Abstract
Microorganisms have developed a resistance against some of the most conventional antibiotics. These microorganisms can be self-assembled, forming a microbial biofilm. A microbial biofilm formation is an inherent event on almost any surface, causing countless side effects on human health and the environment. Therefore, multiple scientific proposals have been developed based on renewable sources such as natural polymers. Natural polymers or biopolymers include cellulose, chitosan, starch, collagen, gelatin, hyaluronic acid, alginates, fibrin, and pectin, which are widely found in nature. The biopolymers have displayed many interesting properties, including biocompatibility and biodegradability. Nonetheless, these materials usually have no antimicrobial properties (except for the chitosan) by themselves. Therefore, antimicrobial agents have been incorporated into the natural polymeric matrix, providing an antimicrobial property to the biocomposite. Biocomposites consist of two different materials (one of natural origin) studied as biocompatible and biodegradable drug carriers of antimicrobial agents. In addition, due to the incorporation of antimicrobial agents, biocomposites can inhibit biofilm formation and bacteria proliferation on many surfaces. This review describes this using natural polymers as a platform of antimicrobial agents to form a biocomposite to eliminate or reduce biofilm formation on different surfaces.
1. Introduction
Microorganisms are clustered, forming a microbial biofilm on almost any surface. Biofilms can develop from one or many microbial species [1,2]. Mixed biofilms guarantee prolonged survival due to their high resistance to common antibiotics, oxidative stress and lack of nutrients, denser and thicker mature biofilms, and even cooperation and competition between species [1,3,4]. Staphylococcus aureus (S. aureus) is a primary reason for chronic biofilm formation in surgical settings, impacting cardiology and orthopedics [5]. In addition, the microbial organisms have somehow developed antibiotic microbial resistance (AMR). If not addressed, AMR will become a major global challenge as it constitutes a severe threat to global public health [6] and the economy [7]. Since the presence of harmful microorganisms is humanity’s central concern nowadays, scientists are working hard to develop antimicrobial biocomposites [8]. A new kind of antimicrobial must not only be effective against bacteria but also resist the possible development of bacterial resistance [9].
A biocomposite is a material composed of two or more distinct constituent materials (one being naturally derived) combined to yield new materials with improved performance over individual constituent materials [10]. Natural polymers or biopolymers are the first biodegradable biomaterials to develop biocompatible biomaterials with tuned degradability and specific structure–function relationships [11]. Naturally occurring biopolymers films have attracted more attention for multiple applications due to their versatile properties, such as nontoxicity, biocompatibility, and biodegradability, along with their abundance and sustainability [12,13,14,15]. Polymers usually do not have intrinsic antibacterial properties, except chitosan, which has been proven to present antibacterial effects [16] due to the positively charged amine groups in its structure. Positively charged moieties are critical in defining antibacterial activity [17].
Therefore, natural polymers have been studied as polymeric matrices for incorporating antimicrobial agents [18]. In this case, the characteristics of the polymer, such as its hydrophilicity or its molecular weight, greatly influence the final antimicrobial activity concerning aspects from the rate of biocide release to even conferring synergistic activities [19]. An antimicrobial agent can be defined as an agent that kills microorganisms or inhibits their growth [20]. Antimicrobial agents emerge as a possible alternative to eliminate or reduce possible microorganisms [21], improving material performance. The slow release of the antimicrobial agents added to the biocomposites can be used to inhibit or kill biofilms [22]. These new families of composites exhibit remarkable improvements in mechanical and material properties when compared with virgin polymers or conventional micro-and macro-composites [23].
The study and understanding of new composite materials with antibacterial features to inhibit bacterial growth and reduce bacterial adhesion is fundamental [7]. It is essential to understand how biofilms are formed to develop materials that can effectively kill microbes or inhibit microbial growth and biofilm formation [24]. In this paper review, we describe the process of biofilm formation. In addition, the main natural polymers for fabricating antimicrobial biocomposites to inhibit bacterial growth are detailed.
2. Biofilm Formation
A biofilm is a community of bacteria enclosed in a self-produced exopolysaccharide matrix that adheres to a biotic or abiotic surface [25]. There are two forms of microbial life in the environment: (a) planktonic microbial cells (independent and free-living) and (b) microbial biofilms, which is an aggregate of sessile microorganisms developed in a dense extracellular matrix [26]. Changing from a planktonic state to a biofilm state confers various advantages to microorganisms, such as antimicrobial resistance, evasion of host immune response, resistance to oxidative stress, and compensation for lack of nutrients [26,27,28,29,30,31]. Several genetic studies have shown that the formation of biofilms depends on factors such as the mobility of microorganisms [31,32,33], the synthesis of exopolysaccharides [33,34,35], environmental conditions: temperature, pH, oxygen availability [36,37], and nutrient availability [38]. Several steps detail the process of biofilm formation. Figure 1 illustrates the biofilm formation.
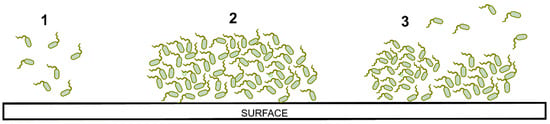
Figure 1.
Representation of biofilm formation: (1) adhesion, (2) growing, (3) detachment.
(1) The appropriate conditions must provide a favorable deposition of the microorganisms onto the surface substrate [39]. It has been determined that there is a greater binding affinity between microbial cells and the surfaces of non-polar hydrophobic materials (e.g., Teflon) compared to hydrophilic surfaces (e.g., metals, glass) [40,41]. Bacterial cells with structures such as type IV flagella and pili can move across the surface of the substrate to associate with other bacteria and form microcolonies [32,33,42]. Cells that do not have flagella and pili use Brownian motion (random, uncontrolled movement of particles in a fluid) to move on the surface [43]. During initial adhesion, microbial cells attach to the substrate surface through membrane proteins called adhesins [31,33].
(2) The union of the microbial cells and the substrate’s surface activate the genes to synthesize extracellular polysaccharides and form the extracellular matrix (EPS). This matrix traps other microbial cells due to its viscous consistency [44]. The EPS matrix also contains eDNA (extracellular DNA), proteins, nutrients, and other components that circulate internally through a complex of diffusion channels; with this system, the microorganisms develop, grow, and eliminate waste [29]. After the recruitment and production of the initial EPS matrix, the microbial community begins to produce an adhesive matrix that facilitates other external cells to adhere to each other and form a differentiated multilayer biofilm. This thick biofilm that protects microorganisms is called a mature biofilm matrix [45,46].
(3) Several causes can generate the dispersion of biofilm cells: detachment of daughter cells from active cells, detachment due to nutrient scarcity, or shearing of biofilm portions [39,47]. The cells return to a planktonic state and detach from the surface to disperse in their surrounding environment. When the planktonic cell is in contact with the contact surface, it is ready to recolonize and start the biofilm formation process again.
Several strategies to control biofilm growth on any surface have been suggested in the last decade. Due to the easy self-aggregation of multiple bacteria to the surface, natural polymers have been studied to prevent biofilm formation or inhibit the cell growth of many microorganisms. In addition, additional agents such as drugs or nanoparticles (NPs) hinder cell-reproduction and prevent biofilm formation [24].
3. Natural Polymers
Natural polymers are essential to daily life as our human forms are based on them [48]. These polymers, also known as biopolymers, are found widely in nature or extracted from plants or animals [49]. Some examples of the chemical structure of biopolymers are shown in Figure 2. Natural polymers exhibit high biocompatibility, biodegradability, accessibility, stability, lack of toxicity, and low cost [48]. These polymers produce fewer toxic effects when compared with synthetic polymers [50]. In addition, these biopolymers are recognized as safe materials for food coatings in air contact [51]. Biopolymers are helpful for their excellent retention and release properties, but the main character is their higher WVP related to their hydrophilic nature [52]. Despite these advantages, biopolymers possess low stability in vitro and in vivo, poor mechanical properties, and disintegrate rapidly [53], which could be improved through cross-linking strategies [54]. These limitations are somewhat resolvable by their surface modification, and combination with other materials [55]. A cross-linking polymer can serve as a matrix to incorporate functional materials to enhance both properties synergistically. Generally, natural polymers incorporate antimicrobial agents and are used as drug carriers [56]. However, using natural polymers as drug carriers is challenging because of their broad molecular weight distributions and batch-to-batch variability [57].
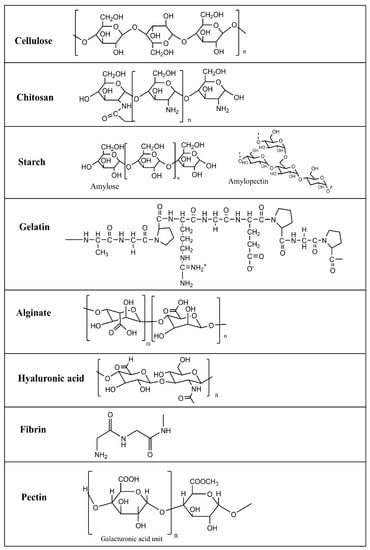
Figure 2.
Chemical structure of biopolymers.
4. Biocomposite-Based on Natural Polymers
Biopolymers are materials obtained from renewable resources [58,59]. Despite the exciting properties of biopolymers, such as biocompatibility and environmental sustainability, they do not present antimicrobial properties (except chitosan). However, this lack of antimicrobial properties can be solved by incorporating or encapsulating antimicrobial agents [60]. Antimicrobial agents must fulfill several requirements, such as a broad antimicrobial spectrum at a short contact time, ease of preparation at low cost; high stability at the intended applications and storage; and regeneration after the loss of activity [61].
In addition, biopolymers have been extensively studied as carriers of antimicrobial agents. The most common fillers with antimicrobial activity are metals, chemicals, essential oils (EOs), natural extracts, and NPs. The synergic union between antimicrobial agents and polymers can form new materials known as composites [62]. Natural fibers comprise several biopolymers and polysaccharides, making the fiber a composite [63]. They are highly used for promising characteristics such as nontoxic, nonabrasive, higher specific strength, lower density, minimal environmental impact, and biodegradability [64]. Natural polymers and hydrogels are cross-linked networks that absorb large amounts of water without dissolving [65].
Natural polymers possess low stability in aqueous media and limited mechanical strength, which could be improved through cross-linking strategies [55]. Hydrogels are biocompatible materials that can be synthesized from natural polymers, forming a cross-linking material. Alginate, collagen, fibrin, chitosan, gelatin, and hyaluronic acid are some natural polymers used to synthesize hydrogels [66]. Hydrogels are three-dimensional (3D) cross-linked polymer networks that can absorb and retain a large amount of water [67]. These materials usually present interesting properties such as mechanical strength, biocompatibility, biodegradability, swellability, and stimuli sensitivity [68]. Hydrophilic polymers might be considered as those polymers that contain polar functional groups such as hydroxyl (-OH), carboxyl (-COOH), and amino (-NH2) groups that make them soluble or swelled by water [69]. Despite lacking in mechanical properties, natural polymers remain attractive for their inherent biocompatibility, encouraging greater cellular attachment and matrix deposition than any other material class [65]. Different materials have been deposited in the polymeric matrix. Table 1 summarizes the content related to the biocomposites employed to inhibit many different bacteria.

Table 1.
Summary of natural polymers used to inhibit bacteria growth.
