin synthesized using NIPU chemistry using cyclic carbonate of bisphenol-based epoxy resin and polyether amine was used for hydrophilic textile treatment. NIPU developed from plant sources has gained vast popularity recently. Moritz Bahr et al., studied linseed and soy-oil-based NIPU, synthesized by curing the carbonated linseed and carbonated soybean oil with different diamines such as ethylene diamine; they revealed a new-route to prepare the crosslinked as well as linear terpene-based non-isocyanate poly(hydroxypolyurethanes) (NIPU) and prepolymers obtained from novel cyclic limonene dicarbonate [113]. Bio-based NIPUs synthesized from carbonated sunflower oil have been studied by Simanta Doley and Swapan K. Dolui [110]. Carbonated vegetable oil was derived from a solvent-free reaction mixture of carbon dioxide with epoxidized sunflower oil. Javani et al. [114] documented that aromatic amine can be utilized as hardeners for various NIPU systems. P-xylenediamine, m-xylenediamine, and aliphatic/cycloaliphatic diamines were used by them for curing the reaction of cyclic carbonated soyabean oil. Xylene diamine led to increased tensile strength, with an inferior elongation in comparison to aliphatic diamine [114].
NIPU-based products can also be employed as sealants, owing to their brilliant adhesive properties. Dendro-aminosilane hardeners can also be used in NIPU sealant production, which enhances their thermal characteristics. High adhesion to glass and steel and high impact resistance can also be acquired based on organosilanepentacyclo-carbonate [115].
4. Recycling and Disposing of Polyurethanes
Polyurethane is the world’s sixth most widely used polymer, with a yearly production of 18 million tonnes, equating to each day’s production of PU speciality of more than 1 million m3. As a coordinate effect of its commercial victory, a plethora of waste is produced as a direct result of its commercial achievement and its management is an environmental and pressing concern. Land filling was once the quick fix to the obstacle; however, because of the long life cycle of polyurethanes (>10 years), limited knowledge is available about their activity in landfills. One of the problems confronting the current world is the recycling of any type of plastic in order to transform waste into profitable items. Furthermore, if the recycling procedure is green within itself, that would be an incredible accomplishment; this fact, combined with new environmental standards, makes the development of environmentally viable recycling processes critical.
Primary and secondary recycling are the two types of physical recycling processes, that are commonly used. These methods do not alter the structural features of the polymer. Polymeric waste is converted into powder, flakes, or granules for utilization in new material production in physical processes. The following are the most important physical recycling procedures for polyurethane:
- Rebonding: Flexible PU foam is cut into smaller pieces and it is used in the manufacture of sports mats and carpet lay [115].
- Regrind or Powdering: Powdered PU waste is blended with one of the virgin reagents (usually with the polyol up to 30% wt) to build new PU goods [115].
- Compression Molding: Powdered PU waste is exposed to high heat and pressures in the mold. It can enable upto approximately 100% recycled substance to be obtained [115].
These physical procedures are beneficial with polymers that are thermoplastic, but due to the thermostable nature of thermoset PU, they are ineffective with them. The process adopted, from the initial efforts of re-purposing polyurethane (PU) leftovers, as a cushion filler to one of the most recent chemical processes involving recycling agents which are green and sustainable. Chemical recycling enables the production of fundamental hydrocarbon entities called monomers (tertiary recycling) which could be utilized as production resources in the petrochemical industry and chemical synthesis materials. Using this method, it is conceivable to attain items with additional value.
4.1. Hydrolysis
The earliest chemical approach for recycling polyurethane waste, particularly flexible foams, was hydrolysis. It is the result of a reaction between polyurethane waste and water, which can be steam or liquid [116]. Amine intermediates, carbon compounds, and Polyols, are amidst the end products (Figure 12).
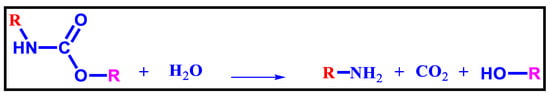
Figure 12.
Hydrolysis of Polyurethane [117].
It is essential to note that the hydrolysis technologies have merely been ramped up to pilot plant scale, and they’ve never made it to the commercial level because of the large energy and pressure input demand in the reactor [117].
4.2. Aminolysis
Aminolysis is a sort of transesterification reaction during which the amine group replaces the ester group attached to the carbonyl carbon of the carbamate linkage of urethane [115], as shown in Figure 13. Products containing polyurethanes are generally crushed or dissolved in a specific solvent, such as cyclic ether or a nitrogen-containing chlorinated hydrocarbon solvent; they are then aminolyzed with a molecule that contains at least one –NH2 group; this reaction takes place at a temperature of 80–190 °C, and it is widely practiced with catalysts such asaluminium hydroxide, sodium methoxide, and sodium hydroxide. Polyfunctional alcohols and amines can be produced according to the diamine or amino alcohol employed; they can be used to synthesize polycarbonates, melamine resins, polyurethanes, polyesters, epoxy resins, etc. Two or more of the resulting functional phenols can also be used to recover the associated isocyanates; this method has also not gained any commercial success yet.
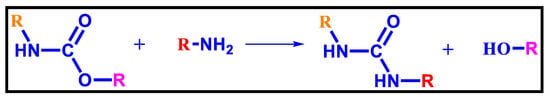
Figure 13.
Aminolysis of Polyurethanes [115].