4.1. Cellulose-Based Composites
Cellulose is the most common polymer on earth because it can be easily obtained from the cell wall of plants and is even produced by some bacteria [70]. There is a 9–25% cellulose content in primary cell walls and 40–80% in secondary cell walls [71]. Therefore, cellulose is considered a renewable polymer [72] and semi-crystal material with high molecular weight homopolymer β-D-glucopyranose units, with a dimer of glucose (cellobiose) as a repeat unit [135,136]. It could be obtained as different derivatives such as Ethyl cellulose (EC), methylcellulose (ME), Cellulose acetate (CA), Cellulose sulfate (CS), Cellulose nitrate (CN) [73], and nanocellulose [137]. Cellulose and its derivatives could present high thermal resistance, protection against ultraviolet agents, low cost, biodegradability, and non-toxicity. However, cellulose has limitations such as high-water absorption capacity and insufficient interfacial adhesion [138]. Besides, cellulose has been studied as a carrier of antimicrobial agents, either as nanocapsules or nanofiber, as shown in Figure 3. Antimicrobial activity is one of cellulose fibers’ most critical functional properties [139,140,141,142,143].
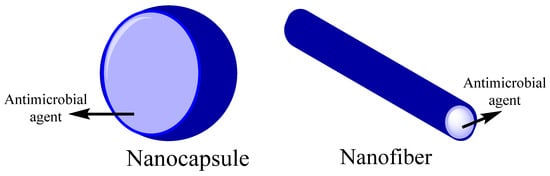
Figure 3.
Biopolymer nanocarriers structures for encapsulation of antimicrobial agents.
The nanocelluloses combine important cellulose properties such as high specific strength, modulus, hydrophilicity [144], biodegradability, nontoxic, extremely high surface area, and tunable surface chemistry [145]. Nanocellulose-based antimicrobial materials can be synthesized through surface modification with biocidal agents, making them effective against wound infection [78]. The antibacterial assays have confirmed the efficient antibacterial activity of several nanocellulose-based to inhibit bacterial growth (in both liquid medium and agar plates). They can kill several logs of microbial cells [146].
Nanofibrillated cellulose has been used to carry chitosan capsules [73]. Its high antimicrobial activity studied nanofibrillated cellulose (NFC) against gram-positive and gram-negative bacteria. It presents the ability of the polymeric grafts to penetrate the thick cell wall and destabilize the cellular membrane [147]. Nanofibrous scaffolds were prepared from polyurethane and CA and could contain reduced graphene oxide/silver nanocomposites (rGO/Ag), curcumin, or both, which can present antimicrobial properties [74]. Good antimicrobial properties were obtained from the composite of cellulose/keratin, where there was a comparison between silver nanoparticles (AgNPs) and ionic silver as an active agent. AgNPs present a higher activity against Escherichia coli (E. coli), S. aureus, Enterococcus faecalis, and Pseudomonas aeruginosa (P. aeruginosa) [72].
Cinnamon essential oil has been studied as an antimicrobial agent in a cellulose polymeric matrix. Cinnamon was more effective against S. aureus than E. coli due to protecting outer membrane proteins or cell walls, which are more resistant to lipophilic substances of gram-negative bacteria [75]. Orlando et al. proposed the functionalization of cellulose with glycidyl trimethylammonium chloride and glycidyl hexadecyl ether for antibacterial wound dressing. These active agents were used for the covalent derivatization of the hydroxyl groups of glucose through a heterogeneous reaction in basic aqueous conditions. The resulting material reduces S. aureus and E. coli by 53 and 43%, respectively, within the first 24 h [76]. Silva et al. reported the casting method’s synthesis of an active film based on cellulose derivatives (hydroxyethylcellulose and CA), using hydroxypropyl-γ-cyclodextrin as active agents. The antimicrobial activity of these films was evaluated against Campylobacter jejuni, Campylobacter coli, and Arcobacter butzleri. The results indicated that this system is a good approach for Campylobacter coli reduction [77].
4.2. Chitosan-Based Composites
Chitosan is the second polysaccharide more abundant in nature. It is structurally a linear polysaccharide made up of arbitrarily distributed β-(1–4)-linked d-glucosamine (deacetylated) and N-acetyl-d-glucosamine (acetylated) [148]. The principal source is shrimps shell wastes, crab peritrophic membranes, lobsters, and cocoons of insects [79]. Chitosan has a great potential for a wide range of applications due to its biodegradability, biocompatibility, antimicrobial activity, non-toxicity, and versatile chemical and physical properties [149]. Nonetheless, chitosan is insoluble at neutral pH or above. Therefore, it is necessary to modify natural polymers such as chitosan to become partially water solubility and enhance their antimicrobial activity [150]. The presence of amino and hydroxyl groups on the structure makes the chemical modification of chitosan possible to improve its solubility and electric change [151,152].
Chitosan exhibits an intrinsic antibacterial activity, inhibiting bacteria and fungi growth [153]. A significant number of amino groups on the surface of chitosan aid in generating positive zeta potentials [154]. Protonation of these amine groups on chitosan glucosamine monomers is facilitated at pH below 6.5 (pKa of chitosan), thus conferring cationic properties on chitosan [155]. Therefore, chitosan can interact with negatively charged cell wall bacteria [156]. This interaction reduces microbial cell membranes’ permeability [157]. In addition, another mechanism has been proposed in which the chitosan selectively binds with metals, inhibiting various metabolic enzymes of microbial cells by blocking their active centers and reducing microbial growth [158]. It has been determined that small-sized chitosan can block RNA and protein synthesis, thus inhibiting bacterial growth [159]. Characteristics such as molecular weight, degree of deacetylation, and environmental conditions of experimentation, such as pH, temperature, and ionic strength, also influence the antimicrobial capacity of chitosan [160,161,162,163].
Chitosan can serve as a matrix for the deposition of antibacterial agents. For instance, including oil compounds could improve the surface adhesive properties and prolong the safety of foods [84]. EOs have been used as antimicrobial agents in a biopolymeric matrix based on chitosan NPs. The antimicrobial activity depends on the volatility, the release rate of EOs, and the matrix. The incorporation of EOs can be directly or by encapsulation. Different EOs such as rosemary, tea, tree, clove, oregano, and eucalypt have been evaluated for food packaging and showed improved functional properties [164], as described in Figure 4. Chitosan NPs and nanofibers were studied as nanocarriers in conjunction with nisin, antimicrobial peptide temporin B, and Cinnamaldehyde as antimicrobial agents [80].
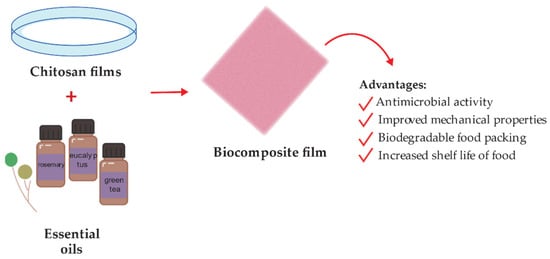
Figure 4.
Schematic representation of chitosan-essential oil films preparation for food packing.
Similarly, montmorillonite and rosemary essential oil were incorporated in a chitosan matrix to enhance the antimicrobial properties against Listeria monocytogenes (L. monocytogenes) and Streptococcus agalactiae. The results showed that the antimicrobial films improved by rosemary essential oil incorporation [81]. In general, adding EOs into chitosan improves chitosan’s effectiveness against some bacteria commonly found in food.
A higher antimicrobial effect was reported in chitosan films with grape seed extract and carvacrol microcapsules (CMF) than in control samples (CS) or just the chitosan control film (CCF). These samples were tested in salmon packed to increase the shelf-life of refrigerated salmon to 4–7 days of storage [165]. Another research conducted the antimicrobial activity evaluated in bio-nanocomposites based on graphene oxide (GO) and chitosan. The studied material decreases the growth of S aureus and E. coli. The composite release reactive oxygen species (ROS), which increase the bactericidal properties of the bio-nanocomposites [82]. Ao et al. reported the hydroxypropyltrimethyl ammonium chloride chitosan (HACC) based multilayer modified plasma-sprayed porous titanium coating generated via the layer-by-layer covalent-immobilized method. They determined the inhibition of the colonization and biofilm formation of several bacterial strains, including S. aureus, methicillin-resistant S. aureus (MSRA), and clinical isolates of methicillin-resistant S. epidermidis (MRSE), in vitro [83].
4.3. Starch-Based Composites
Starch is a semi-crystalline polysaccharide natural polymer with a complex structure that consists of two-component polymers: amylose (AM) and amylopectin (AP) [166]. Since most plants contain around 25% amylose and 75% amylopectin, and the ratio of these two related polymers directly influences solution, and structural properties, control of this ratio has a tremendous impact on the properties of starches [167]. Depending on its botanical origin (potato, maize, rice, etc.) and genetic background, starch has different chemical structures and functional groups, making it a useful natural polymer for different applications [85]. However, starch does not present inherent antimicrobial properties and is commonly used as a carrier [80].
The interest in starch is accrued from its high molecular weight and film-forming properties. Using a high molecular weight (37,000 kg mol−1) [168] polymer as a carrier of antimicrobial polymer eliminates the problem of leaching via entanglement and other interactions with the baseline polymer [169]. The starch polymer was studied with tea polyphenol (TP) for active food packaging, which presents inhibition efficiency against S. aureus and E. coli [86]. Biopolymer composite based on starch and carboxymethylcellulose (CMC) was used as an antimicrobial agent polymeric matrix of turmeric oil. It was reported that the increase in the film thickness increases the release of antimicrobial agents for low volumes of turmeric oil [170]. Starch has been reported in conjunction with poly-hexamethylene guanidine hydrochloride (PHGH). The composite studied showed surfaces with high antimicrobial potency against Bacillus subtilis (B. subtilis) and E. coli [87].
Another area of study is dental health. Starch NPs are used as carriers for curcumin, which has antimicrobial characteristics. Their interactions were studied through molecular dynamics simulation software with which molecular docking was obtained. The experimental and simulated studies reported a minimum inhibitory concentration (MIC) of curcumin against Streptococcus mutans [88]. Starch encapsulated biogenic AgNPs were tested to study the starch encapsulation effect. Results showed that encapsulation increases antimicrobial activity and reduces the toxicity of the NPs [89]. Chitosan NPs were synthesized via ionic gelation and used to prepare starch-based nanocomposite films. The antimicrobial properties of starch/chitosan NPs films were evaluated in vitro and in vivo against B. cereus, S. aureus, E. coli, and Salmonella typhimurium. Those films containing chitosan NPs were more effective than those with starch-based films [91]. Do Evangelho and coworkers reported corn starch films containing orange (Citrus sinensis) essential oil through the casting method. These films showed higher antibacterial activity against S. aureus and L. monocytogenes [92].
On the other hand, Li et al. [90] reported synthesizing ultrafine composites starch/polyvinyl alcohol, using glutaraldehyde as a cross-linking agent. AgNPs were loaded to the composites to provide a better mechanical and antimicrobial performance against E. coli and S. aureus. The development of this composite suggests a new route for producing less costly antibacterial fiber materials.
4.4. Collagen-Based Composites
Collagen is a naturally occurring matrix polymer highly conserved across species [171]. Collagen is a protein with biological properties that make it a suitable biomaterial for biomedical applications. It is the most abundant animal protein, providing mechanical strength to the tissues. At least 29 types of collagen have been identified in vertebrates [172]. Based on the structure, collagens can be classified into different groups such as fibrils, beaded filaments, networks, anchoring fibrils, and fibril-associated collagen [173]. Collagen I is the most abundant structural protein of connective tissues such as skin, bone, and tendon [93], assembled into fibrils.
Collagen-based materials have received significant attention in medical applications ranging from drug/gene delivery to tissue engineering [100] because it possesses outstanding properties such as tensile stiffness to resist plastic deformation and rupture, biocompatibility, biodegradability, and cell growth potential [173,174]. In addition, collagen can be prepared into cross-linked compacted solids or lattice-like gels [175]. Collagen gels are flowable, suggesting the possibility of an easily injectable, biocompatible drug delivery matrix [100]. However, collagen has no antimicrobial properties despite its properties [99]. Therefore, it is necessary to incorporate antimicrobial agents to obtain the desired antibacterial. For instance, AgNPs present interesting antimicrobial effects. Therefore, AgNPs can be incorporated into a collagen matrix providing bactericidal effects [94].
The antibacterial activity can also be bestowed to the collagen by adding NPs such as AgNPs. Recently, the development of a collagen-carboxymethylcellulose biocomposite containing AgNPs for wound dressings was proposed by Neacsu et al. [95]. During this experiment, the antimicrobial assessments showed the antimicrobial potential against gram-negative (E. coli), gram-positive (S. aureus) bacteria, and yeast (Candida albicans). These results agreed well with the literature that reports the potential of AgNPs as an antimicrobial agent [96]. Alvarez et al. developed silica–collagen type I biocomposite hydrogels loaded with gentamicin and rifamycin to prevent infection in chronic wounds. The biocomposites were evaluated against P. aeruginosa and S. aureus. Nonetheless, only gentamycin-loaded hydrogels showed bactericidal activity [97]. Vladkova et al. performed new collagen composites, Collagen/(Silver (Ag)/Reduced Graphene Oxide (RGO)) and Collagen/(Ag/RGO/Silicium oxide (SiO2)). These composites were tested against E. coli, S. epidermidis, B. cereus, and a fungus C. Lusistaniae. The biological activity found for the Collagen/(Ag/RGO/SiO2) composites is better expressed than that of Collagen/(Ag/RGO) composites with the same level of antimicrobial agent loading [98]. Thymol is an antimicrobial compound in the composition of thyme and oregano EOs [176]. The antimicrobial activity of collagen/thymol films was studied for wound dressing applications by Michalska-Sionkowska et al. The bacterial tests showed the antimicrobial efficiency against E. coli, B. subtilis, Enterobacter aerogenes, Candida albicans, and S. aureus, the latter being the most sensitive microorganisms to thymol action [99].
4.5. Gelatin-Based Composites
Gelatin is a nontoxic natural biomacromolecule comprised of bioactive polypeptides derived from collagen in animal skin, bones, and connective tissues [177]. It is a protein obtained through controlled partial hydrolysis of collagen [101]. Gelatin has many glycine, proline, and 4-hydroxy proline residues [178]. Depending on the process employed, two types of gelatin can be obtained: type A gelatin produced by acid hydrolysis and type B obtained by an alkaline or lime process [101].
Gelatin has long attracted interest in food, packaging, pharmaceutical, and photographic industries, because of its physical and functional properties such as the reversible gel-to-sol transition of aqueous solution; viscosity behavior; protective colloid function, biodegradability, and solubility in hot water but insolubility in cold water. The gelatin-based film has a suitable matrix and compatibility that allows it to act as a medium for incorporating antimicrobial and antioxidant agents [179]. There is a considerable number of publications on the preparation of gelatin-based films with antimicrobial activity by incorporating naturally occurring and synthetic antimicrobials such as organic acids [180], proteins [181], enzymes [182], chelating agents [183], and EOs [184]. For instance, the effect of incorporating tannic acid (TA) and cellulose nanocrystals (CNC) on gelatin films was evaluated by Leite et al. [102]. The gelatin films containing nonoxidized TA and CNC (G-nTA-CNC) exhibited antimicrobial activity against S. aureus and E. coli due to the incorporation of TA. Moreover, G-nTA-CNC films showed an improvement in the gelatin’s antioxidant capacity antioxidant capacity, UV barrier, tensile strength, and water vapor barrier properties. Thus, the resulting approach is suitable for different applications, particularly food packaging.
Developing wound dressing loaded with antimicrobial agents has also received much interest in reducing wound bacterial colonization [107]. Figure 5 shows a schematic illustration for the design of bioactive agent-loaded gelatin-based materials by electrospinning for the wound healing process. Recently, the design of a copper peroxide-loaded gelatin sponge with pH-controllable •OH delivery and effective antimicrobial activity for wound healing was reported. The experiments showed that the as-prepared wound dressing could release •OH, specifically in the bacterial-infected skin wound. In addition, in vitro experiments revealed that the wound dressing has good bactericidal properties against E. coli, S. aureus, and P. aeruginosa [103]. Pereda et al. reported the synthesis of biodegradable composite films based on gelatin and chitosan. Composite obtained showed a uniformity due to a compact structure indicating good compatibility between components, which could interact by strong hydrogen bonding. The researchers tested these films against E. coli and L. monocytogenes strains. However, only E. coli resulted be sensitive to the gelatin-chitosan composite [104]. Thongsrikhem and coworkers developed an antibacterial gelatin-bacterial cellulose nanocomposite (GCB) film using cinnamaldehyde as a crosslinker and an antibacterial additive. These films were evaluated using S. aureus and E. coli, resulting in a vigorous antibacterial activity against both bacteria strains [105]. In addition, Roy et al. synthesized Gelatin-based multifunctional composite films reinforcing various amounts of copper sulfide nanoparticles (CuSNPs). The gelatin/CuSNP composite film presented effective antibacterial activity against E. coli and some activity against L. monocytogenes, suggesting their use in food packaging [106].
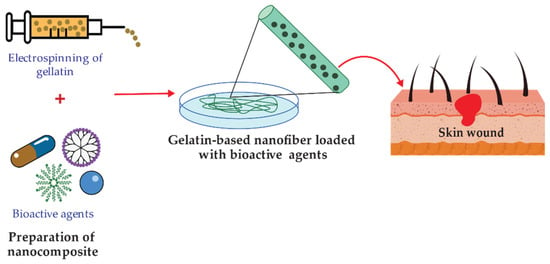
Figure 5.
Representation of functionalized gelatin-based nanofibers preparation for wound treatment applications.
4.6. Hyaluronic Acid-Based Composites
Hyaluronic acid (HA) is a natural polymeric polysaccharide that contains N-acetyl glucosamine and glucuronic acid groups [185]. It is present in nature, mainly in mammalian connective tissues. HA is a highly reactive, biocompatible, biodegradable, no-inflammatory, and non-toxic polymer. However, HA has poor biomechanical properties in its native form, and various chemical modifications have been devised to provide mechanically and chemically robust materials [186]. HA can be cross-linked or conjugated with assorted biomacromolecules, and it is optimal to encapsulate different active agents [187]. Hyaluronic acid hydrogels are readily fabricated as microspheres, sponges, and fibers depending on the intended application [188]. However, unmodified HA has a poor residence time in vivo, which can be tailored via cross-linking reactions [189].
Among various polymers tested as antibacterial coatings, HA and some of its composites offer a well-established long-term safety profile and a proven ability to reduce bacterial adhesion and biofilm formation [190]. HA can interfere with bacterial adhesion to a cellular substrate concentration-dependent [191]. HA is bacteriostatic but not bactericidal and exhibits dose-dependent effects on different microorganisms in the planktonic phase [189]. HA and its derivate may offer a solution and long-term safety with a known ability to retard bacterial adhesion and biofilm formation [192]. However, some studies have shown that the bacteriostatic effect of soluble HA in vitro may be attributed to the saturation of the bacterial hyaluronidase by an excess of HA in the medium [193].
To impart antimicrobial properties, the polymeric matrix is commonly functionalized with antimicrobial agents such as quaternary ammonium compounds (QACs), improving antimicrobial efficiency through a contact killing mechanism [108]. The surface-functionalized scheme is present in Figure 6. HA carboxylic acid groups are modified by ester formation, while hydroxyl groups can be modified utilizing glutaraldehyde [194]. It is applied in ophthalmic treatments as a visual carrier material in a long-term antibiotic release.
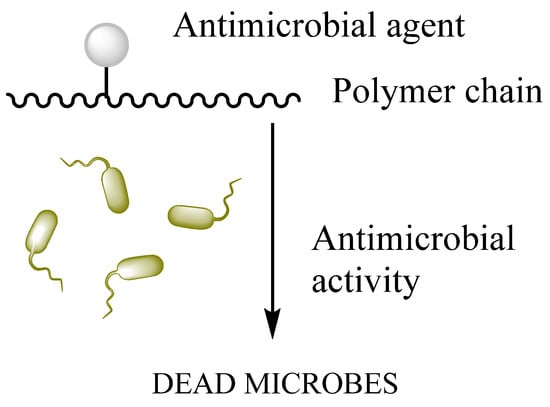
Figure 6.
Surface functionalization of biopolymer.
Multiple antimicrobial agents have been studied with HA as a composite to improve antimicrobial activity. For instance, ciprofloxacin and vancomycin were used in HA particles against P. aeruginosa, S. aureus, and B. subtilis [109]. In addition, it is reported that antimicrobial multilayers based on HA and chitosan developed onto the activated surface of polyethylene terephthalate, obtain polyelectrolyte multilayers (PEMs). Triclosan (TRI) and rifampicin (RIF) were used as bactericidal and antibiotic agents, respectively [195]. A composite of hyaluronic acid-oleylamine (HA-OLA) was studied as a promising nano-carrier to delivery agents for the treatment of bacterial/Methicillin-resistant Staphylococcus aureus (MRSA) infections [110]. D-α-tocopherol polyethylene glycol 1000 succinate (TPGS)-poly(lactic-co-glycolic acid) (PLGA) with azithromycin are studied to improve the antimicrobial activity against P. aeruginosa [111]. Nisin (an antimicrobial peptide) has been attached to HA to obtain an antimicrobial biopolymer under solution or gel, showing a great activity against S. epidermidis, S., and P. aeruginosa bacteria [112]. Harris and Richard coated titanium surfaces with HA for orthopedic applications, showing a decrease in S. aureus attachment on the surface [113]. Felgueiras et al. use HA as an antifouling agent, previously modified with thiol groups (HA-SH), using polydopamine as the binding agent. The polyurethane films were coated with the HA. Octadecyl acrylate was subsequently used to bind thiol groups to attract albumin, allowing the system to selectively bind albumin, a protein responsible for bacterial adhesion, thus granting an effective antimicrobial activity. The results showed that these films decreased the S. aureus adhesion [114].