4.3. Phosphorolysis
Phosphorolysis is a process comparable to hydrolysis where esters of phosphoric acids or phosphonic display similar behaviour as water with the production of phosphate (Figure 14); this method allowed a combination of phosphorus-containing oligo-urethanes to be created. Because of the phosphate functionality of the recovered product, phosphorolysis can provide glycolyzates that can be employed in the manufacture of polyurethanes with flame retardant characteristics; this approach has the privilege of being able to create novel polyurethanes with enhanced adhesive characteristics, flame retardancy (comparable to industrial goods with larger concentrations of flame retardants), and UV resistance [117].
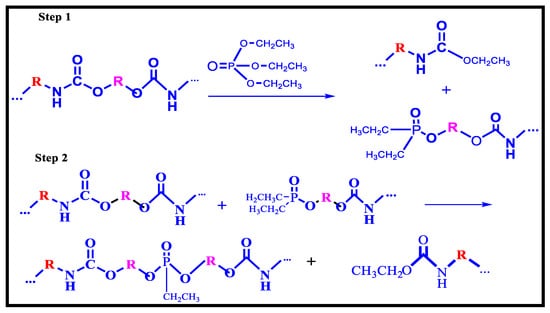
Figure 14.
Phosphorolysis of Polyurethane [117].
4.4. Glycolysis
The most extensively utilised chemical recycling procedure for PU is glycolysis. It comprises a transesterification reaction wherein a glycol’s hydroxyl group replaces the ester group attached to the carbonylic carbon of the urethane [118]. The primary reaction is as shown in Figure 15.
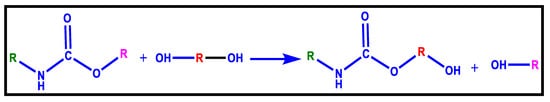
Figure 15.
Glycolysis of Polyurethanes [118].
This reaction creates polyols with properties that can be tweaked to a few degrees and are potentially similar to the original substance; they can be used to manufacture polyurethanes. Glycolysis can go in two different directions. The first allows polyols to be recovered and used to make flexible PU foam. Split-phase glycolysis (SPG), on the other hand, produces flexible or rigid polyols. SPG is built on diethylene glycol (DEG) glycolysis processing of the bottom and top layers separately. The upper layer, which primarily consists of flexible polyols, is washed with DEG, while the lower layer is allowed to react with propylene oxide to produce rigid polyols. The biggest impediment of glycolysis is the disparity in process parameters between flexible and rigid foams, which forces a waste separation. Due to the great susceptibility of the process to the presence of contaminants that commonly exist in consumer trash, this approach is also substantially more successful when employed in post-production waste [118]. Table 5 reviews the glycolysis of various polyurethane materials. The following agents contribute significantly toward the glycolysis of polyurethane materials:

Table 5.
Glycolysis of Various Polyurethane Materials.
- Catalysts: The catalysts engaged are amines such as diethanolamine and ethanolamine; alkaline acetates such as sodium, potassium, or lithium acetate; hydroxides such as potassium or sodium hydroxides; metallic octoates such as stannous or lithium octoates and organometallic compounds such as titanium butoxide [115].
- Cleavage Agents: DPG, DEG, MPG, and MEG, and are some of the most commonly utilised cleavage agents, with DEG being the most common; however, crude glycerol is now the most assuring [115].
- Glycolysis agent: PU ratios: In transesterification reaction, the most frequent glycolysis agent: PU ratios used. It can be seen that the glycolysis agent to PU ratio is often more than 1, in order to ensure optimal split-phase glycolysis and, as an outcome, a good quality glycolyzate that may be used in the generation of a fresh PU product [115].
- Temperature: the best temperature for carrying out the glycolysis reaction is between 160 and 250 °C, because cooler temperatures result in a slow recovery course and a low polyol proportion, whereas higher temperatures result in excessive evaporation of cleavage agent and a rise in the extension of the secondary reactions [115].
4.5. Gasification
Gasification is the partial oxidation of carbonate compounds which is a highly exothermic process, resulting in the formation of Syngas (a combination of mostly hydrogen and carbon monoxide) and ash. One of the most notable benefits of gasification is that it eliminates the requirement for waste segregation. Polyurethanes that have been combined with some other raw materials can also be employed in the course of action. The profitability of this method, nevertheless, completely depends on the probable use of syngas asraw material and a source of energy for the production of acetic acid, ammonia, methanol, carbohydrates, and other chemicals [119].
4.6. Pyrolysis
Pyrolysis is the anaerobic, high-pressure thermal disintegration of complex polymeric chains into simpler molecules [120]. According to a study, polyurethane pyrolysis occurs in at least two stages, which corresponds to the sequential thermal decomposition of the isocyanate and polyol parts of the polyurethane. Over half of the polymer weight is lost in the first stage, which runs at temperatures lying in the range of 100–300 degrees Celsius. The next stage of degradation begins between 300 and 800 degrees Celsius. The amount of ash left over after pyrolysis is even less than 3% of the original polyurethane [121]. Pyrolysis has undeniable advantages, including the little quantity of waste produced and the ability to use the resultant chemicals in other petrochemical operations. Unfortunately, due to the failure to define the ratio of individual items and the complexity of getting products with the appropriate qualities, this is not always practicable. The resultant gas has a high calorific value and can be used to heat and power gas engines. Furthermore, gas products carry hazardous chemicals such as aniline, benzene, and hydrogen cyanide [117].