4.7. Alginates-Based Composites
Alginate (ALG) is a natural polymer comprising β-D-mannuronic acid and α-L- guluronic acid extracted from brown seaweed [115]. This biomaterial exhibits several properties, including biocompatibility, gelation capability, low toxicity [196], mild gelation conditions, and simple modifications to prepare alginate derivatives with new properties [197], suggesting its use in biomedical and food industry applications. Alginate can absorb water and body fluids up to 20 times its weight, resulting in a hydrophilic gel [198]. The formed gel is weak, but it maintains a moist wound healing environment [198]. Linear Alginate polymer chains contain multiple carboxyl groups that can bind to divalent cations (Ca2+, Ba2+) to promote the formation of cross-linked structures [199]. Applications within biotechnology and medicine are mainly based on the temperature-independent sol-gel transition in multivalent cations (e.g., Ca2+), making alginates highly suitable as an immobilization matrix for living cells [200].
Several studies have investigated the effectiveness of incorporating antimicrobial agents such as EOs and NPs into alginate-based materials to induce an antimicrobial activity. Ahmed and Boateng reported the development of antimicrobial films for treating bacterial infections [116]. The calcium alginate films were loaded with ciprofloxacin and tested against E. coli, S. aureus, and P. aeruginosa. The results indicated a bacterial kill within 24 h and were highly biocompatible with human keratinocyte cells. In another study, biodegradable alginate films were prepared by adding zinc oxide nanoparticles (ZnONPs) and citronella essential oil (CEO) for cheese packaging. The ZnONPs act as a reinforcing agent and arrange the alginate polymer chains around them (Figure 7). Microbiological studies revealed a synergic effect between antibacterial activities of ZnO and CEO against two gram-negative (E. coli and Salmonella typhi) and two gram-positive (B. cereus and S. aureus) bacterial strains. Moreover, alginate/ZnO/CEO films showed better UV light barrier properties and lowered water vapor permeability (WVP) than pure alginate film [117]. Very similar research was reported by using spherical AgNPs and lemongrass essential oil (LGO) as antimicrobial agents, and the result indicated the feasibility of using alginate/Ag NPs/LGO films as antibacterial packaging to preserve the color, surface texture, and softness of cheese for 14 days [118].
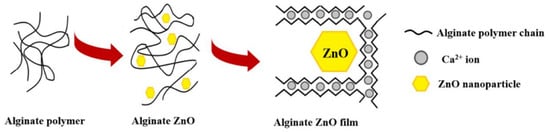
Figure 7.
Schematic representation of alginate films with ZnONPs. Reprinted with permission from ref [182], MDPI, 2022.
Furthermore, new and affordable alternatives have been proposed to prevent microbial infections. In a recent study, incorporating a low percentage of carbon nanofibers enhanced the antibacterial properties of alginate films against the Gram-positive bacterial model S. aureus. In addition, the films showed no cytotoxicity, suggesting their potential use in antimicrobial biomedical materials [119].
Alginate fibers cross-linked with zinc ions have also been proposed for wound dressings [185]. Zinc ions may generate immunomodulatory and antimicrobial effects, enhanced keratinocyte migration, and increased endogenous growth factors [120]. Asadpoor et al. developed films containing alginate oligosaccharides (AOS) as an alternative for antibiotic treatment. AOS decreases the biofilm of Streptococcus agalactiae and S. aureus strains by determining the MIC [121].
4.8. Fibrin-Based Composites
Fibrin, derived from critical proteins involved in blood clotting (fibrinogen and thrombin), is a self-assembling biopolymer [122]. Fibrin is a critical component of the blood clot that accelerates wound healing, prevents hemorrhage, and protects against bacterial infection [123]. Fibrin alone, or in combination with other biomaterials, was employed as a biological scaffold to promote stem or primary cells to regenerate [129]. In comparison to alginate-only gel-laden constructs, fibrin has an advantage in cytocompatibility due to cell adhesion moieties within the fibrin structure [201].
Several studies have reported the antimicrobial effect of leukocyte- and platelet-rich fibrin (L-PRF) against periodontal pathogens. Castro et al. assessed the antimicrobial properties of L-PRF against pathogens grown on agar plates and in planktonic cultures. A potent inhibition was found against Prevotella intermedia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and especially against Porphyromonas gingivalis [128]. The research conducted by Venante and co-workers [124] exhibited the effectiveness of fibrin biopolymer incorporating antimicrobial agents such as digluconate chlorhexidine and Punica granatum alcoholic extract to prevent the development of Candida albicans biofilm. In vitro results displayed the inhibition of the growth of C. albicans biofilm on poly(methyl methacrylate) (PMMA) substrates for up to 72 h, which suggests the excellent performance of the modified fibrin biopolymer as a drug delivery system, preventing the formation of denture biofilm.
Fibrin sealant was used as a matrix for teicoplanin as an antimicrobial carrier applied externally to control infection sites [125]. Vancomycin impregnated fibrin sealant was developed to measure antibacterial activity and antibiotic release. This study uses fibrin sealant as a topical hemostat for post-operatory treatments in surgical fields [126]. AgNPs were studied in metal/fibrin nanocomposites, recognized as suitable materials for wound healing. AgNPs produce an antimicrobial effect related to the easy oxidation of silver. The action over the bacteria occurs due to the interaction between AgNPs/Ag+ and the cell membrane of the bacteria. The reaction was tested against E. coli and S. aureus [127]. A bioartificial human dermis substitute was developed for the treatment of infected wounds. It was based on a fibrin-agarose matrix with sodium colistimethate (SCM) and amikacin (AMK) as antimicrobial agents [202].
4.9. Pectin-Based Composites
Pectin is a polymer with a linear structure in which a few hundred to thousand galacturonic acid monomer units are linked via α-(1→4)-glycosidic bond-forming a backbone [203], with variations in composition, structure, and molecular weight [204]. Pectin is one of the significant and more complex components of the primary cell walls of most plants, with a structure rich in galacturonic acid units [205]. Many carboxyl groups are esterified with methanol to form methoxy groups; these determine the gelling ability of the pectin used in jam, marmalade, and jelly and preserve production [130].
The word ‘pectin’ comes from the Greek word pektos which means firm and hard, reflecting pectin’s ability to form gels [206]. One of the most remarkable properties of pectin is its ability to form gels, making it suitable for its use in food design and the pharmaceutical industry [207,208]. In addition, the gelation ability, biocompatibility and nontoxicity make pectin a suitable material for producing NPs, healing agents, edible coating, and bio-based films. Pectin is semi-soluble in liquids, which can take up some liquid. This is especially important in cooking fruits and vegetables because it softens them when cooked [209]. Pectin has demonstrated antimicrobial activity against gram-positive and gram-negative microorganisms [210]. Its antimicrobial activity likely involves the carboxylic acid groups’ binding action in the main pectin’s backbone [211]. In this regard, Presentato et al. reported that pectin extracted via hydrodynamic cavitation in water only from waste lemon peel and further isolated via freeze-drying displays significant antibacterial activity against S. aureus at a pH of 6 [212]. Due to its exciting properties, pectin can be a suitable carrier for antimicrobial agents.
The combination of different antimicrobial agents has gained increasing interest. Therefore, a wide range of antimicrobial materials, including plant extracts [213,214], NPs [215], lysozymes [216], salicylic acid [217], and others, have been tested to impart antimicrobial properties in pectin derivatives. Moreover, employing ions with an antimicrobial character as a cross-linking agent, such as Zn ions, is also an exciting strategy to impart antimicrobial activity to pectin [131]. For instance, Hari et al. [132] developed pectin-based antibacterial bionanocomposite films and evaluated the effects of ZnONPs at different concentrations (0.5%, 1%, and 1.5% w/w, based on pectin). The results revealed that adding ZnONPs improved the UV barrier and the mechanical properties of the bionanocomposite films. Moreover, the films incorporating 1.5% ZnONPs exhibited intense antibacterial activity against food-borne pathogenic bacteria such as E. coli and S. aureus inhibiting over 99% of the bacteria because of the inherent antimicrobial activity of ZnONPs. Similarly, Otoni et al. reported the synthesis of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. The cinnamaldehyde provided antimicrobial properties against E. coli, Salmonella enterica, L. monocytogenes, and S. aureus [133]. In addition, pectin–linoleate, pectin–oleate, and pectin–palmitate composites systems were evaluated against S. aureus and E. coli, inhibiting 50–70% of the bacteria growth. The best results were obtained against S. aureus [134]. New materials can be achieved based on natural sources for active food packaging. Based on this principle, Trejo-Gonzáles et al. developed pectin-based films containing citrus pectin/gellan gum/glycerol/calcium chloride and five mM/ethylenediaminetetraacetic acid (EDTA) and an antimicrobial concentrated supernatant (ACS) from fermentation culture broths of the lactic acid bacterium, Streptococcus infantarius. The functional films inhibited the growth of L. monocytogenes, E. coli, and S. aureus in the “Barbacoa” medium in 7-day cultures at 35 °C, attributed to the synergy between ACS, EDTA, and pectin [135].
5. Prospects, Challenges and Future Perspectives
Despite the advantages of the use of biopolymers, their commercialization is limited. It is necessary to raise awareness about the impact of replacing polymers of fossil origin with natural polymers. The use of biodegradable polymers in place of conventional plastics is increasing due to environmental and economic concerns. As is known, polystyrene and other conventional polymers cause land and water pollution. Therefore, scientists and engineers have focused on biodegradable polymers of natural origin with antimicrobial properties to address these problems. Biopolymers can be extracted from the most varied natural sources; however, it is necessary to assure a green and sustainable extraction for obtaining an environmentally friendlier product. In addition, more research and development are required to improve its efficacy, production, and reproducibility and ensure its safety during clinical trials.
In addition, many studies have searched for new methodologies to face the growth of antibiotic-resistant bacteria. The use of natural biopolymers is considered a friendly environmental route. To identify the microbial activity of biopolymers, agar diffusion tests [159,218,219] and chitosan microdilution tests with the MIC method [159] have generally been used. Other studies have analyzed membrane integrity using ultraviolet absorption, potential membrane assays, and flow cytometric analysis to assess the antimicrobial activity of biopolymers [220]. Nonetheless, the microbial action mechanisms of biopolymers are not exactly clear so far. The method used to provide antimicrobial effects is based on the insertion of active compounds in polymeric structures such as capsules, fibrils, foams, aerogels, or other solid materials containing closed assemblies. The mechanisms of action are related to the change of permeability, disruption, change of the bacterial water osmosis gradient, or destruction of bacterial cell membranes. The inhibition of ATP production, DNA synthesis, DNA replication, and the respiratory system could conduce to direct bacterial killing [146]. In the case of EOs, the mechanism of action is related to the degradation of the cell membrane, leakage of cell components, damage of membrane proteins, coagulations of cytoplasm, or the collapse of the proton motive force [164]. The generation of ROS is another route principally caused by the release of metallic ions, which disrupt cellular function and cell death. The biocompatibility characteristic of biopolymers is related to their chemical structures, similar to extracellular matrix components. Polymeric surfaces are usually functionalized through covalent attachment. The action route is usually through quaternary ammonium groups, which can interact hydrophobically with the bacterial cell wall to provoke cell death [76]. A polymeric matrix has a cationic nature due to the positive charge density, which can reduce the transmission of external nutrients necessary for cell viability [59], or the matrix can diffuse into the nucleus to inhibit their synthesis [79].