4.7. Hydrogenation
Pyrolysis and gasification are both processes that are analogous to hydrogenation. Gas and oil are formed as a result of this process. The use of high-pressure hydrogen rather than inert gas is the primary distinction between this technique and pyrolysis. This strategy relies on addressing two key issues: the composition of the obtained gas and oil, as well as the cost of converting them into functional products that may be utilised as substrates and energy materials in chemical methods. Only gasification and glycolysis have been executed on a wide scale among chemical processes of polyurethane recycling, while others are still in the research phase. Furthermore, just glycolysis results in raw material recovery [122].
5. Biological Degradation of Polyurethane
The decomposition of organic compounds by various living organisms or their enzymes is referred to as biodegradation. It leads to the shortening of polymeric chains and the eradication of some of their components leading to the decrease in its molecular mass, and under the appropriate conditions, it can also lead to total mineralization of deteriorated material. Since the process does not demand elevated temperatures or sophisticated chemicals, biodegradation is generally more environmentally pleasant than chemical degradation. It can also be used for the decomposition of post-consumer waste. Bacterial biodegradation, fungal biodegradation, and enzymatic degradation are the three types of polyurethane modifications. Polyester polyurethanes are substantially more biologically degradable than polyether polyurethanes. The use of fungal strains in research yields more promising outcomes for polyether ones. Mechanical cracking of more obstinate polyether urethanes, which are commonly used in foams, can be linked to mycelium accessing material pores and causing mechanical cracking. On the other side, microorganisms are used in the majority of PU coating degradation studies. It is possible that this is due to bacteria’s ability to develop biofilm on the sleek coated surface.
5.1. Degradation by Fungi
Fungi are microbes that are recognised to be the primary cause of PU degradation in experimentally tested species. The majority of studies focus on soil microorganisms involved in polyurethane breakdown [123,124]. Irrespective of isolation circumstances, soil source, or polyurethane employed by scientists, the majority of isolated species that exhibit promising results in PU breakdown fall into one of a few categories such as Aspergillus, Cladosporium, Chaetomium, Penicillium, and Trichoderma; however, most of the fungi responsible for degrading polyurethane are filamentous, with only a few yeasts yielding positive results [125]; this disparity could be due to abiotic outcomes of fungal biodegradation. Growing filaments enter the material, causing the pore size to expand, perhaps leading to material fracture; however, the mechanism of this course, as well as the role of abiotic and biotic components of fungal degradation, remain unknown. All types of polyurethanes, including coatings, foams, polyether, polyester, and thermoplastic PU, have been found to be biodegradable by fungus strains and communities [117].
5.2. Degradation by Bacteria
Though fungi are responsible for the majority of the encouraging outcomes, some studies point to bacteria as possible polyurethane degrading microorganisms. Acinetobactergerneri, Bacillus subtilis, Comamonasacidovorans, and Pseudomonas chlororaphis were found as microbes capable of degrading polyester polyurethane Impranil DLN in research [117].
5.3. Degradation by Enzymes
The fundamental benefit of enzymatic degradation over microbial degradation is the ability to manage bond breakage, which results in the synthesis of building blocks that can be recycled or used as a substrate in the manufacture of other materials. The key problem in this approach is the wide variety of raw materials utilised in polyurethane synthesis, which necessitates treating each type of PU separately.
Figure 16 summarises the degradation of different polyurethanes by various enzymes.
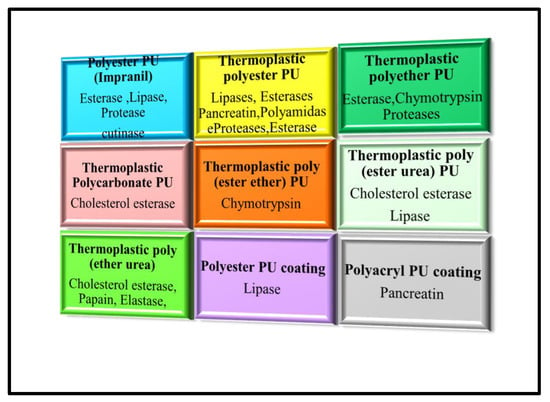
Figure 16.
Degradation of Different Polyurethanes by Various Enzymes.
The hydrolysis of the polyester component of polyester-based polyurethanes by esterases (Figure 17) is responsible for the majority of favourable biodegradation outcomes. According to certain research, esterases are also accountable for the hydrolysis of urethane linkages, resulting in alcohol chain-ends and carbamic acid; however, because acid is unstable and dissociates quickly, the release of carbon dioxide and amine is a more likely event. Furthermore, because most polyurethane degradation assays use polyester types, they do not distinguish between urethane hydrolysis and ester bond hydrolysis. Researchers studying urease degradation of polyurethane encounter a similar issue, but the majority of their observations are favourable as long as the polyurethane contains urea linkages. Those linkages could be a piece of the soft section or the result of water foaming. Those in long, stretchy chains, on the other hand, are much simpler to disintegrate. The generation of alcohol, amine, and the release of carbon dioxide, proved to be extremely fruitful for urethane bond hydrolysis by peptidases and amidases [122].
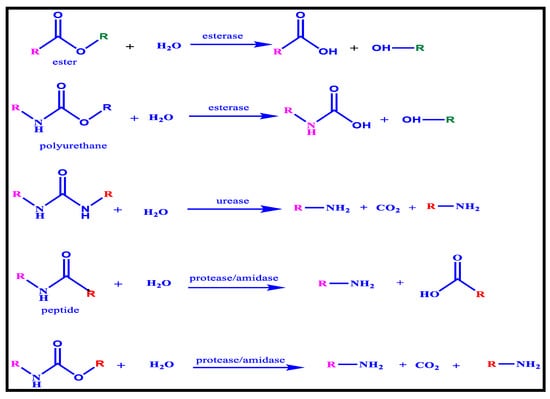
Figure 17.
Enzymatic Degradation of Polyurethane.
6. Conclusions
Attempts have been devoted in the recent past to manufacture green PU materials from renewable resources to satisfy the specified qualities for social, technological, and economic criteria. Different compound strategies for the integration of PU have been offered to study the use of renewable resources rather than petroleum base requirements in both research and industry. The bio-based PU’s promise as a long-term contender for upgrading bio-based derivatives via different processes. The bio-based polyurethanes have significant potential to be used as future PU products, with a partial or total replacement of petroleum-based polyurethanes; however, the literature studies have revealed a few problems, such as, the low bio-based content, lack of comprehension of individual formulations and a lack of insight into catalyst toxicity and degradation products. Nonetheless, the overall energy/cost imbalance is an open difficulty associated with these reaction processes; this gave rise to the sustainable alternative for the conventional polyurethanes i.e., Non-Isocyanate polyurethanes. The NIPUs are proving to be very efficient in a diverse range of applications owing to their hydrolytic stability, moisture insensitivity, and green pathway. To deal with the problem of post-consumption PU wastage, different recycling techniques are employed and reviewed in detail. Special emphasis has been given to the biodegradation of polyurethane wastes. Biological degradation requires a moderate temperature and does not necessitate the use of toxic chemicals, but there is still a long journey to go before it can be employed on a large basis. Biodegradation, in particular, is the most attractive due to a large number of available options and accessible alterations. By employing suitably altered building blocks while preparing, PU can be made 100% degradable; this approach could be a realistic alternative to deal with the accumulation of polyurethane waste. In nutshell, the path of polyurethane synthesis progresses from hazardous to non-toxic, and finally to sustainable.
Author Contributions
Conceptualization, R.K.; Methodology, P.S.; Validation, R.K.; Formal Analysis, S.T.; Investigation, P.S.; Resources, S.Y.; Data Curation, S.T.; Writing—original Draft Preparation, P.S. and G.V.; Writing—Review and Editing, G.V. and S.Y.; Supervision, R.K.; Project Administration, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Delhi Technological University for providing technical and financial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2020, 278, 111527. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Cornille, A.; Guillet, C.; Benyahya, S.; Negrell, C.; Boutevin, B.; Caillol, S. Room temperature flexible isocyanate-free polyurethane foams. Eur. Polym. J. 2016, 84, 873–888. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Paraskar, P.M. Synthesis of Isostearic Acid/Dimer Fatty Acid-Based Polyesteramide Polyol for the Development of Green Polyurethane Coatings. J. Polym. Environ. 2021, 29, 54–70. [Google Scholar]
- Cheng, Z.; Li, Q.; Yan, Z.; Liao, G.; Zhang, B.; Yu, Y.; Yi, C.; Xu, Z. Design and synthesis of novel aminosiloxane crosslinked linseed oil-based waterborne polyurethane composites and its physicochemical properties. Prog. Org. Coat. 2018, 127, 194–201. [Google Scholar] [CrossRef]
- Kovács, E.; Turczel, G.; Szabó, L.; Varga, R.; Tóth, I.; Anastas, P.T.; Tuba, R. Synthesis of 1,6-Hexandiol, Polyurethane Monomer Derivatives via Isomerization Metathesis of Methyl Linolenate. ACS Sustain. Chem. Eng. 2017, 5, 11215–11220. [Google Scholar] [CrossRef]
- Liang, H.; Feng, Y.; Lu, J.; Liu, L.; Yang, Z.; Luo, Y.; Zhang, Y.; Zhang, C. Bio-based cationic waterborne polyurethanes dispersions prepared from different vegetable oils. Ind. Crops Prod. 2018, 122, 448–455. [Google Scholar] [CrossRef]
- Gomez-Jimenez-Aberasturi, O.; Gomez, J.R. New approaches to producing polyols from biomass. J. Chem. Technol. Biotechnol. 2017, 92, 705–711. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Fan, Y. Preparation of Soy-Based Adhesive Enhanced by Waterborne Polyurethane: Optimization by Response Surface Methodology. Adv. Mater. Sci. Eng. 2018, 2018, 9253670. [Google Scholar] [CrossRef] [Green Version]
- Zuber, M.; Shah, S.A.A.; Jamil, T.; Asghar, M.I. Performance behavior of modified cellulosic fabrics using polyurethane acrylate copolymer. Int. J. Biol. Macromol. 2014, 67, 254–259. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crop. Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Vanbésien, T.; Monflier, E.; Hapiot, F. Hydroformylation of vegetable oils: More than 50 years of technical innovation, successful research, and development. Eur. J. Lipid Sci. Technol. 2015, 118, 26–35. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- Peyrton, J.; Chambaretaud, C.; Sarbu, A.; Avérous, L. Biobased Polyurethane Foams Based on New Polyol Architectures from Microalgae Oil. ACS Sustain. Chem. Eng. 2020, 8, 12187–12196. [Google Scholar] [CrossRef]