There is no consensus antimicrobial mechanism since the investigations are emphasized a specific polymer or application; most of the specific antimicrobial mechanisms of each biopolymer have been postulated [136,221]. Such is the case of chitosan, whose most accepted antimicrobial mechanism consists of electrostatic interactions between chitosan with amino sites (positively charged) and microbial cell membranes (negatively charged). This interaction changes the osmotic balance and affects the permeability of the microbial cell, causing the release of intracellular material [163] or causing the formation of a surface layer that prevents the entry of external nutrients [157]. HA has anti-adherent or antibacterial biofilm activity because the negative charges of this biopolymer do not interact with the microbial membranes [222,223]. Other biopolymers described in this review do not have an inherent microbial action. Still, they can be supplemented with antimicrobial components such as Zn ions [131], EOs [75], AgNPs [224], or used as vehicles such as this is the case starch [86,131,221], cellulose [225], collagen, gelatin [179], alginates, fibrin, pectin and even the chitosan [84] as well.
The unique properties of biopolymers, such as biocompatibility and biodegradability, make them ideal for medical applications. Biopolymers play a relevant role in controlling pathogenic microorganisms, which becomes a challenge for the pharmacological and physiological industry. Their use in biomedicine is remarkable due to their high biocompatibility associated with their chemical structure. One of the most important uses is related to wound healing due to its capacity to promote the regeneration of new tissues. Moreover, biopolymers can be tailored with predefined properties for applications such as drug release targeting, medicine regenerative, sterilization of medical devices, etc. In fact, most of their uses are related to the controlled drug release, based on the improved solubility in water medium. The combination of biopolymers with antimicrobial agents can mimic the natural function of tissues and offer significant benefits for their use in the biomedical field. Nonetheless, despite all the research, there are still some drawbacks to solve. Food packaging is particularly interesting among all the potential applications of antimicrobial biopolymers. Even though antimicrobial agents for the packaging industry have many exceptional features, more comprehensive research is still required to maintain food quality, meet consumer sensory preferences, and avoid the risk of serious health consequences. However, there are a few obstacles to overcome to meet the packaging industry’s demanding requirements. The relationship between various antimicrobial agents and polymeric matrix must be fully comprehended. For instance, in the case of NPs embedded into polymers, toxicological tests must be conducted to ensure long-term human and animal safety without side effects or adverse events. Moreover, antimicrobial agents such as EOs may present a high loss rate owing to their rapid volatilization.
6. Conclusions
During the last two decades, the increase of antibiotic-resistant microorganisms has prompted the interest in the development of new approaches and strategies for healthcare. Materials based on natural polymers have attracted attention as promising alternatives in various food and biomedical applications. Natural polymers are found in renewable sources of natural origin. They have been extensively studied due to their biocompatibility and biodegradability. Nonetheless, biopolymers have weak mechanical properties and durability compared with synthetic polymers. In addition, except for chitosan, biopolymers have no antimicrobial properties to face microorganism proliferation in their applications. Chitosan is a natural positive-charged polymer, that can interact electrostatically with a negative membrane of bacteria, reducing the permeability of the microbial cell membranes. Recent research has demonstrated that using biopolymers combined with antimicrobial agents has revolutionized composites manufacturing.
Using different techniques, these biopolymers can serve as a polymeric matrix for incorporating antimicrobial agents. Natural polymers such as cellulose, chitosan, starch, collagen, gelatin, HA, alginates, fibrin, and pectin showed dynamic structures capable of containing antimicrobial compounds such as metals, chemicals, EOs, natural extracts, or NPs to inhibit bacterial growth and improve mechanical material properties. Those antimicrobial agents have shown a suitable combination with natural polymers to form biocomposites, providing an antimicrobial property to the polymeric system. Therefore, antimicrobial biocomposite acts as a drug carrier of antimicrobial agents as a great solution to reduce or eradicate the biofilms produced by many microbial species. The obtained characteristics of these biocomposites are helpful for application daily-use industries.
Author Contributions
M.B.-T., B.A.-V., J.E.-N., H.Y.-V. and E.B. participated in the review, writing, and revision. E.B. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México under Grant IN202320.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No applicable.
Acknowledgments
This work was supported by Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México under Grant IN202320 (México).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adam, B.; Baillie, G.; Douglas, L. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 2002, 51, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Hansen, M.; Roder, H.; Wang, N.; Burmolle, M.; He, G. Mixed-species biofilms in the food industry: Current knowledge and novel control strategies. Crit. Rev. Food Sci. Nutr. 2019, 60, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms with Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Yahid, I.; Ha, S. The Paradox of Mixed-Species Biofilms in the Context of Food Safety. Compr. Rev. Food Sci. Food Saf. 2014, 13, 990–1011. [Google Scholar] [CrossRef]
- Joshi Navare, K.; Eggermont, L.; Rogers, Z.; Mohammed, H.; Colombani, T.; Bencherif, S. Antimicrobial Hydrogels: Key Considerations and Engineering Strategies for Biomedical Applications. In Racing for the Surface, 1st ed.; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 511–542. ISBN 978-3-030-34475-7. [Google Scholar]
- Garza-Cervantes, J.; Mendiola-Garza, G.; de Melo, E.; Dugmore, T.; Matharu, A.; Morones-Ramirez, J. Antimicrobial activity of a silver-microfibrillated cellulose biocomposite against susceptible and resistant bacteria. Sci. Rep. 2020, 10, 7281. [Google Scholar] [CrossRef] [PubMed]
- Marcello, E.; Maqbool, M.; Nigmatullin, R.; Cresswell, M.; Jackson, P.; Basnett, P.; Knowles, J.; Boccaccini, A.; Roy, I. Antibacterial Composite Materials Based on the Combination of Polyhydroxyalkanoates with Selenium and Strontium Co-substituted Hydroxyapatite for Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 647007. [Google Scholar] [CrossRef]
- Mondal, S.; Sharif, A. Antimicrobial Biocomposites. In Green Biocomposites for Biomedical Engineering, 1st ed.; Hoque, M., Sharif, A., Jawaid, M., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 37–63. ISBN 9780128215548. [Google Scholar]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.d.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [Green Version]
- Rudin, A.; Choi, P. Biopolymers. In The Elements of Polymer Science and Engineering, 3rd ed.; Rudin, A., Choi, P., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 521–535. ISBN 978-0-12-382178-2. [Google Scholar]
- Simionescu, B.; Ivanov, D. Natural and Synthetic Polymers for Designing Composite Materials. In Handbook of Bioceramics and Biocomposites, 1st ed.; Antoniac, I., Ed.; Springer: Cham, Switzerland, 2016; pp. 233–286. ISBN 978-3-319-12460-5. [Google Scholar]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Panda, P.; Dash, P.; Yang, J.; Chang, Y. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Olmos, D.; González-Benito, J. Polymeric Materials with Antibacterial Activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, S.; Cong, Z.; Zhang, H.; Lu, Z.; Shao, N.; Bhatti, S.; Zhou, C.; Cheng, J.; Gellman, S.; et al. Secondary Amine Pendant β-Peptide Polymers Displaying Potent Antibacterial Activity and Promising Therapeutic Potential in Treating MRSA-Induced Wound Infections and Keratitis. J. Am. Chem. Soc. 2022, 144, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Hidalgo-Bonilla, S.; Bucio, E. Natural Antimicrobial Materials. In Advanced Antimicrobial Materials and Applications, 1st ed.; Inamuddin, Ahamed, M.I., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 149–169. ISBN 978-981-15-7098-8. [Google Scholar]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Visakh, P.; Mathew, A. Advances in Natural Polymers, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-20940-6. [Google Scholar]
- Sonnleitner, D.; Sommer, C.; Scheibel, T.; Lang, G. Approaches to inhibit biofilm formation applying natural and artificial silk-based materials. Mater. Sci. Eng. C 2021, 131, 112458. [Google Scholar] [CrossRef]
- Schachter, H. Glycobiology of Caenorhabditis elegans. In Comprehensive Glycoscience, 1st ed.; Kamerling, H., Ed.; Elsevier Science: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany; London, UK; New York, NY, USA, 2007; Volume 4, pp. 81–100. ISBN 978-0-444-51967-2. [Google Scholar]
- Costerton, J.; Stewart, P.; Greenberg, E. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and Control Strategies of Antibiotic Resistance in Pathological Biofilms. J. Microbiol. Biotechnol. 2021, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Ciofu, O.; Johansen, H.; Song, Z.; Moser, C.; Jensen, P.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Watnick, P.; Kolter, R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 1999, 34, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Watnick, P.; Lauriano, C.; Klose, K.; Croal, L.; Kolter, R. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 2001, 39, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Poulin, M.; Kuperman, L. Regulation of Biofilm Exopolysaccharide Production by Cyclic Di-Guanosine Monophosphate. Front. Microbiol. 2021, 12, 2506. [Google Scholar] [CrossRef]
- Whiteley, C.; Lee, D. Bacterial diguanylate cyclases: Structure, function and mechanism in exopolysaccharide biofilm development. Biotechnol. Adv. 2015, 33, 124–141. [Google Scholar] [CrossRef]
- Iliadis, I.; Daskalopoulou, A.; Simões, M.; Giaouris, E. Integrated combined effects of temperature, pH and sodium chloride concentration on biofilm formation by Salmonella enterica ser. Enteritidis and Typhimurium under low nutrient food-related conditions. Food Res. Int. 2018, 107, 10–18. [Google Scholar] [CrossRef]
- Petrin, S.; Mancin, M.; Losasso, C.; Deotto, S.; Olsen, J.; Barco, L. Effect of pH and Salinity on the Ability of Salmonella Serotypes to Form Biofilm. Front. Microbiol. 2022, 13, 821679. [Google Scholar] [CrossRef]
- Kim, K.; Frank, J. Effect of Nutrients on Biofilm Formation by Listeria monocytogenes on Stainless Steel. J. Food Prot. 1995, 58, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.; Loeb, G. Influence of Substratum Characteristics on the Attachment of a Marine Pseudomonad to Solid Surfaces. Appl. Environ. Microbiol. 1979, 37, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Nishad, K.; Bhosle, N. Effects of DNP on the cell surface properties of marine bacteria and its implication for adhesion to surfaces. Biofouling 2007, 23, 171–177. [Google Scholar] [CrossRef]