- Devi, K.; Nurul, P.P.; Ain, H.; Tuan Noor Maznee, T.I.; MohdNorhisham, S.; Srihanum, A.; Norhayati, M.N.; Yeong, S.K.; Hazimah, A. Green Polyurethane for Ornamental Products; MPOB Information Series, MPOB TT No. 522; MPOB: Bandar Baru Bangi, Malaysia, 2012. [Google Scholar]
- De Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. ACS Symp. Ser. 2021, 1380, 1–24. [Google Scholar]
- KhoonPoh, A.; Choy Sin, L.; Foon, C.; Hock, C. Polyurethane wood adhesive from palm oil-based polyester polyol. J. Adhes. Sci. Technol. 2014, 28, 1020–1033. [Google Scholar] [CrossRef]
- Ang, K.P.; Lee, C.S.; Cheng, S.F.; Chuah, C.H. Synthesis of palm oil-based polyester polyol for polyurethane adhesive production. J. Appl. Polym. Sci. 2013, 131, 6. [Google Scholar] [CrossRef]
- Li, N.; Qi, G.; Sun, X.S.; Stamm, M.J.; Wang, D. Physicochemical Properties and Adhesion Performance of Canola Protein Modified with Sodium Bisulfite. J. Am. Oil Chem. Soc. 2011, 89, 897–908. [Google Scholar] [CrossRef]
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive. Ind. Crops Prod. 2014, 60, 177–185. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.; Aung, M.; Salleh, M.; Biak, D.A.; Basri, M.; Jusoh, E.; Mamat, S.; Al Edrus, S.O. Chemical and Thermo-Mechanical Properties of Waterborne Polyurethane Dispersion Derived from Jatropha Oil. Polymers 2021, 13, 795. [Google Scholar] [CrossRef]
- Wang, C.-S.; Yang, L.-T.; Ni, B.-L.; Shi, G. Polyurethane networks from different soy-based polyols by the ring opening of epoxidized soybean oil with methanol, glycol, and 1,2-propanediol. J. Appl. Polym. Sci. 2009, 114, 125–131. [Google Scholar] [CrossRef]
- Valero, M.; Gonzalez, A. Polyurethane adhesive system from castor oil modified by a transesterification reaction. J. Elastomers Plast. 2012, 44, 433–442. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, L.; Ding, M.; Tan, H.; Li, J.; Fu, Q. Preparation and rapid degradation of nontoxic biodegradable polyurethanes based on poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) and l-lysine diisocyanate. Polym. Cem. 2011, 2, 601–607. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Evaluation of biodegradability of green polyurethane/nanosilica composite synthesized from transesterified castor oil and palm oil based isocyanate. Int. Biodeterior. Biodegrad. 2017, 117, 278–288. [Google Scholar] [CrossRef]
- Rodriguez, R.; Pérez, B.; Florez, S. Effect of Different Nanoparticles on Mechanical Properties and Curing Behavior of Thermoset Polyurethane Adhesives. J. Adhes. 2014, 90, 848–859. [Google Scholar] [CrossRef]
- Mirabedini, S.; Mohseni, M.; PazokiFard, S.; Esfandeh, M. Effect of TiO2 on the mechanical and adhesion properties of RTV silicone elastomer coatings. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 80–86. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M. Brief Overview on Bio-Based Adhesives and Sealants. Polymers 2019, 11, 1685. [Google Scholar] [CrossRef] [Green Version]
- Mapari, S.; Mestry, S.; Mhaske, S.T. Developments in pressure-sensitive adhesives: A review. Polym. Bull. 2021, 78, 4075–4108. [Google Scholar] [CrossRef]
- Liu, X.; Hong, W.; Chen, X. Continuous Production of Water-Borne Polyurethanes: A Review. Polymers 2020, 12, 2875. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martin-Martínez, J.M. Thermoplastic polyurethane pressure sensitive adhesives made with mixtures of polypropylene glycols of different molecular weights. Int. J. Adhes. Adhes. 2019, 88, 81–90. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Yang, J. Design strategies and applications of tissue bioadhesives. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Tu, W.; Hu, J. Preparation and Characterization of Two-component Waterborne Polyurethane Comprised of Water-soluble Acrylic Resin and HDI Biuret. Chin. J. Chem. Eng. 2006, 14, 99–104. [Google Scholar] [CrossRef]
- Malik, M.; Kaur, R. Mechanical and Thermal Properties of Castor Oil–Based Polyurethane Adhesive: Effect of TiO2 Filler. Adv. Polym. Technol. 2016, 37, 24–30. [Google Scholar] [CrossRef]
- Dai, J.; Liu, X.; Ma, S.; Wang, J.; Shen, X.; You, S.; Zhu, J. Soybean oil-based UV-curable coatings strengthened by crosslink agent derived from itaconic acid together with 2-hydroxyethyl methacrylate phosphate. Prog. Org. Coat. 2016, 97, 210–215. [Google Scholar] [CrossRef]
- Raydan, N.D.V.; Leroyer, L.; Charrier, B.; Robles, E. Recent Advances on the Development of Protein-Based Adhesives for Wood Composite Materials—A Review. Molecules 2021, 26, 7617. [Google Scholar] [CrossRef]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Processes 2016, 4, 11. [Google Scholar] [CrossRef]
- Silva, B.B.R.; Santana, R.M.C.; Forte, M.C. A solventless castor oil-based PU adhesive for wood and foam substrates. Int. J. Adhes. Adhes. 2010, 30, 559–565. [Google Scholar] [CrossRef]