- O’Toole, G.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Floyd, K.; Eberly, A.; Hadjifrangiskou, M. Adhesion of bacteria to surfaces and biofilm formation on medical devices. In Biofilms and Implantable Medical Devices, 1st ed.; Deng, Y., Lv, W., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 47–95. ISBN 978-0-08-100398-5. [Google Scholar]
- Yang, L.; Hu, Y.; Liu, Y.; Zhang, J.; Ulstrup, J.; Molin, S. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ. Microbiol. 2011, 13, 1705–1717. [Google Scholar] [CrossRef]
- De Kievit, T. Biofilms. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2011; pp. 547–558. ISBN 9780080885049. [Google Scholar]
- Laverty, G.; Gorman, S.; Gilmore, B. Biofilms and implant-associated infections. In Biomaterials and Medical Device—Associated Infections, 1st ed.; Barnes, L., Cooper, I.R., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 19–45. ISBN 978-0-85709-722-4. [Google Scholar]
- Gilbert, P.; Evans, D.; Brown, M. Formation and dispersal of bacterial biofilms in vivo and in situ. J. Appl. Bacteriol. 1993, 74, 67S–78S. [Google Scholar] [CrossRef]
- Khan, M.; Svedberg, A.; Singh, A.; Ansari, M.; Karim, Z. Use of Nanostructured Polymer in the Delivery of Drugs for Cancer Therapy. In Nanostructured Polymer Composites for Biomedical Applications, 1st ed.; Kumar Swain, S., Jawaid, M., Eds.; Elsevier: Oxford, UK, 2019; pp. 261–276. ISBN 9780128168929. [Google Scholar]
- Shrivastava, A. Introduction to Plastics Engineering, 1st ed.; William Andrew: Norwich, NY, USA, 2018; pp. 1–16. ISBN 9780323396196. [Google Scholar]
- Doppalapudi, S.; Katiyar, S.; Domb, A.; Khan, W. Biodegradable Natural Polymers. In Advanced Polymers in Medicine, 1st ed.; Puoci, F., Ed.; Springer: Cham, Switzerland, 2014; pp. 33–66. ISBN 978-3-319-12478-0. [Google Scholar]
- Randazzo, W.; Fabra, M.; Falcó, I.; López-Rubio, A.; Sánchez, G. Polymers and Biopolymers with Antiviral Activity: Potential Applications for Improving Food Safety. Compr. Rev. Food Sci. Food Saf. 2018, 17, 754–768. [Google Scholar] [CrossRef] [Green Version]
- Wicochea-Rodríguez, J.; Chalier, P.; Ruiz, T.; Gastaldi, E. Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release. Front. Chem. 2019, 7, 398. [Google Scholar] [CrossRef]
- George, A.; Shah, P.; Shrivastav, P. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Boffito, M.; Tonda-Turo, C.; Ciardelli, G. Design of electrospun fibrous patches for myocardium regeneration. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices, 1st ed.; Guarino, V., Ambrosio, L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 221–250. ISBN 9780081017463. [Google Scholar]
- Mallakpour, S.; Behranvand, V.; Tabesh, F. Natural polymer–based organic–inorganic hybrid nanosorbents. In Natural Polymers-Based Green Adsorbents for Water Treatment; Susheel, K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 159–193. ISBN 978-0-12-820541-9. [Google Scholar]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.K.; Huang, P.K.; Law, W.C.; Chu, C.H.; Chen, N.T.; Lo, L.W. Biodegradable Polymers for Gene-Delivery Applications. Int. J. Nanomed. 2020, 15, 2131–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, R.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Gkountela, C.I.; Vouyiouka, S.N. Enzymatic Polymerization as a Green Approach to Synthesizing Bio-Based Polyesters. Macromol 2022, 2, 30–57. [Google Scholar] [CrossRef]
- Akhter, R.; Masoodi, F.; Wani, T.; Rather, S. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Maruthapandi, M.; Saravanan, A.; Gupta, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial Activities of Conducting Polymers and Their Composites. Macromol 2022, 2, 78–99. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef]
- Liotier, P.; Pucci, M.; Drapier, S. Fibre/matrix interface. In Biocomposites for High-Performance Applications, 1st ed.; Ray, D., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 165–180. ISBN 978-0-08-100794-5. [Google Scholar]
- Saba, N.; Jawaid, M. Epoxy resin based hybrid polymer composites. In Hybrid Polymer Composite Materials, 1st ed.; Vijay Thakur, K., Thakur, M.K., Pappu, A., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 57–82. ISBN 978-0-08-100788-4. [Google Scholar]
- Pawelec, K.; White, A.; Best, S. Properties and characterization of bone repair materials. In Bone Repair Biomaterials, 2nd ed.; Pawelec, K., Planell, J., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 65–102. ISBN 9780081024522. [Google Scholar]
- Catoira, M.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Narain, R.; Zeng, H. Hydrogels. In Polymer Science and Nanotechnology, 1st ed.; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–244. ISBN 9780128168073. [Google Scholar]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.; Mujtaba, M.; Alghamdi, N.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Brigham, C. Biopolymers. In Green Chemistry, 1st ed.; Török, B., Dransfield, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 753–770. ISBN 9780128095492. [Google Scholar]
- Yahya, E.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Khalil, H. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.; Nada, A.; Kumbar, S. Natural Polymers. In Natural and Synthetic Biomedical Polymers, 1st ed.; Kumbar, S., Laurencin, C.T., Deng, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 67–89. ISBN 9780123972903. [Google Scholar]
- Carvalho, J.; Silva, A.; Silvestre, A.; Freire, C.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Orlando, I.; Basnett, P.; Nigmatullin, R.; Wang, W.; Knowles, J.; Roy, I. Chemical Modification of Bacterial Cellulose for the Development of an Antibacterial Wound Dressing. Front. Bioeng. Biotechnol. 2020, 8, 557885. [Google Scholar] [CrossRef] [PubMed]
- Silva, Â.; Duarte, A.; Sousa, S.; Ramos, A.; Domingues, F. Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. LWT 2016, 73, 481–489. [Google Scholar] [CrossRef]
- Alvarado, D.; Argyropoulos, D.; Scholle, F.; Peddinti, B.; Ghiladi, R. A facile strategy for photoactive nanocellulose-based antimicrobial materials. Green Chem. 2019, 21, 3424–3435. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Joz Majidi, H.; Babaei, A.; Arab Bafrani, Z.; Shahrampour, D.; Zabihi, E.; Jafari, S. Investigating the best strategy to diminish the toxicity and enhance the antibacterial activity of graphene oxide by chitosan addition. Carbohydr. Polym. 2019, 225, 115220. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Yang, S.; Nie, B.; Fan, Q.; Zhang, Q.; Zong, J.; Guo, S.; Zheng, X.; Tang, T. Improved antibacterial properties of collagen I/hyaluronic acid/quaternized chitosan multilayer modified titanium coatings with both contact-killing and release-killing functions. J. Mater. Chem. B 2019, 7, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Hutmacher, D. Polymers from Biotechnology. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Jürgen, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E., Mahajan, S., Veyssière, P., Eds.; Pergamon: Oxford, UK, 2001; pp. 7680–7683. ISBN 9780080431529. [Google Scholar]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ward, V.; Mekonnen, T. Functionalized starch microparticles for contact-active antimicrobial polymer surfaces. Carbohydr. Polym. 2020, 229, 115422. [Google Scholar] [CrossRef]
- Rezapour, N.; Rasekh, B.; Mofradnia, S.; Yazdian, F.; Rashedi, H.; Tavakoli, Z. Molecular dynamics studies of polysaccharide carrier based on starch in dental cavities. Int. J. Biol. Macromol. 2019, 121, 616–624. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sriram, B.; Sathiyaseelan, A.; Mariadoss, A.; Hu, X.; Han, K.; Vishnupriya, V.; MubarakAli, D.; Wang, M. Synthesis, characterization, and cytotoxicity of starch-encapsulated biogenic silver nanoparticle and its improved anti-bacterial activity. Int. J. Biol. Macromol. 2021, 182, 1409–1418. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kong, W.; Zhou, J.; Hou, T.; Zhang, X.; Zhou, L.; Sun, M.; Liu, S.; Yang, B. Cross-Linking of Centrifugally Spun Starch/Polyvinyl Alcohol (ST/PVA) Composite Ultrafine Fibers and Antibacterial Activity Loaded with Ag Nanoparticles. ACS Omega 2022, 7, 7706–7714. [Google Scholar] [CrossRef]
- Shapi’i, R.; Othman, S.; Nordin, N.; Kadir Basha, R.; Nazli Naim, M. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef]
- do Evangelho, J.; da Silva Dannenberg, G.; Biduski, B.; el Halal, S.; Kringel, D.; Gularte, M.; Fiorentini, A.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Chung, H.; Steplewski, A.; Chung, K.; Uitto, J.; Fertala, A. Collagen Fibril Formation. Int. J. Biol. Chem. 2008, 283, 25879–25886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, L.; Xu, Y.; Li, X.; Yuan, L.; Tan, H.; Li, D.; Mu, C. Fabrication of Antibacterial Collagen-Based Composite Wound Dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166. [Google Scholar] [CrossRef]
- Neacsu, I.; Leau, S.; Marin, S.; Holban, A.; Vasile, B.; Nicoara, A.; Ene, V.; Bleotu, C.; Albu Kaya, M.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Alvarez, G.; Hélary, C.; Mebert, A.; Wang, X.; Coradin, T.; Desimone, M. Antibiotic-loaded silica nanoparticle–collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. J. Mater. Chem. B 2014, 2, 4660. [Google Scholar] [CrossRef] [Green Version]
- Vladkova, T.; Ivanova, I.; Staneva, A.; Albu-Kaya, M.; Shalaby, A.; Moskova-Doumanova, V.; Kostadinova, A. Preparation and Biological Activity of New Collagen Composites, Part III. Collagen/(Ag/RGO) and Collagen/(Ag/RGO/SiO2) Composites. J. Arch. Mil. Med. 2017, 5, e57454. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 2017, 63, 360–366. [Google Scholar] [CrossRef]
- David, G. Collagen-based 3D structures—Versatile, efficient materials for biomedical applications. In Biopolymer-Based Formulations, 1st ed.; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W., Dubey, N.K., Majumder, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 881–906. ISBN 9780128168981. [Google Scholar]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.; Khadheer Pasha, S.; Bhagat, P.; Chidambaram, K. Biopolymer Composites With High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics, 1st ed.; Sadasivuni, K., Ponnamma, D., Kim, J., Cabibihan, J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. ISBN 9780081009741. [Google Scholar]
- Leite, L.; Pham, C.; Bilatto, S.; Azeredo, H.; Cranston, E.; Moreira, F.; Mattoso, L.; Bras, J. Effect of Tannic Acid and Cellulose Nanocrystals on Antioxidant and Antimicrobial Properties of Gelatin Films. ACS Sustain. Chem. Eng. 2021, 9, 8539–8549. [Google Scholar] [CrossRef]
- Cui, H.; Liu, M.; Yu, W.; Cao, Y.; Zhou, H.; Yin, J.; Liu, H.; Que, S.; Wang, J.; Huang, C.; et al. Copper Peroxide-Loaded Gelatin Sponges for Wound Dressings with Antimicrobial and Accelerating Healing Properties. ACS Appl. Mater. Interfaces 2021, 13, 26800–26807. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Y.; Wang, Y.; Cao, Y. Tissue Engineering of Blood Vessels. In Encyclopedia of Tissue Engineering and Regenerative Medicine, 1st ed.; Reis, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 1, pp. 413–424. ISBN 9780128137000. [Google Scholar]
- Thongsrikhem, N.; Taokaew, S.; Sriariyanun, M.; Kirdponpattara, S. Antibacterial activity in gelatin-bacterial cellulose composite film by thermally crosslinking with cinnamaldehyde towards food packaging application. Food Packag. Shelf Life 2022, 31, 100766. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications. Appl. Sci. 2021, 11, 6307. [Google Scholar] [CrossRef]
- Khosravimelal, S.; Chizari, M.; Farhadihosseinabadi, B.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application. J. Mater. Sci. Mater. Med. 2021, 32, 114. [Google Scholar] [CrossRef]
- Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.; Ashrafizadeh, M.; Zare, E.; Agarwal, T.; Padil, V.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 12. [Google Scholar] [CrossRef]
- Zhang, Z.; Suner, S.; Blake, D.; Ayyala, R.; Sahiner, N. Antimicrobial activity and biocompatibility of slow-release hyaluronic acid-antibiotic conjugated particles. Int. J. Pharm. 2020, 576, 119024. [Google Scholar] [CrossRef]
- Walvekar, P.; Gannimani, R.; Salih, M.; Makhathini, S.; Mocktar, C.; Govender, T. Self-assembled oleylamine grafted hyaluronic acid polymersomes for delivery of vancomycin against methicillin resistant Staphylococcus aureus (MRSA). Colloids Surf. B Biointerfaces. 2019, 182, 110388. [Google Scholar] [CrossRef] [PubMed]
- Kłodzińska, S.; Wan, F.; Jumaa, H.; Sternberg, C.; Rades, T.; Nielsen, H. Utilizing nanoparticles for improving anti-biofilm effects of azithromycin: A head-to-head comparison of modified hyaluronic acid nanogels and coated poly (lactic-co-glycolic acid) nanoparticles. J. Colloid Interface Sci. 2019, 555, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Lequeux, I.; Ducasse, E.; Jouenne, T.; Thebault, P. Addition of antimicrobial properties to hyaluronic acid by grafting of antimicrobial peptide. Eur. Polym. J. 2014, 51, 182–190. [Google Scholar] [CrossRef]
- Harris, L.; Richards, R. Staphylococcus aureus adhesion to different treated titanium surfaces. J. Mater. Sci. Mater. Med. 2004, 15, 311–314. [Google Scholar] [CrossRef]
- Felgueiras, H.; Wang, L.; Ren, K.; Querido, M.; Jin, Q.; Barbosa, M.; Ji, J.; Martins, M. Octadecyl Chains Immobilized onto Hyaluronic Acid Coatings by Thiol–ene “Click Chemistry” Increase the Surface Antimicrobial Properties and Prevent Platelet Adhesion and Activation to Polyurethane. ACS Appl. Mater. Interfaces 2017, 9, 7979–7989. [Google Scholar] [CrossRef]
- Preman, N.; Jain, S.; Sanjeeva, S.; Johnson, R. Alginate derived nanoassemblies in drug delivery and tissue engineering. In Polysaccharide Nanoparticles, 1st ed.; Venkatesan, J., Kim, S., Anil, S., Rekha, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–280. ISBN 9780128223567. [Google Scholar]
- Ahmed, A.; Boateng, J. Calcium alginate-based antimicrobial film dressings for potential healing of infected foot ulcers. Ther. Deliv. 2018, 9, 185–204. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.; Andronescu, E.; Holban, A. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Ene, V.; Vasile, B.; Andronescu, E.; Holban, A. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín-Santos, I.; Gandía-Llop, S.; Salesa, B.; Martí, M.; Lillelund Aachmann, F.; Serrano-Aroca, Á. Enhancement of Antimicrobial Activity of Alginate Films with a Low Amount of Carbon Nanofibers (0.1% w/w). Appl. Sci. 2021, 11, 2311. [Google Scholar] [CrossRef]
- Agren, M. Zinc in Wound Repair. Arch. Dermatol. 1999, 135, 1273–1274. [Google Scholar] [CrossRef]
- Asadpoor, M.; Ithakisiou, G.; van Putten, J.; Pieters, R.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I. Fibrin. In Comprehensive Biomaterials, 1st ed.; Ducheyne, P., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2011; Volume 2, pp. 303–328. ISBN 9780080552941. [Google Scholar]
- Ahmed, T.; Dare, E.; Hincke, M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Venante, H.; Chappuis-Chocano, A.; Marcillo-Toala, O.; da Silva, R.; da Costa, R.; Pordeus, M.; Barraviera, B.; Ferreira Junior, R.; Lara, V.; Neppelenbroek, K.; et al. Fibrin Biopolymer Incorporated with Antimicrobial Agents: A Proposal for Coating Denture Bases. Materials 2021, 14, 1618. [Google Scholar] [CrossRef]
- Tan, R.; Lee, H.; Ma, H.; Lee, H.; Han, S. Antibacterial Effect of Antibiotic-Saturated Fibrin Sealant; In Vitro Study. J. Wound Manag. Res. 2018, 14, 12–17. [Google Scholar] [CrossRef]
- Shin, D.; Sohn, M.; Cho, C.; Koo, H.; Yoon, S. Evaluation of Cumulative and Conditional Antibiotic Release from Vancomycin-Embedded Fibrin Sealant and Its Antibacterial Activity: An In Vitro Study. J. Korean Neurosurg. Soc. 2020, 63, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Zahran, M.; Marei, A. Innovative natural polymer metal nanocomposites and their antimicrobial activity. Int. J. Biol. Macromol. 2019, 136, 586–596. [Google Scholar] [CrossRef]
- Castro, A.; Herrero, E.; Slomka, V.; Pinto, N.; Teughels, W.; Quirynen, M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci. Rep. 2019, 9, 8188. [Google Scholar] [CrossRef] [Green Version]
- Climov, M.; Leavitt, T.; Molnar, J.; Orgill, D. Natural Biomaterials for Skin Tissue Engineering. In Skin Tissue Engineering and Regenerative Medicine, 1st ed.; Albanna, M., Holmes, J.H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 145–161. ISBN 9780128017975. [Google Scholar]
- Lanza, C. CITRUS FRUITS|Processed and Derived Products of Oranges. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 1346–1354. ISBN 9780080917917. [Google Scholar]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Hari, K.; Garcia, C.; Shin, G.; Kim, J. Improvement of the UV Barrier and Antibacterial Properties of Crosslinked Pectin/Zinc Oxide Bionanocomposite Films. Polymers 2021, 13, 2403. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.; Moura, M.; Aouada, F.; Camilloto, G.; Cruz, R.; Lorevice, M.; Soares, N.; Mattoso, L. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Calce, E.; Mignogna, E.; Bugatti, V.; Galdiero, M.; Vittoria, V.; De Luca, S. Pectin functionalized with natural fatty acids as antimicrobial agent. Int. J. Biol. Macromol. 2014, 68, 28–32. [Google Scholar] [CrossRef]
- Trejo-González, L.; Rodríguez-Hernández, A.; del Rocío López-Cuellar, M.; Martínez-Juárez, V.; Chavarría-Hernández, N. Antimicrobial pectin-gellan films: Effects on three foodborne pathogens in a meat medium, and selected physical-mechanical properties. CYTA J. Food 2018, 16, 469–476. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.; Fernández-García, M. Bio-Based Polymers With Antimicrobial Properties Towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norrrahim, M.; Nurazzi, N.; Jenol, M.; Farid, M.; Janudin, N.; Ujang, F.; Yasim-Anuar, T.; Syed Najmuddin, S.; Ilyas, R. Emerging development of nanocellulose as an antimicrobial material: An overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Szostak-Kotowa, J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Vigo, T.L. Protection of Textiles from Biological Attack. In Handbook of Fibre Science and Technology; Lewin, M., Sello, S.B., Eds.; Marcel Dekker, INC.: New City, NY, USA, 1983; Volume 2, pp. 367–426. ISBN 9781351442732. [Google Scholar]
- Schindler, W.; Hauser, P. Chemical Finishing of Textiles, 1st ed.; Schindler, W., Hauser, P., Eds.; Woodhead Publishing: Cambridge, UK, 2004; pp. 165–174. ISBN 9781845690373. [Google Scholar]
- Simončič, B.; Tomšič, B. Recent Concepts of Antimicrobial Textile Finishes. In Textile Finishing: Recent Developments and Future Trends; Mital, K.L., Bahners, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1, pp. 3–68. ISBN 978-1-119-42685-1. [Google Scholar]
- Malis, D.; Jeršek, B.; Tomšič, B.; Štular, D.; Golja, B.; Kapun, G.; Simončič, B. Antibacterial Activity and Biodegradation of Cellulose Fiber Blends with Incorporated ZnO. Materials 2019, 12, 3399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopakumar, D.A.; Thomas, S.; Grohens, Y. Nanocelluloses as Innovative Polymers for Membrane Applications. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements, 1st ed.; Puglia, D., Fortunati, E., Kenny, J.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 253–275. ISBN 9780323417396. [Google Scholar]
- Kumar, R.; Kumar, G. Nanocellulose: Fascinating and sustainable nanomaterial for papermaking. In Nanotechnology in Paper and Wood Engineering, 1st ed.; Bhat, R., Kumar, A., Nguyen, T.A., Sharma, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 389–407. ISBN 9780323859639. [Google Scholar]
- Rashki, S.; Shakour, N.; Yousefi, Z.; Rezaei, M.; Homayoonfal, M.; Khabazian, E.; Atyabi, F.; Aslanbeigi, F.; Safaei Lapavandani, R.; Mazaheri, S.; et al. Cellulose-Based Nanofibril Composite Materials as a New Approach to Fight Bacterial Infections. Front. Bioeng. Biotechnol. 2021, 9, 732461. [Google Scholar] [CrossRef] [PubMed]
- Littunen, K.; Snoei de Castro, J.; Samoylenko, A.; Xu, Q.; Quaggin, S.; Vainio, S.; Seppälä, J. Synthesis of cationized nanofibrillated cellulose and its antimicrobial properties. Eur. Polym. J. 2016, 75, 116–124. [Google Scholar] [CrossRef]
- Arakere, U.; Jagannath, S.; Krishnamurthy, S.; Chowdappa, S.; Konappa, N. Microbial bio-pesticide as sustainable solution for management of pests. In Biopesticides, 1st ed.; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Kumar, A., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 183–200. ISBN 9780128236147. [Google Scholar]
- Dutta, P.; Tripathi, S.; Mehrotra, G.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Panda, P.; Yang, J.; Chang, Y.; Su, W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Samadi, F.; Mohammadi, Z.; Yousefi, M.; Majdejabbari, S. Synthesis of raloxifene–chitosan conjugate: A novel chitosan derivative as a potential targeting vehicle. Int. J. Biol. Macromol. 2016, 82, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Liakos, E.V.; Lazaridou, M.; Michailidou, G.; Koumentakou, I.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review. Macromol 2021, 1, 130–154. [Google Scholar] [CrossRef]
- Barbosa, M.; Pêgo, A.; Amaral, I. Chitosan. Compr. Biomater. 2011, 2, 221–237. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Revuri, V.; Huh, K.; Lee, Y. Polysaccharide based nano/microformulation: An effective and versatile oral drug delivery system. In Nanostructures for Oral Medicine, 1st ed.; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 409–433. ISBN 9780323477215. [Google Scholar]
- Jean, M.; Alameh, M.; De Jesus, D.; Thibault, M.; Lavertu, M.; Darras, V.; Nelea, M.; Buschmann, M.; Merzouki, A. Chitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetes. Eur. J. Pharm. Sci. 2012, 45, 138–149. [Google Scholar] [CrossRef]
- Das, B.; Patra, S. Antimicrobials. In Nanostructures for Antimicrobial Therapy, 1st ed.; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. ISBN 9780323461511. [Google Scholar]
- Kaczmarek, M.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Tsai, G.; Su, W. Antibacterial Activity of Shrimp Chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.; Selvakumar, D.; Kadirvelu, K.; Kumar, N. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef]
- Varghese, S.; Siengchin, S.; Parameswaranpillai, J. Essential oils as antimicrobial agents in biopolymer-based food packaging—A comprehensive review. Food Biosci. 2020, 38, 100785. [Google Scholar] [CrossRef]