- Patel, M.R.; Shukla, J.M.; Patel, N.K.; Patel, K.H. Biomaterial based novel polyurethane adhesives for wood to wood and metal to metal bonding. Mater. Res. 2009, 12, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Radojčić, D.; Ionescu, M.; Petrović, Z.S. Novel potentially biodegradable polyurethanes from bio-based polyols. Contemp. Mater. 2013, 4, 9–21. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Bio-Renewable Sources for Synthesis of Eco-Friendly Polyurethane Adhesives—Review. Open J. Polym. Chem. 2017, 7, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Börcsök, Z.; Pásztory, Z. The role of lignin in wood working processes using elevated temperatures: An abbreviated literature survey. Eur. J. Wood Wood Prod. 2021, 79, 511–526. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2019, 3, 8–18. [Google Scholar] [CrossRef]
- Sudoł, E.; Kozikowska, E. Mechanical Properties of Polyurethane Adhesive Bonds in a Mineral Wool-Based External Thermal Insulation Composite System for Timber Frame Buildings. Materials 2021, 14, 2527. [Google Scholar] [CrossRef]
- Calpena, E.O.; Francisca, A.A.; Sánchez, M.A.M. Adhesives in the Footwear Industry: A Critical Review. Rev. Adhes. Adhes. 2019, 7, 69–91. [Google Scholar] [CrossRef]
- Bao, Z.; Gao, M.; Sun, Y.; Nian, R.; Xian, M. The recent progress of tissue adhesives in design strategies, adhesive mechanism and applications. Mater. Sci. Eng. C 2020, 111, 110796. [Google Scholar] [CrossRef]
- Liu, L.; Lu, J.; Zhang, Y.; Liang, H.; Liang, D.; Jiang, J.; Lu, Q.; Quirino, R.L.; Zhang, C. Thermosetting polyurethanes prepared with the aid of a fully bio-based emulsifier with high bio-content, high solid content, and superior mechanical properties. Green Chem. 2018, 21, 526–537. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Kothapalli, R.V. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Sawpan, M.A. Polyurethanes from vegetable oils and applications: A review. J. Polym. Res. 2018, 25, 184. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Shang, Q.; An, R.; Liu, C.; Hu, L.; Zhou, Y. Rubber Seed Oil-Based UV-Curable Polyurethane Acrylate Resins for Digital Light Processing (DLP) 3D Printing. Molecules 2021, 26, 5455. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.M.; Jirimali, H.D.; Gite, V.V.; Jagtap, R.N. Synthesis and performance of bio-based hyperbranched polyol in polyurethane coatings. Prog. Org. Coat. 2020, 149, 105895. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Noh, H.-J.; Baek, J.-B. Hyperbranched Macromolecules: From Synthesis to Applications. Molecules 2018, 23, 657. [Google Scholar] [CrossRef] [Green Version]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Saif, M.J. Recent trends in environmentally friendly water-borne polyurethane coatings: A review. Korean J. Chem. Eng. 2015, 33, 388–400. [Google Scholar] [CrossRef]
- Anıl, D.; Berksun, E.; Durmuş-Sayar, A.; Sevinis-Özbulut, E.B.; Ünal, S. Chapter 11—Recent advances in waterborne polyurethanes and their nanoparticle-containing dispersions. In Handbook of Waterborne Coatings; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–302. [Google Scholar]
- Zhang, Y.; Zhang, W.; Wang, X.; Dong, Q.; Zeng, X.; Quirino, R.L.; Lu, Q.; Wang, Q.; Zhang, C. Waterborne polyurethanes from castor oil-based polyols for next generation of environmentally-friendly hair-styling agents. Prog. Org. Coat. 2020, 142, 105588. [Google Scholar] [CrossRef]
- Agnol, L.D.; Dias, F.T.G.; Ornaghi, H.L.; Sangermano, M.; Bianchi, O. UV-curable waterborne polyurethane coatings: A state-of-the-art and recent advances review. Prog. Org. Coat. 2021, 154, 106156. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Cashew nut shell liquid terminated self-healable polyurethane as an effective anticorrosive coating with biodegradable attribute. Prog. Org. Coat. 2019, 139, 105472. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A.; D’Mello, D. Isocyanate free polyurethanes from new CNSL based bis-cyclic carbonate and its application in coatings. Eur. Polym. J. 2014, 57, 99–108. [Google Scholar] [CrossRef]
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153. [Google Scholar] [CrossRef]
- Kinaci, E.; Can, E.; La Scala, J.J.; Palmese, G.R. Epoxidation of Cardanol’s Terminal Double Bond. Polymers 2020, 12, 2104. [Google Scholar] [CrossRef]
- Asif, A.H.; Mahajan, M.S.; Sreeharsha, N.; Gite, V.V.; Al-Dhubiab, B.E.; Kaliyadan, F.; Nanjappa, S.H.; Meravanige, G.; Aleyadhy, D.M. Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles. Materials 2022, 15, 2308. [Google Scholar] [CrossRef] [PubMed]
- Stachak, P.; Łukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of Non-Isocyanate Polyurethane-Based Composite Materials. Materials 2021, 14, 3497. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, G.; Jin, C.; Kong, Z. Preparation and antimicrobial activity of terpene-based polyurethane coatings with carbamate group-containing quaternary ammonium salts. Prog. Org. Coat. 2015, 80, 150–155. [Google Scholar] [CrossRef]
- Llevot, A.; Meier, M. Perspective: Green polyurethane synthesis for coating applications. Polym. Int. 2019, 68, 826–831. [Google Scholar] [CrossRef]
- Olcay, H.; Kocak, E.D.; Yıldız, Z. Sustainability in Polyurethane Synthesis and Bio-based Polyurethanes BT. In Sustainability in the Textile and Apparel Industries: Sourcing Synthetic and Novel Alternative Raw Materials; Springer International Publishing: Cham, Switzerland, 2020; pp. 139–156. [Google Scholar]
- Lee, S.T.; Park, C.B.; Ramesh, N.S. Polymeric foams. In Polymers, 1st ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; Volume 6. [Google Scholar]
- Szpiłyk, M.; Lubczak, R.; Lubczak, J. The biodegradable cellulose-derived polyol and polyurethane foam. Polym. Test. 2021, 100, 107250. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Kirpluks, M.; Priya, A.; Kaur, G. Conversion of food waste-derived lipid to bio-based polyurethane foam. Case Stud. Chem. Environ. Eng. 2021, 4, 100131. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Influence of NCO/OH ratio on preparation of four agricultural wastes liquefied polyols based polyurethane foams. Polym. Degrad. Stab. 2020, 179, 109256. [Google Scholar] [CrossRef]
- Jonjaroen, V.; Ummartyotin, S.; Chittapun, S. Algal cellulose as a reinforcement in rigid polyurethane foam. Algal Res. 2020, 51, 102057. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. A fully bio-based coating made from alginate, chitosan and hydroxyapatite for protecting flexible polyurethane foam from fire. Carbohydr. Polym. 2020, 246, 116641. [Google Scholar] [CrossRef]
- Thangavelu, S.A.G.; Mukherjee, M.; Layana, K.; Kumar, C.D.; Sulthana, Y.R.; Kumar, R.R.; Ananthan, A.; Muthulakshmi, V.; Mandal, A.B. Biodegradable polyurethanes foam and foam fullerenes nanocomposite strips by one-shot moulding: Physicochemical and mechanical properties. Mater. Sci. Semicond. Process. 2020, 112, 105018. [Google Scholar] [CrossRef]
- Moon, J.; Kwak, S.B.; Lee, J.Y.; Kim, D.; Ha, J.U.; Oh, J.S. Recycling of bio-polyurethane foam using high power ultrasound. Polymer 2019, 186, 122072. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Ryszkowska, J.; Szczepkowski, L.; Kurańska, M.; Prociak, A.; Leszczyński, M.K.; Gloc, M.; Antos-Bielska, M.; Mizera, K. Cooperative effect of rapeseed oil-based polyol and eggshells on the structure and properties of rigid polyurethane foams. Polym. Test. 2020, 90, 106696. [Google Scholar] [CrossRef]
- Mirski, R.; Dukarska, D.; Walkiewicz, J.; Derkowski, A. Waste Wood Particles from Primary Wood Processing as a Filler of Insulation PUR Foams. Materials 2021, 14, 4781. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Kaur, R.; Walia, R.S. PU foam derived from renewable sources: Perspective on properties enhancement: An overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, M. Function of silicon oil in the castor oil based rigid polyurethane foams. J. Polym. Eng. 2013, 33, 875–880. [Google Scholar] [CrossRef]
- Kumar, M.; Kaur, R. Effect of Different Formulations of MDI on Rigid Polyurethane Foams based on Castor Oil. Int. J. Sci. Res. Rev. 2013, 2, 29–42. [Google Scholar]
- Agrawal, A.; Kaur, R.; Walia, R.S. Investigation on flammability of rigid polyurethane foam-mineral fillers composite. Fire Mater. 2019, 43, 917–927. [Google Scholar] [CrossRef]
- Kumar, M.; Kaur, R. Glass fiber reinforced rigid polyurethane foam: Synthesis and characterization. e-Polymers 2017, 17, 517–521. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R. Development of vegetable oil-based conducting rigid PU foam. e-Polymers 2019, 19, 411–420. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. Flame retardancy of ceramic-based rigid polyurethane foam composites. J. Appl. Polym. Sci. 2019, 136, 48250. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, M. Addition of anti-flaming agents in castor oil based rigid polyurethane foams: Studies on mechanical and flammable behaviour. Mater. Res. Express 2020, 7, 015333. [Google Scholar] [CrossRef]
- Shin, E.J.; Choi, S.M. Advances in Waterborne Polyurethane-Based Biomaterials for Biomedical Applications. Adv. Exp. Med. Biology. 2018, 1077, 251–283. [Google Scholar]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendels, S.; Avérous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef]
- Baheiraei, N.; Gharibi, R.; Yeganeh, H.; Miragoli, M.; Salvarani, N.; Di Pasquale, E.; Condorelli, G. Electroactive polyurethane/siloxane derived from castor oil as a versatile cardiac patch, part I: Synthesis, characterization, and myoblast proliferation and differentiation. J. Biomed. Mater. Res. Part A 2016, 104, 775–787. [Google Scholar] [CrossRef]
- Tan, L.; Hu, J.; Huang, H.; Han, J.; Hu, H. Study of multi-functional electrospun composite nanofibrous mats for smart wound healing. Int. J. Biol. Macromol. 2015, 79, 469–476. [Google Scholar] [CrossRef]
- Tan, L.; Hu, J.; Zhao, H.-F. Design of bilayered nanofibrous mats for wound dressing using an electrospinning technique. Mater. Lett. 2015, 156, 46–49. [Google Scholar] [CrossRef]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Ebrahimibarough, S.; Azami, M.; Vahdat, S.; Baharvand, H. Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3179–3187. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.C. Facile Synthesis of Degradable and Electrically Conductive Polysaccharide Hydrogels. Biomacromolecules 2011, 12, 2601–2609. [Google Scholar] [CrossRef]