- Alves, V.; Rico, B.; Cruz, R.; Vicente, A.; Khmelinskii, I.; Vieira, M. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT 2018, 89, 525–534. [Google Scholar] [CrossRef] [Green Version]
- McKeen, L. The effect of heat aging on the properties of sustainable polymers. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers, 2nd ed.; William Andrew: Norwich, NY, USA, 2021; pp. 313–332. ISBN 978-0-323-85437-5. [Google Scholar]
- Winkler, S.; Kaplan, D. Biosynthesized Materials: Properties and Processing. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Jürgen, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E., Mahajan, S., Veyssière, P., Eds.; Pergamon: Oxford, UK, 2001; pp. 609–615. ISBN 9780080431529. [Google Scholar]
- Van Soest, J.; Benes, K.; De Wit, D.; Vliegenthart, J. The influence of starch molecular mass on the properties of extruded thermoplastic starch. Polymer 1996, 37, 3543–3552. [Google Scholar] [CrossRef] [Green Version]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Mustapha, F.; Jai, J.; Nik Raikhan, N.; Sharif, Z.; Yusof, N. Response surface methodology analysis towards biodegradability and antimicrobial activity of biopolymer film containing turmeric oil against Aspergillus niger. Food Control 2019, 99, 106–113. [Google Scholar] [CrossRef]
- Cheema, U.; Ananta, M.; Muder, V. Collagen: Applications of a Natural Polymer in Regenerative Medicine. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-51-4450-2. [Google Scholar]
- Balasubramanian, P.; Prabhakaran, M.; Sireesha, M.; Ramakrishna, S. Collagen in Human Tissues: Structure, Function, and Biomedical Implications from a Tissue Engineering Perspective. In Polymer Composites—Polyolefin Fractionation—Polymeric Peptidomimetics—Collagens, 1st ed.; Abe, A., Kausch, H.H., Möller, M., Pasch, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 173–206. ISBN 978-3-642-34330-8. [Google Scholar]
- Lin, K.; Zhang, D.; Macedo, M.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2018, 29, 1804943. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical applications. J. Food Sci. Technol. 2014, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patino, M.; Neiders, M.; Andreana, S.; Noble, B.; Cohen, R. Collagen as an Implantable Material in Medicine and Dentistry. J. Oral Implantol. 2002, 28, 220–225. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Kanagesan, S.; Pandurangan, A.; Padmanabhan, P. Basics to different imaging techniques, different nanobiomaterials for image enhancement. In Nanobiomaterials in Medical Imaging, 1st ed.; Grumezescu, A.M., Ed.; William Andrew: Norwich, NY, USA, 2016; Volume 8, pp. 101–129. ISBN 9780323417389. [Google Scholar]
- Pereda, M.; Ponce, A.; Marcovich, N.; Ruseckaite, R.; Martucci, J. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Said, N.; Howell, N.; Sarbon, N. A Review on Potential Use of Gelatin-based Film as Active and Smart Biodegradable Films for Food Packaging Application. Food Rev. Int. 2021, 1–23. [Google Scholar] [CrossRef]
- Clarke, D.; Molinaro, S.; Tyuftin, A.; Bolton, D.; Fanning, S.; Kerry, J. Incorporation of commercially-derived antimicrobials into gelatin-based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control 2016, 64, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Jridi, M.; Hajji, S.; Ayed, H.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Bower, C.; Avena-Bustillos, R.; Olsen, C.; McHugh, T.; Bechtel, P. Characterization of Fish-Skin Gelatin Gels and Films Containing the Antimicrobial Enzyme Lysozyme. J. Food Sci 2006, 71, M141–M145. [Google Scholar] [CrossRef]
- Abarca, R.; Medina, J.; Alvarado, N.; Ortiz, P.; Carrillo López, B. Biodegradable gelatin-based films with nisin and EDTA that inhibit Escherichia coli. PLoS ONE 2022, 17, e0264851. [Google Scholar] [CrossRef] [PubMed]
- Martucci, J.; Gende, L.; Neira, L.; Ruseckaite, R. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop. Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Guvendiren, M.; Purcell, B.; Burdick, J. Photopolymerizable Systems. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 413–438. ISBN 9780080878621. [Google Scholar]
- Burd, A. Hyaluronan and Scarring. In Chemistry and Biology of Hyaluronan, 1st ed.; Garg, H.G., Hales, C.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 367–394. ISBN 9780080472225. [Google Scholar]
- Bayer, I. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Burdick, J.; Stevens, M. Biomedical hydrogels. Biomaterials, Artificial Organs and Tissue Engineering, 1st ed.; Hench, L., Jones, J., Eds.; Woodhead Publishing: Cambridge, UK, 2005; pp. 107–115. ISBN 9781845690861. [Google Scholar]
- Zamboni, F.; Okoroafor, C.; Ryan, M.; Pembroke, J.; Strozyk, M.; Culebras, M.; Collins, M. On the bacteriostatic activity of hyaluronic acid composite films. Carbohydr. Polym. 2021, 260, 117803. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.; Vecchi, E.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Drago, L.; Cappelletti, L.; De Vecchi, E.; Pignataro, L.; Torretta, S.; Mattina, R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. APMIS 2014, 122, 1013–1019. [Google Scholar] [CrossRef]
- Zamboni, F.; Wong, C.; Collins, M. Hyaluronic acid association with bacterial, fungal and viral infections: Can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioact. Mater. 2022, 19, 458–473. [Google Scholar] [CrossRef]
- Carlson, G.; Dragoo, J.; Samimi, B.; Bruckner, D.; Bernard, G.; Hedrick, M.; Benhaim, P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem. Biophys. Res. Commun. 2004, 321, 472–478. [Google Scholar] [CrossRef]
- Schanté, C.; Zuber, G.; Herlin, C.; Vandamme, T. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Azua, I.; Benito, V.; Bilbao, A.; Vilas-Vilela, J. Development of multiactive antibacterial multilayers of hyaluronic acid and chitosan onto poly (ethylene terephthalate). Eur. Polym. J. 2019, 112, 31–37. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.; Nasaruddin, R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mooney, D. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P. Introduction to biomaterials for wound healing. In Wound Healing Biomaterials, 1st ed.; Agren, M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; Volume 2, pp. 3–38. ISBN 9780081006061. [Google Scholar]
- Hamai, R.; Anada, T.; Suzuki, O. Novel scaffold composites containing octacalcium phosphate and their role in bone repair. In Octacalcium Phosphate Biomaterials, 1st ed.; Suzuki, O., Insley, G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 121–145. ISBN 9780081025123. [Google Scholar]
- Skjåk-Bræk, G.; Draget, K. Alginates. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 213–220. [Google Scholar]
- Carrow, J.; Kerativitayanan, P.; Jaiswal, M.; Lokhande, G.; Gaharwar, A. Polymers for Bioprinting. In Essentials of 3D Biofabrication and Translation, 1st ed.; Atala, A., Yoo, J.J., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 229–248. ISBN 9780128010150. [Google Scholar]
- Chato-Astrain, J.; Chato-Astrain, I.; Sánchez-Porras, D.; García-García, Ó.; Bermejo-Casares, F.; Vairo, C.; Villar-Vidal, M.; Gainza, G.; Villullas, S.; Oruezabal, R.; et al. Generation of a novel human dermal substitute functionalized with antibiotic-loaded nanostructured lipid carriers (NLCs) with antimicrobial properties for tissue engineering. J. Nanobiotechnol. 2020, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D. The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for The Prevention of Cardiovascular Disease, 1st ed.; Samaan, R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. ISBN 9780128052754. [Google Scholar]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willats, W.; McCartney, L.; Mackie, W.; Knox, J. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Flutto, L. PECTIN | Properties and Determination. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 4440–4449. ISBN 9780080917917. [Google Scholar]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, V.; Sharma, P.; Malviya, R. Pectins and Their Role in Food and Pharmaceutical Industry: A Review. J. Chronother Drug Deliv. 2015, 6, 65–77. [Google Scholar]
- Marcus, J. Food Science Basics: Healthy Cooking and Baking Demystified. In Culinary Nutrition, 1st ed.; Marcus, J., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 51–97. ISBN 9780123918833. [Google Scholar]
- Minzanova, S.; Mironov, V.; Arkhipova, D.; Khabibullina, A.; Mironova, L.; Zakirova, Y.; Milyukov, V. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.; Pagliaro, M. Pectin: A Long-Neglected Broad-Spectrum Antibacterial. Chemmedchem 2020, 15, 2228–2235. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. Chemistryopen 2020, 9, 628–630. [Google Scholar] [CrossRef]
- Nastasi, J.; Kontogiorgos, V.; Daygon, V.; Fitzgerald, M. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.; Shetty, N. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Ardjoum, N.; Shankar, S.; Chibani, N.; Salmieri, S.; Lacroix, M. In situ synthesis of silver nanoparticles in pectin matrix using gamma irradiation for the preparation of antibacterial pectin/silver nanoparticles composite films. Food Hydrocoll. 2021, 121, 107000. [Google Scholar] [CrossRef]
- Lopes, N.; Barreto Pinilla, C.; Brandelli, A. Antimicrobial activity of lysozyme-nisin co-encapsulated in liposomes coated with polysaccharides. Food Hydrocoll. 2019, 93, 1–9. [Google Scholar] [CrossRef]
- Kurczewska, J.; Ratajczak, M.; Gajecka, M. Alginate and pectin films covering halloysite with encapsulated salicylic acid as food packaging components. Appl. Clay Sci. 2021, 214, 106270. [Google Scholar] [CrossRef]
- Tarusha, L.; Paoletti, S.; Travan, A.; Marsich, E. Alginate membranes loaded with hyaluronic acid and silver nanoparticles to foster tissue healing and to control bacterial contamination of non-healing wounds. J. Mater. Sci. Mater. Med. 2018, 29, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, P.; Yao, Z.; Fang, Q.; Feng, L.; Guo, R.; Cheng, B. Preparation of Antimicrobial Hyaluronic Acid/Quaternized Chitosan Hydrogels for the Promotion of Seawater-Immersion Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 360. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Liu, Z.; Pan, F.; Meng, R.; Bu, X.; Xing, H.; Deng, Y.; Guo, N.; Yu, L. Antibacterial activity and mode of action of ε-polylysine against Escherichia coli O157:H7. J. Med. Microbiol. 2018, 67, 838–845. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Int. Food Res. J. 2020, 136, 109327. [Google Scholar] [CrossRef]
- Cassinelli, C.; Morra, M.; Pavesio, A.; Renier, D. Evaluation of interfacial properties of hyaluronan coated poly(methylmethacrylate) intraocular lenses. J. Biomater. Sci. Polym. Ed. 2000, 11, 961–977. [Google Scholar] [CrossRef]
- Morra, M.; Cassineli, C. Non-fouling properties of polysaccharide-coated surfaces. J. Biomater. Sci. Polym. Ed. 1999, 10, 1107–1124. [Google Scholar] [CrossRef] [PubMed]
- Gómez Chabala, L.; Cuartas, C.; López, M. Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe vera and Silver Nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khojastehfar, A.; Mahjoub, S. Application of Nanocellulose Derivatives as Drug Carriers; A Novel Approach in Drug Delivery. Anti-Cancer Agents Med. Chem. 2021, 21, 692–702. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).