- Ganji, Y.; Kasra, M.; Salahshour, K.S.; Bagheri, H.M. Synthesis and characterization of gold nanotube/nanowire–polyurethane composite based on castor oil and polyethylene glycol. Mater. Sci. Eng. C 2014, 42, 341–349. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Hung, K.-C.; Hung, S.-C.; Hsu, S.-H. Evaluation of biodegradable elastic scaffolds made of anionic polyurethane for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2015, 125, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Tseng, C.-S.; Dai, L.-G.; Hsu, S.-H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, R.; Ge, J.; Guo, B.; Ma, P.X. Biocompatible, Biodegradable, and Electroactive Polyurethane-Urea Elastomers with Tunable Hydrophilicity for Skeletal Muscle Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 28273–28285. [Google Scholar] [CrossRef]
- Chang, C.-J.; Hsu, S.-H. The effect of high outflow permeability in asymmetric poly(dl-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. Biomaterials 2006, 27, 1035–1042. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Chang, W.-C.; Yen, C.-T. Novel flexible nerve conduits made of water-based biodegradable polyurethane for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2017, 105, 1383–1392. [Google Scholar] [CrossRef]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef]
- Adolph, E.J.; Guo, R.; Pollins, A.C.; Zienkiewicz, K.J.; Cardwell, N.L.; Davidson, J.M.; Guelcher, S.A.; Nanney, L.B. Injected biodegradable polyurethane scaffolds support tissue infiltration and delay wound contraction in a porcine excisional model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1679–1690. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Yang, C.; Darvell, B.W.; Wu, J.; Lin, K.; Chang, J.; Pan, H.; Lu, W. Alkaline biodegradable implants for osteoporotic bone defects--importance of microenvironment pH. Osteoporos. Int. 2016, 27, 93–104. [Google Scholar] [CrossRef]
- González Paz, R.J.; Lligadas, G.; Ronda, J.C.; Galià, M.; Ferreira, A.M.; Boccafoschi, F.; Ciardelli, G.; Cadiz, V. Enhancement of fatty acid-based polyurethanes cytocompatibility by non-covalent anchoring of chondroitin sulfate. Macromol. Biosci. 2012, 12, 1697–1705. [Google Scholar] [CrossRef]
- Blakney, A.K.; Simonovsky, F.I.; Suydam, I.T.; Ratner, B.D.; Woodrow, K.A. Rapidly Biodegrading PLGA-Polyurethane Fibers for Sustained Release of Physicochemically Diverse Drugs. ACS Biomater. Sci. Eng. 2016, 2, 1595–1607. [Google Scholar] [CrossRef] [Green Version]
- Żółtowska, K.; Piotrowska, U.; Oledzka, E.; Kuras, M.; Zgadzaj, A.; Sobczak, M. Biodegradable Poly(ester-urethane) Carriers Exhibiting Controlled Release of Epirubicin. Pharm. Res. 2017, 34, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, A.K.; Mittal, V.; Singh, N.; Dash, D.; Malik, S.; Kumar, M.; Maiti, P. Polyurethane-Grafted Chitosan as New Biomaterials for Controlled Drug Delivery. Macromolecules 2015, 48, 2654–2666. [Google Scholar] [CrossRef]
- Yu, C.; Tan, X.; Xu, Z.; Zhu, G.; Teng, W.; Zhao, Q.; Liang, Z.; Wu, Z.; Xiong, D. Smart drug carrier based on polyurethane material for enhanced and controlled DOX release triggered by redox stimulus. React. Funct. Polym. 2020, 148, 104507. [Google Scholar] [CrossRef]
- Aluri, R.; Jayakannan, M. Development of l-Tyrosine-Based Enzyme-Responsive Amphiphilic Poly(ester-urethane) Nanocarriers for Multiple Drug Delivery to Cancer Cells. Biomacromolecules 2017, 18, 189–200. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar] [CrossRef]
- Malik, M.; Kaur, R. Synthesis of NIPU by the carbonation of canola oil using highly efficient 5,10,15-tris(pentafluorophenyl)corrolato-manganese(III) complex as novel catalyst. Polym. Adv. Technol. 2018, 29, 1078–1085. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Javni, I.; Hong, D.P.; Petrović, Z.S. Polyurethanes from soybean oil, aromatic, and cycloaliphatic diamines by nonisocyanate route. J. Appl. Polym. Sci. 2013, 128, 566–571. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Motokucho, S.; Nakayama, Y.; Morikawa, H.; Nakatani, H. Environment-friendly chemical recycling of aliphatic polyurethanes by hydrolysis in a CO2 -water system. J. Appl. Polym. Sci. 2017, 135, 14–17. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, W.; Wang, L.; Hao, J. Comparative study of nitrogen migration among the products from catalytic pyrolysis and gasification of waste rigid polyurethane foam. J. Anal. Appl. Pyrolysis 2016, 120, 144–153. [Google Scholar] [CrossRef]
- AnuarSharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Jomaa, G.; Goblet, P.; Coquelet, C.; Morlot, V. Kinetic modeling of polyurethane pyrolysis using non-isothermal thermogravimetric analysis. Thermochim. Acta 2015, 612, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Osman, M.; Satti, S.M.; Luqman, A.; Hasan, F.; Shah, Z.; Shah, A.A. Degradation of Polyester Polyurethane by Aspergillus sp. Strain S45 Isolated from Soil. J. Polym. Environ. 2018, 26, 301–310. [Google Scholar] [CrossRef]
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).